Introduction
Osteoclasts are important cells involved in bone
resorption. They are multinucleated cells derived from
hematopoietic cells (1–3). In the presence of receptor-activated
nuclear factor κB ligand (RANKL) and macrophage colony-stimulating
factor (M-CSF), hematopoietic precursor cells differentiate into
mature osteoclasts, which are fused polykaryons arising from
multiple monocytic cells (3,4).
Numerous in vitro models of osteoclast
differentiation have been developed. It has been suggested that
bone marrow cells (5), spleen
cells (6) and blood monocytes
(7) are able to differentiate into
osteoclasts in the presence of certain specific factors.
Tartrate-resistant acid phosphatase 5b (TRACP 5b) is one of several
bone metabolic biomarkers that are specifically secreted by
osteoclasts (8,9) and has been used as biomarker of bone
resorption and cancer metastasis. Alatalo et al (10) and Rissanen et al (11) have shown that TRACP 5b is a good
indicator of osteoclast number in mouse bone marrow-derived
osteoclasts and human blood monocyte-derived osteoclasts.
The mouse macrophage cell line RAW 264.7 is a
transfectable monocyte/macrophage cell line that retains the
capacity to differentiate into osteoclast-like cells in the
presence of RANKL (12,13). RAW 264.7 cells have been widely
used and accepted as a cellar model of osteoclast formation and
function in biology and pharmacology (14,15).
However, to the best of our knowledge, no studies have investigated
whether TRACP 5b can be used as an indicator of osteoclast number
in RAW 264.7 cell-derived osteoclasts. In the present study, using
the RAW 264.7 cell line, the association of TRACP 5b and the number
of osteoclasts was investigated.
Materials and methods
RAW 264.7 cell culture
The RAW 264.7 mouse monocyte/macrophage cell line
(American Type Culture Collection number, TIB-71) was obtained as a
gift from Dr Hui Sheng (Department of Orthopedics and Traumatology,
The Chinese University of Hong Kong, Hong Kong, China). The cells
have the capacity to differentiate into osteoclast-like cells in
the presence of RANKL. The cells were cultured in α-MEM containing
10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA),
100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in a
humidified atmosphere of 95% air and 5% CO2 with a
change of medium every two days.
TRACP staining
RAW 264.7 cells were cultured with α-MEM containing
RANKL (40 ng/ml) for 3, 5 and 7 days with a change of medium every
2 days. The cells were fixed and stained for TRACP using an Acid
Phosphatase, Leukocyte (TRAP) staining kit (catalog number, 387A;
Sigma-Aldrich) according to the manufacturer’s instructions. Cells
that were stained red were considered to be differentiated
osteoclast-like cells and multinucleated cells were those
comprising ≥3 nuclei. The number of TRACP+ cells was
counted using Simple PCI imaging software (Compix Inc., Cranberry,
PA, USA) by two individuals who were blinded to the previous
treatment of the cells.
Scanning electron microscope (SEM)
examination
RAW 264.7 cells were cultured on glass slides with
α-MEM containing RANKL (40 ng/ml) for 3, 5 and 7 days with a change
of medium every two days. The RAW 264.7 cells were fixed using 4%
paraformaldehyde and SEM analysis was conducted using a Hitachi
S520 SEM (Hitachi Ltd., Tokyo, Japan). The slices were mounted on
the SEM stub with carbon tape and carbon coated prior to
analysis.
TRACP 5b assay
RAW 264.7 cells were cultured with α-MEM containing
RANKL (40 ng/ml) for 3, 5 and 7 days with a change of medium every
two days and the supernatants on days 3, 5 and 7 were collected and
stored at −80°C. A MouseTRAP™ enzyme immunoassay (EIA) kit
(Immunodiagnostic Systems Ltd., Boldon, UK) was used in the
determination of the level of TRACP 5b in the supernatants
according to the instructions provided by the manufacturer. The
intra-assay variation of the method for TRACP 5b was <6.5% and
the inter-assay variation was <8%. The standard sample supplied
by the kit was 2.0 (1.6–2.5) U/l, and the obtained result in our
laboratory was 1.94 U/l.
Statistical analysis
SPSS software, version 11.5 (SPSS Inc., Chicago, IL,
USA) was used for statistical analysis. Data are represented as the
mean ± standard deviation. The correlation of the TRACP 5b level
and the number of osteoclasts was analyzed using Pearson
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
TRACP-positive cell formation
The results of the TRACP staining assay indicated
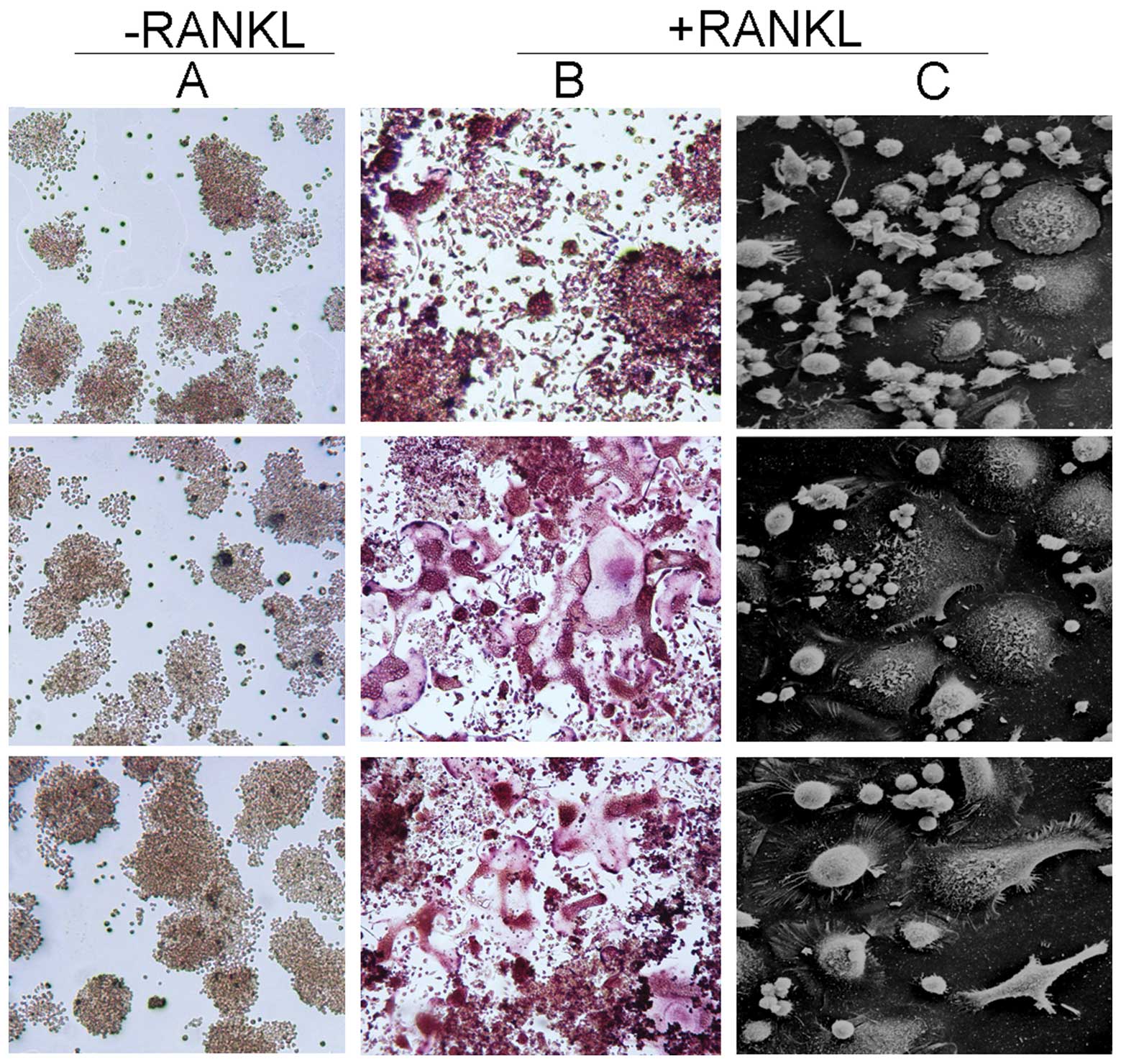
that RANKL induced the formation of osteoclasts from RAW 264.7
cells. No clearly TRAP+ cells were observed in the
untreated RAW 264.7 cells (Fig.
1A). A number of multinucleated (≥3 nuclei) giant cells were
formed in the RAW 264.7 cells that were treated with 40 ng/ml RANKL
for 5 and 7 days (Fig. 1B). SEM
showed the osteoclast-like cells were larger, darker, branched
cells with a number of microvilli (Fig. 1C). Furthermore, the number of
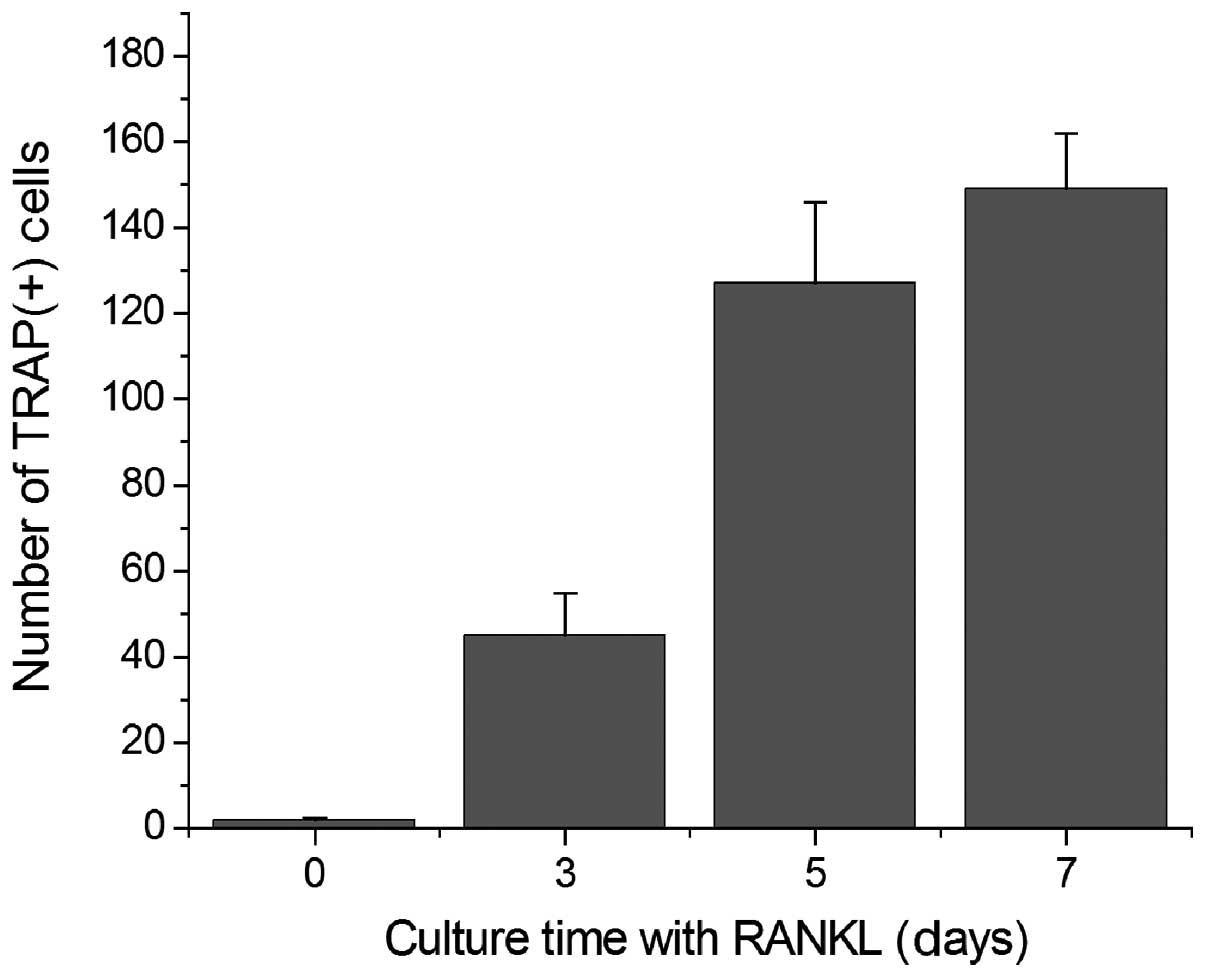
TRAP+ cells was determined. The results indicated that
RANKL induced a marked increase in the number of TRAP+
cells (Fig. 2).
TRACP 5b level
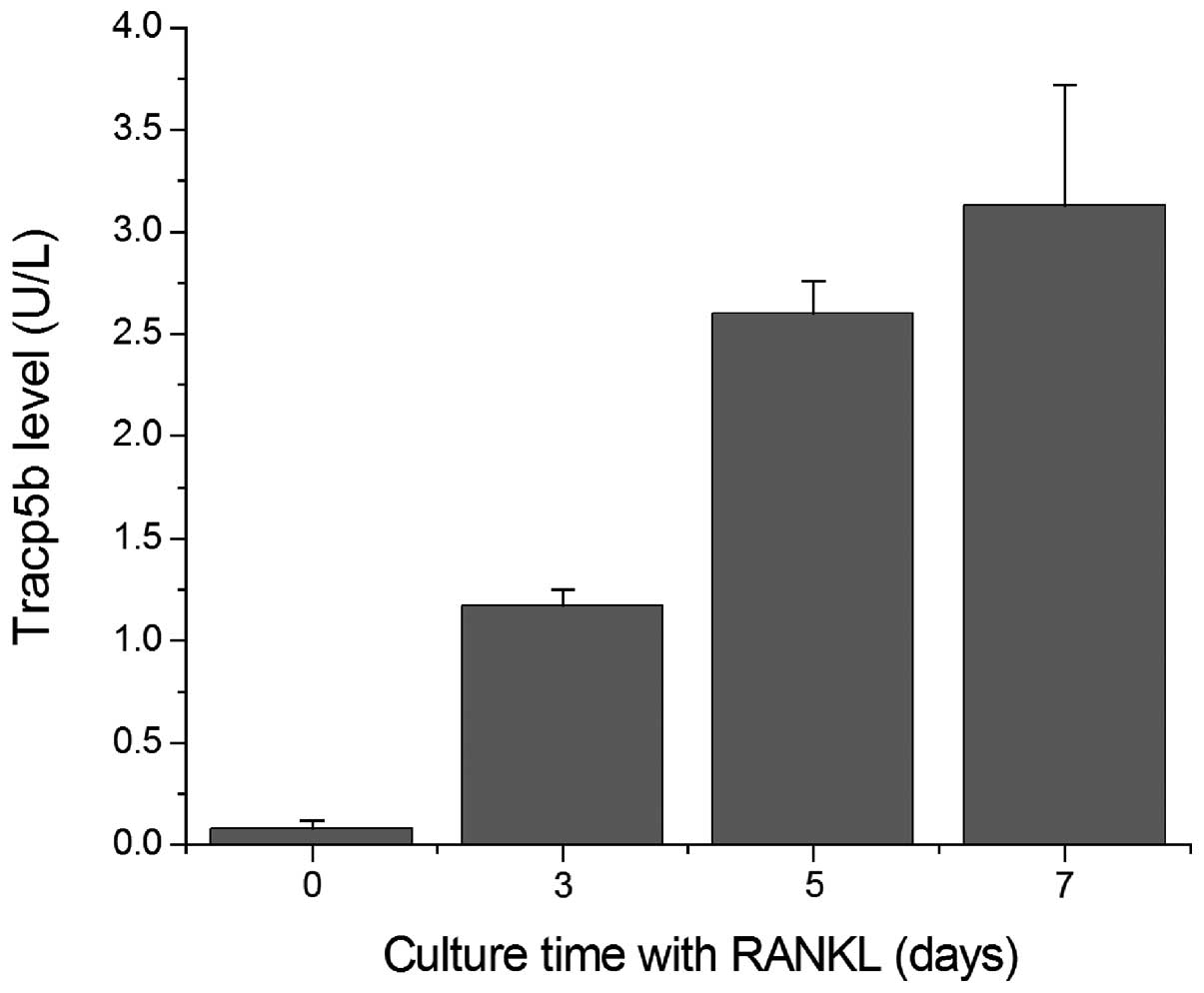
The TRACP 5b level in the RAW 264.7 cells was
examined using an EIA kit. TRACP 5b was detected in the RAW 264.7
cells following treatment with RANKL for 3, 5 and 7 days, and the
level of TRACP 5b increased as the culture time increased (Fig. 3).
Correlation of TRACP 5b with osteoclast
number and volume
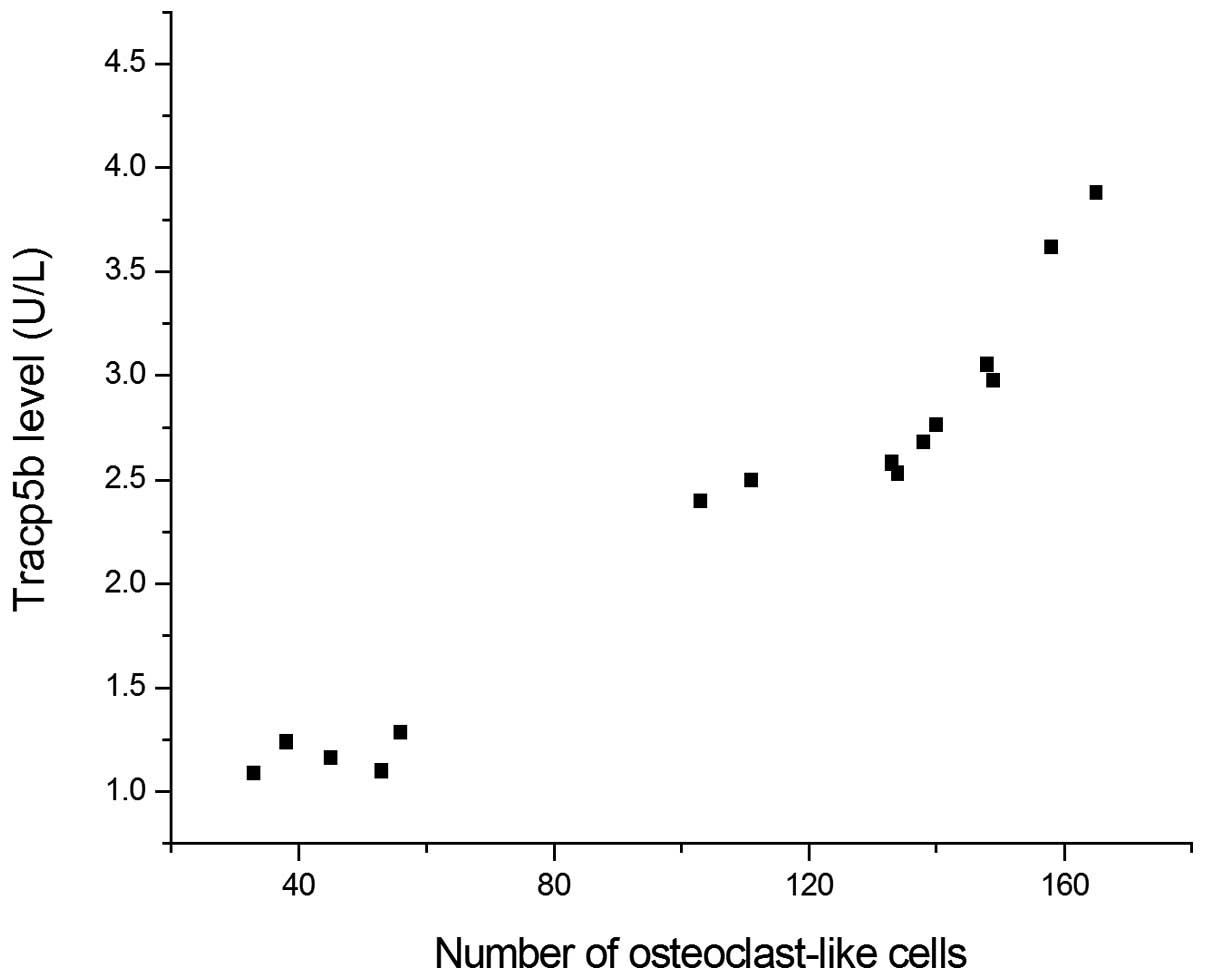
The results of the correlation analysis, presented
in Figs. 4 and 5, indicate that number and volume of
osteoclast-like cells was significantly correlated with the level
of TRACP 5b released into the medium (r=0.95 and 0.92,
respectively; P<0.001).
Discussion
In the present study, it was demonstrated that RANKL
induced RAW 264.7 cell fusion to form mature osteoclasts. The level
of TRACP 5b secreted into the culture medium by the RAW 264.7
cell-derived osteoclast-like cells was significantly correlated
with the number of osteoclastic-like cells formed. These results
suggest that TRACP 5b analysis may be used as an alternative to the
microscopic counting of osteoclasts differentiated from RAW 264.7
cells with the presence of RANKL.
TRACP 5b has been used in the diagnosis of bone
diseases (16,17), such as osteoporosis and bone
metastasis, and for monitoring antiresorptive treatment (18). It has been suggested that TRACP 5b
levels may reflect the status of bone resorption (9). TRACP 5b levels have been shown to
correlate with the number of osteoclasts in patients with
osteopetrosis (19) and renal bone
disease (20). However, to the
best of our knowledge, no studies have investigated the correlation
of TRACP 5b levels with osteoclast number in vitro.
In the present study, it was observed that RAW 264.7
cells cultured with RANKL secreted TRACP 5b constitutively into the
medium. The TRACP 5b level was correlated with the number of
osteoclast-like cells. The data were consistent with the results
observed by Alatalo et al (10). However, there was a certain
difference in the findings. The plot of osteoclast number and TRACP
5b data in the present study formed a curve, not a straight line as
was observed for the data provided by Alatalo et al
(10) and Rassanen et al
(11). The data appeared to fit a
binomial curve. This may be due to the different cell type used in
the present study. In addition, it is speculated that the TRACP 5b
level may not be only associated with the osteoclast number, but
also with osteoclast size. Big osteoclasts may secrete greater
amounts of TRACP 5b into the culture medium compared with that
secreted by smaller osteoclasts.
An increasing number of studies have adopted RAW
264.7 cell as a source of osteoclasts to study the effects of
potential therapeutic agents on bone diseases (21,22)
and to conduct osteoclast-related research (23–25).
Thus, it would be of benefit to develop new methods for quantifying
osteoclasts as an alternative to osteoclast counting using light
microscopy. The data from the present study indicate that TRACP 5b
immunoassay may be a fast and reliable method to replace
time-consuming osteoclast counting.
References
|
1
|
Väänänen HK, Zhao H, Mulari M and Halleen
JM: The cell biology of osteoclast function. J Cell Sci.
113:377–381. 2000.PubMed/NCBI
|
|
2
|
Blair HC and Athanasou NA: Recent advances
in osteoclast biology and pathological bone resorption. Histol
Histopathol. 19:189–199. 2004.PubMed/NCBI
|
|
3
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cuetara BV, Crotti TN, O’Donoghue AJ and
McHugh KP: Cloning and characterization of osteoclast precursors
from the RAW264.7 cell line. In Vitro Cell Dev Biol Anim.
42:182–188. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
MacDonald BR, Takahashi N, et al:
Formation of multinucleated cells that respond to osteotropic
hormones in long term human bone marrow cultures. Endocrinology.
120:2326–2333. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Udagawa N, Takahashi N, Akatsu T, et al:
Origin of osteoclasts: mature monocytes and macrophages are capable
of differentiating into osteoclasts under a suitable
microenvironment prepared by bone marrow-derived stromal cells.
Proc Natl Acad Sci USA. 87:7260–7264. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Higuchi S, Tabata N, Tajima M, et al:
Induction of human osteoclast-like cells by treatment of blood
monocytes with anti-fusion regulatory protein-1/CD98 monoclonal
antibodies. J Bone Miner Res. 13:44–49. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Halleen JM, Alatalo SL, Suominen H, et al:
Tartrate-resistant acid phosphatase 5b: a novel serum marker of
bone resorption. J Bone Miner Res. 15:1337–1345. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Halleen JM, Tiitinen SL, Ylipahkala H,
Fagerlund KM and Väänänen HK: Tartrate-resistant acid phosphatase
5b (TRACP 5b) as a marker of bone resorption. Clin Lab. 52:499–509.
2006.PubMed/NCBI
|
|
10
|
Alatalo SL, Halleen JM, et al: Rapid
screening method for osteoclast differentiation in vitro that
measures tartrate-resistant acid phosphatase 5b activity secreted
into the culture medium. Clin Chem. 46:1751–1754. 2000.PubMed/NCBI
|
|
11
|
Rissanen JB, Suominen MI, Peng ZQ and
Halleen JM: Secreted tartrate-resistant acid phosphatase 5b is a
marker of osteoclast number in human osteoclast cultures and the
rat ovariectomy model. Calcif Tissue Int. 82:108–115. 2008.
View Article : Google Scholar
|
|
12
|
Hsu H, Lacey DL, Dunstan CR, et al: Tumor
necrosis factor receptor family member RANK mediates osteoclast
differentiation and activation induced by osteoprotegerin ligand.
Proc Natl Acad Sci USA. 96:3540–3545. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thompson CD, Frazier-Jessen MR, Rawat R,
Nordan RP and Brown RT: Evaluation of methods for transient
transfection of a murine macrophage cell line, RAW 264.7.
Biotechniques. 27:824–832. 1999.PubMed/NCBI
|
|
14
|
Yu X, Huang Y, Collin-Osdoby P and Osdoby
P: CCR1 chemokines promote the chemotactic recruitment, RANKL
development, and motility of osteoclasts and are induced by
inflammatory cytokines in osteoblasts. J Bone Miner Res.
19:2065–2077. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Battaglino R, Fu J, Späte U, et al:
Serotonin regulates osteoclast differentiation through its
transporter. J Bone Miner Res. 19:1420–1431. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mose S, Menzel C, Kurth AA, et al:
Evaluation of tartrate-resistant acid phosphatase (TRACP) 5b as
bone resorption marker in irradiated bone metastases. Anticancer
Res. 25:4639–4645. 2005.PubMed/NCBI
|
|
17
|
Qin YJ, Zhang ZL, Zhang H, et al:
Age-related changes of serum tartrate-resistant acid phosphatase 5b
and the relationship with bone mineral density in Chinese women.
Acta Pharmacol Sin. 29:1493–1498. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nenonen A, Cheng S, Ivaska KK, et al:
Serum TRACP 5b is a useful marker for monitoring alendronate
treatment: comparison with other markers of bone turnover. J Bone
Miner Res. 20:1804–1812. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alatalo SL, Ivaska KK, Waguespack SG, et
al: Osteoclast-derived serum tartrate-resistant acid phosphatase 5b
in Albers-Schonberg disease (type II autosomal dominant
osteopetrosis). Clin Chem. 50:883–890. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alatalo SL, Ivaska KK, Peng Z, et al:
Serum tartrate-resistant acid phosphatase 5b and osteocalcin in
naturally occurring osteopetrotic rats. J Bone Miner Res.
18(Suppl): S952003. View Article : Google Scholar
|
|
21
|
Xiao XH, Liao EY, Zhou HD, et al: Ascorbic
acid inhibits osteoclastogenesis of RAW264.7 cells induced by
receptor activated nuclear factor kappaB ligand (RANKL) in vitro. J
Endocrinol Invest. 28:253–260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abe K, Yoshimura Y, Deyama Y, et al:
Effects of bisphosphonates on osteoclastogenesis in RAW264.7 cells.
Int J Mol Med. 29:1007–1015. 2012.PubMed/NCBI
|
|
23
|
Teramachi J, Morimoto H, Baba R, et al:
Double stranded RNA-dependent protein kinase is involved in
osteoclast differentiation of RAW264.7 cells in vitro. Exp Cell
Res. 316:3254–3262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
GuezGuez A, Prod’homme V, Mouska X, et al:
3BP2 Adapter protein is required for receptor activator of NF
kappaB ligand (RANKL)-induced osteoclast differentiation of
RAW264.7 cells. J Biol Chem. 285:20952–20963. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishida T, Emura K, Kubota S, Lyons KM and
Takigawa M: CCN family 2/connective tissue growth factor
(CCN2/CTGF) promotes osteoclastogenesis via induction of and
interaction with dendritic cell-specific transmembrane protein
(DC-STAMP). J Bone Miner Res. 26:351–363. 2011. View Article : Google Scholar
|