Introduction
The transmembrane 4 superfamily (TM4SF), which is
ubiquitously expressed in the cells or tissues of mammals,
predominantly regulates cell adhesion and migration. As the most
important member of this family, cluster of differentiation (CD)
151 forms a CD151-α3β1/α6β1 functional complex by binding integrin
α3β1 or α6β1 at extracellular loop-specific sites, thus regulating
the proliferation, migration and adhesion of endothelial cells, as
well as the signaling transduction pathways (1).
It has previously been demonstrated that CD151
promotes angiogenesis of a rat model of hindlimb ischemia (2). The injection of a recombinant
adeno-associated virus (rAAV) vector carrying the CD151 gene into
rat ischemic myocardium results in the expression of CD151 mRNA and
protein, significant densification of local capillaries,
facilitation of angiogenesis and improvement of ventricular
function; however, the molecular mechanisms have yet to be
elucidated (3). Therefore, the aim
of the present study was to investigate the effects of CD151 gene
transfer on vascular endothelial growth factor (VEGF) expression
and the associated molecular mechanism.
Materials and methods
Materials
pZeoSV-CD151 plasmid, pAAV-green fluorescent protein
(GFP) vector and E. coli DH5a strain were obtained from
Polepolar Research Company (Beijing, China). Umbilical vein
endothelial cells (ECV304) were purchased from the China Center for
Type Culture Collection (Wuhan, China). The pAAV-CD151 plasmid was
constructed by our group. Briefly, a pair of primers were designed
using the PzeoSV-CD151 plasmid as the template. The sequences were
as follows: forward: 5′-GAGATCTATGGGTGAGTTCAACGAG-3′; and reverse:
5′-GGAATTCCTCAGTAGTGCTCCAGCTTGAG-3′. The CD151 gene fragment was
amplified by PCR and inserted at the downstream of the CMV promoter
packaging plasmid pAAV. The recombinant plasmid pAAV-CD151 was then
subjected to digestion, identification and sequencing.
Polyvinylidene difluoride (PVDF) membrane was obtained from
Invitrogen Life Technologies (Carlsbad, CA, USA). The
hypersensitive enhanced chemiluminescence (ECL) kit was purchased
from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit
anti-VEGF antibody was obtained from Sigma (St. Louis, MO, USA).
Mouse anti-human CD151 antibody and β-actin antibody were obtained
from Santa Cruz Biotechnology. Horseradish peroxidase-conjugated
goat anti-rabbit immunoglobulin G (IgG) antibody was purchased from
Sigma. The apparatus used herein included a western blotting system
(Trans-Blot® Turbo™ Transfer system; Bio-Rad,
Hercules, CA, USA), western blotting color developing reagent
(Pierce Biotechnology, Inc., Rockford, IL, USA) and GeneTools
density scanning analysis software version 3.02.00 (SynGene,
Frederik, MA, USA). Recombinant AAV (rAAV)-CD151 and rAAV-GFP
viruses were each packaged and copied with human embryonic kidney
epithelial cells (293 cells; obtained from the Pathology Institute
of Chongqing Medical University, Chongqing, China) by the
three-plasmid co-transfection method. The viral titers were
measured using reverse transcriptase PCR (RT-PCR) (4).
Establishment of the myocardial
infarction model, grouping and gene transfer
Healthy adult, male Sprague Dawley rats (clean
grade, weighing 200–250 g) were provided by the Experimental Animal
Center of Chongqing Mecical University (Chongqing, China). All of
the experimental procedures were performed in accordance with the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals (5) and were
approved by the Biomedical Ethics Committee of Chongqing Three
Gorges Medical College (Chongqing, China). The rats were randomly
divided into sham surgery, control, GFP and CD151 groups (n=6). The
rats were anesthetized with 60 mg/kg pentobarbital and catheterized
with ventilator-assisted breathing. A thoracotomy was performed and
the heart was exposed. With the exception of the sham surgery
group, the rats were subjected to ligation of the left anterior
descending coronary arteries, which led to the vertex cordis and
left ventricular anterior walls turning cyanotic. The cyanotic
edges were vertically injected with 4×1011 viral genomes
(6) rAAV-CD151 (CD151 group) or
rAAV-GFP exogenous genes (GFP group) or normal saline (control
group). Five sites were injected, 7 mm apart. The same volume of
normal saline was injected in the control and the sham surgery
groups. The thoracic cavity was then closed and the rats were
attended to until recovery of spontaneous breathing. Finally, the
rats were fed in the animal house after regaining
consciousness.
Detection of CD151 mRNA expression by
RT-PCR
The rats were sacrificed 4 weeks following the
surgery, and the myocardial tissues at the infarction edges were
frozen in liquid nitrogen. ECV304 cells transfected with rAAV-CD151
were used as the positive control, and distilled water was used as
the negative control. Total RNA from the myocardial tissues and
ECV304 cells was extracted and quantified using UV-Vis
spectroscopy. Then 2 μg total RNA was subjected to reverse
transcription, and 1 μl of the product underwent PCR, using GAPDH
as the internal reference. PCR primers used were as follows: human
CD151, upstream, 5′-GAGGTCTATGGGTGAGTTCAACGAG-3′ and downstream,
5′-AATTCCTAGGCGTAGTC-3′, 799 bp; β-actin, upstream,
5′-GGAGAAGGACCCAGATC-3′ and downstream, 5′-GATCTTCATGAGGTA
GTCAG-3′, 300 bp. PCR was performed in a total volume of 25 μl
using a 7500 real-time PCR system (Invitrogen Life Technologies),
under the following conditions: 5 min of pre-denaturation at 94°C
and then 1 min at 94°C, 1 min at 60°C and 40 sec at 72°C, for a
total of 30 cycles, followed by 3 min of extension at 72°C.
Finally, 10 μl PCR product was subjected to 1% agarose gel
electrophoresis, and the resulting images were analyzed.
Detection of CD151 and VEGF expression by
western blot analysis
The rats were sacrificed 4 weeks following the
surgery, and the myocardial tissues of the injected regions were
removed and homogenized in lysis buffer in an ice bath.
Subsequently, the homogenates were centrifuged at 4°C, 12,000 × g
for 30 min and the supernatant was collected. The protein
concentrations were determined using the Coomassie brilliant blue
method. Protein from each group (40 μg) was separated by 12%
SDS-PAGE and then electronically transferred at 4°C to a PVDF
membrane. The membrane was then blocked with TBS-T solution (10
mmol/l Tris-HCl pH 7.5, 100 mmol/l NaCl and 0.1% Tween-20)
containing 5% skimmed milk powder. The PVDF membrane was incubated
with the following primary antibodies at 4°C overnight: rabbit
anti-human VEGF, mouse anti-human CD151 antibody and β-actin
antibodies. It was subsequently incubated at room temperature for 2
h with the following: horseradish peroxidase-conjugated goat
anti-mouse and goat anti-rabbit IgG secondary antibodies. Relative
expression of CD151 and VEGF proteins was detected using ECL
(developing and exposure was performed in accordance with the
manufacturer’s instructions) and grayscale scanning using the gel
imaging analysis system. All data were normalized to β-actin.
Statistical analysis
The categorical data were expressed as the mean ±
standard deviation. Relative optical density (OD) values of protein
bands from the western blot analysis were determined using a gel
imaging analysis system. The experiments for each group were
performed in triplicate, and significant differences were analyzed
by a Student’s t-test. The corrected OD values were subjected to
univariate analysis of variance. The categorical data of two groups
were compared using a Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of exogenous CD151 mRNA
In the present study the CD151 gene was connected to
a downstream coding HA gene fragment (30 bp) that did not affect
the expression or function of CD151 protein. In the PCR, the
upstream primer hybridized with the start segment of CD151 gene,
while the downstream primer hybridized with the end segment of the
HA gene. Therefore, the PCR product originated from exogenous CD151
mRNA.
The results from the RT-PCR (Fig. 1) demonstrated the presence of
specific CD151 and β-actin bands in the CD151 and positive control
(ECV304 cell extract) groups, indicating that the exogenous CD151
gene was expressed in the myocardial tissues of the CD151 group.
Since only one band, corresponding to β-actin, was discerned in the
sham surgery, control and GFP groups, exogenous CD151 gene was not
expressed in these groups. Therefore, this indicates that the CD151
gene carried by the rAAV vector was stably and continuously
expressed in the rats.
Expression of CD151 protein
There were specific bands observed in all groups
four weeks following transfection with the CD151 gene. However, the
bands in the sham surgery, control and GFP groups were narrow and
light, while in the CD151 group the band was broad and darker
(Fig. 2A). A significantly greater
amount of CD151 protein was expressed in the CD151 group compared
with that in the other three groups (P<0.05), while the outcomes
of the sham surgery, control and GFP groups were similar
(P>0.05; Fig. 2B). Hence,
introduction of the human CD151 gene into rats significantly
increased the expression of CD151 protein.
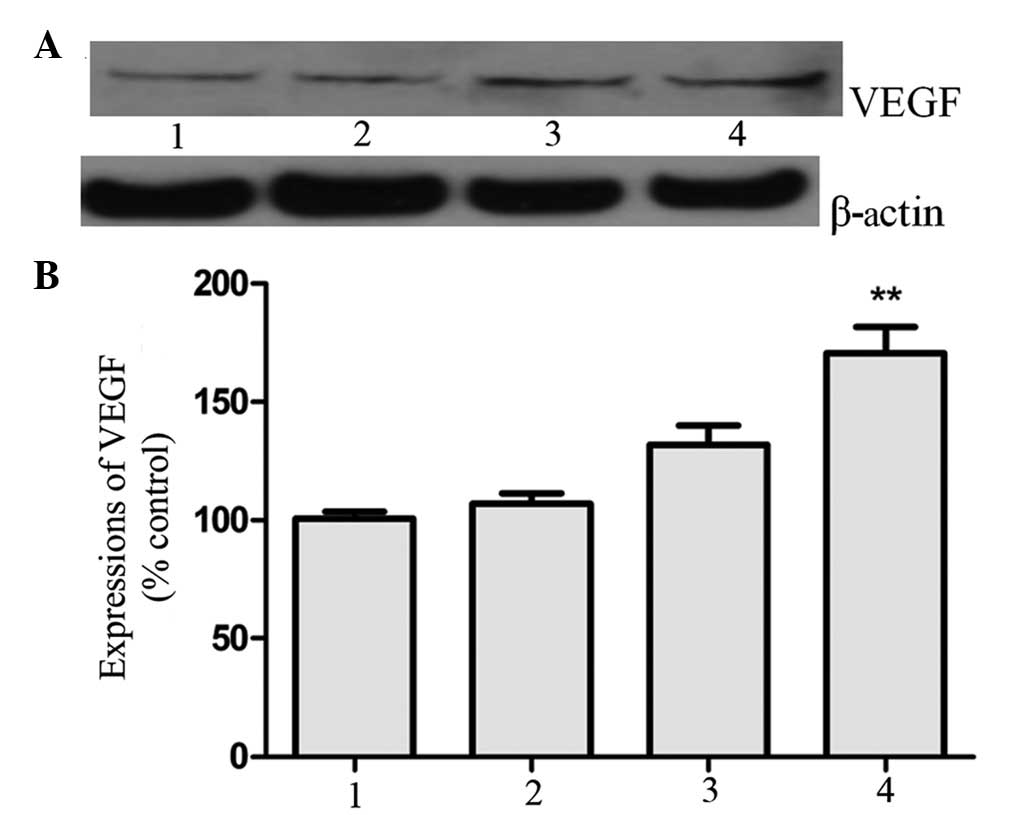
Expression of VEGF
VEGF expression was detected to elucidate whether
VEGF could participate in angiogenesis in the CD151-induced rat
myocardial infarction model. It was observed that the elevated
expression of CD151 was conducive to the expression of VEGF
(P<0.05; Fig. 3).
Discussion
Angiogenic therapy has been investigated in basic
and clinical studies on coronary artery disease. Revascularization
has a crucial role in the recovery of cardiac functions following
myocardial infarction. Angiogenesis in infarcted areas may mitigate
the apoptosis of hypertrophic cardiomyocytes, maintain the activity
of cardiomyocytes and inhibit collagen deposition, thereby
protecting the heart and improving the prognosis. Angiogenesis is a
complicated process leading to the development of new blood
vessels, which involves the proliferation and migration of
endothelial cells, regulation of the expression of proteolytic
enzymes, reconstruction of degraded extracellular matrix and the
formation of endothelial lumen (7).
As a highly specific, strong mitogenic factor for
vascular endothelial cells, VEGF is able to predominantly induce
physiological or pathological angiogenesis. By activating
mitogen-activated protein kinase, stress-activated protein kinase,
protein kinase C and the Akt pathway, VEGF induces organisms to
produce proteases and specific integrins required for decomposition
of the vascular basement membrane. As a result, cells are prone to
proliferation, migration and survival, accompanied by angiogenesis.
Furthermore, due to the activation of metalloproteinase, focal
adhesion kinase and PI3K, endothelial cells are induced to migrate
(8–10).
CD151, as the most important member of the TM4SF, is
expressed in epithelial cells, endothelial cells, skeletal muscle
cells, platelets, megakaryocytes and immature hematopoietic cells.
In addition, CD151 participates in the adhesion, migration and
proliferation of cells, as well as many physiological and
pathological functions, including the interaction with
integrin-mediated angiogenesis (11–14).
It has previously been demonstrated that the rAAV-mediated
expression of the CD151 gene in ischemic lower limbs and myocardial
tissues is capable of promoting angiogenesis (14). However, the detailed mechanisms and
whether VEGF is simultaneously highly expressed have yet to be
established. Therefore, in the present study, western blot analysis
was used to determine VEGF expression levels following myocardial
infarction, in order to elucidate the likely effect on myocardial
angiogenesis and the cardiac function of rats. It was observed that
the transfection resulted in the upregulation of CD151 expression,
and that the overexpression of CD151 facilitated VEGF expression,
suggesting that VEGF expression is dependent on CD151.
In the present study, CD151 was demonstrated to
upregulate the expression of VEGF, which may be responsible for
enhanced angiogenesis of the ischemic myocardium. However, further
investigation is required to determine if angiogenic molecules
other than CD151 also have the same effect. The functions of CD151
and the underlying molecular mechanisms also require further study.
The results in the present study provide information relevant to
the treatment of cardiovascular diseases.
References
|
1
|
Sincock PM, Mayrhofer G and Ashman LK:
Localization of the transmembrane 4 superfamily (TM4SF) member
PETA-3 (CD151) in normal human tissues: comparison with CD9, CD63,
and alpha5beta1 integrin. J Histochem Cytochem. 45:515–525. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lan RF, Liu ZX, Liu XC, Song YE and Wang
DW: CD151 promotes neovascularization and improves blood perfusion
in a rat hind-limb ischemia model. J Endovasc Ther. 12:469–478.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng ZZ, Liang J, Wang MH, et al: The
effect of CD151 gene delivery on the expression of VEGF in rat
myocardial infarction model. Chin J Gerontol. 28:1565–1568.
2008.(In Chinese).
|
|
4
|
Rohr UP, Wulf MA, Stahn S, et al: Fast and
reliable titration of recombinant adeno-associated virus type-2
using quantitative real-time PCR. J Virol Methods. 106:81–88. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guide for the Care and Use of Laboratory.
Animals National Research Council (US) Committee for the Update of
the Guide for the Care and Use of Laboratory Animals. 8th edition.
National Academies Press; Washington D.C: 2011
|
|
6
|
AAV genome loss from dystrophic mouse
muscles during AAV-U7 snRNA-mediated exon-skipping therapy. Mol
Ther. 21:1551–1558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Denekamp J: Review article: angiogenesis,
neovascular proliferation and vascular pathophysiology as targets
for cancer therapy. Br J Radiol. 66:181–196. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gliki G, Wheeler-Jones C and Zachary I:
Vascular endothelial growth factor induces protein kinase C
(PKC)-dependent Akt/PKB activation and phosphatidylinositol
3′-kinase-mediates PKC delta phosphorylation: role of PKC in
angiogenesis. Cell Biol Int. 26:751–759. 2002. View Article : Google Scholar
|
|
9
|
Yahata Y, Shirakata Y, Tokumaru S, et al:
Nuclear translocation of phosphorylated STAT3 is essential for
vascular endothelial growth factor-induced human dermal
microvascular endothelial cell migration and tube formation. J Biol
Chem. 278:40026–40031. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zachary I and Gliki G: Signaling
transduction mechanisms mediating biological actions of the
vascular endothelial growth factor family. Cardiovasc Res.
49:568–581. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ang J, Fang BL, Ashman LK and Frauman AG:
The migration and invasion of human prostate cancer cell lines
involves CD151 expression. Oncol Rep. 24:1593–1597. 2010.PubMed/NCBI
|
|
12
|
Franco M, Muratori C, Corso S, et al: The
tetraspanin CD151 is required for Met-dependent signaling and tumor
cell growth. J Biol Chem. 285:38756–38764. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haeuw JF, Goetsch L, Bailly C and Corvaia
N: Tetraspanin CD151 as a target for antibody-based cancer
immunotherapy. Biochem Soc Trans. 39:553–558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zuo H, Liu Z, Liu X, et al: CD151 gene
delivery after myocardial infarction promotes functional
neovascularization and activates FAK signaling. Mol Med.
15:307–315. 2009. View Article : Google Scholar : PubMed/NCBI
|