Introduction
Osteoporosis is a systemic disorder affecting the
skeletal system and is characterized by a reduced bone mass and
micro-architectural deterioration of bone tissue with a consequent
increase in bone fragility and susceptibility to fracture (1). Bone mineral density (BMD) is commonly
used as a skeletal phenotype in evaluating osteoporosis. The World
Health Organization defines osteoporosis as a BMD value of ≥2.5
standard deviations below the young-adult mean measured by
dual-energy X-ray absorptiometry (DXA) (2). The pathophysiology of osteoporosis is
complex and involves numerous endogenous (genetic and hormonal) and
environmental factors. Twin and family studies have shown that
genetic influences account for 50–80% of the inter-individual
variability of BMD in young adults (3–5).
Various candidate genes have been implicated in the genetic basis
of osteoporosis, including hormones and their receptors, cytokines
and bone-matrix proteins. Polymorphisms in the genes encoding the
calcitonin receptor (CTR), estrogen receptor (ESR) and vitamin D
receptor (VDR) have been studied previously and the results show
that these receptors are positively or negatively associated with
biomarkers of bone turnover, BMD and the incidence of osteoporotic
fracture (6–8). Genome-wide association studies and
meta-analysis have confirmed the association between BMD and ESR or
VDR (9–11). To the best of our knowledge, there
have been no genome-wide association studies or meta-analyses to
assess the association between AluI gene polymorphism and
BMD.
Calcitonin, a 3.4-kDa polypeptide hormone secreted
by thyroid gland parafollicular cells, is an important hormone
regulating calcium metabolism and bone turnover through the CTR.
The CTR, which is expressed in osteoclasts and osteoclast precursor
cells, activates one of the members of the G-protein-coupled
receptor family. By doing this, it regulates bone metabolism and
maintains the calcium balance between bone resorption and formation
(12,13).
In 1997, Nakamura et al (14) described an AluI CTR
polymorphism in the Japanese population, which was characterized by
a single nucleotide difference at position 1,377 of human CTR cDNA,
expressing either proline (CCG) or leucine (CTG) as the amino acid
at position 463. Single nucleotide polymorphisms are used as a tool
for mapping the disease gene. Using this technique, Masi et
al (15) found an association
between the AluI CTR gene C/T polymorphism and BMD in
Italian postmenopausal females. Furthermore, Tsai et al
(16) reported that an AluI
CTR gene polymorphism was associated with a reduced BMD, and
predisposed postmenopausal females to osteoporosis; however, other
studies reported contrasting results. Charopoulos et al
(17) reported that AluI
polymorphism was not associated with BMD in Greek males, as no
significant difference was observed in the BMD between CTR
genotypes. Xu et al (18)
also found that CTR gene polymorphism had no evident effect on
Xinjiang Han and Uygur postmenopausal patients with osteoporosis,
and the authors suggested that CTR gene polymorphism was not
involved in the low bone mass. Consequently, no conclusion about
the association between AluI polymorphism and BMD could be
drawn.
As the small sizes and different ethnicities of
individual studies may be responsible for the contrasting results,
a large-scale study with more subjects is required. Meta-analysis
is an effective tool that is frequently used to compensate for the
limitations of individual studies by pooling all published data
together to obtain sufficient statistical power to detect potential
effects of small to moderate sizes of samples associated with these
polymorphisms. In order to explore the effect of AluI
polymorphism on BMD, a meta-analysis was therefore performed in the
present study to provide a more comprehensive assessment of the
association between AluI CTR gene polymorphisms and BMD in
an elderly population, particularly in China.
Materials and methods
Ethics statement
The present meta-analysis was conducted according to
the Preferred Reporting Items for Systematic Reviews and
Meta-analyses guidance with minor modifications appropriate for
this study (19), and did not
require ethics board approval.
Literature searching
A literature search for eligible studies published
prior to March 31, 2014 was conducted in the following electronic
databases: PubMed, Web of Science, Cochrane Library and the China
National Knowledge Infrastructure. The following combined keywords
and MeSH terms were used: ‘calcitonin receptor’ [All Fields] or
‘CTR’ [All Fields] or ‘AluI’ [All Fields] or ‘rs1801197’ [All
Fields], and ‘genes’ [MeSH Terms] or ‘gene’ [All Fields], and
‘polymorphism, genetic’ [MeSH Terms] or ‘polymorphism’ [All Fields]
or ‘genetic polymorphism’ [All Fields] and ‘bone density’ [MeSH
Terms] or ‘bone density’ [All Fields] or ‘bone mineral density’
[All Fields] or ‘BMD’ [All Fields]. Studies written in English and
Chinese focusing on middle-aged or older subjects were included.
The reference lists of reviews and retrieved articles were manually
screened by two independent authors to identify additional
potential studies.
Inclusion and exclusion criteria
To be included in the analysis, the candidate
studies had to meet the following criteria: i) Genotyping was
performed with validated molecular methods and the possible
genotypes were CC, CT or TT for AluI; ii) lumbar spine and
femoral neck BMD was measured by DXA; and iii) measurements of BMD
at the lumbar spine and/or femoral neck were used to calculate the
mean difference and their corresponding 95% confidence intervals
(95% CI). Studies were excluded for the following reasons: i)
Duplicate publication; and ii) subjects younger than 18 years old.
If a research team reported similar data in different studies, the
study reporting the largest number of subjects was included. In
addition, when raw and adjusted BMD values were available, adjusted
BMD values were used. When the complete information required for
quantitative synthesis was unavailable, the relevant authors were
contacted to obtain the necessary information.
Data extraction
For eligible studies, information was extracted on
authors, publication year, country and region, age, the number of
subjects recruited, genotypes and the BMD of the lumber spine and
femoral neck in each genotype. All data were extracted
independently by two authors using a standard form, and minor
discrepancies were resolved through discussion by the authors.
Statistical analysis
A statistical test (Cochran’s Q statistic) of
heterogeneity was used to evaluate any potential inter-study
heterogeneity: P<0.05 indicated significant inter-study
heterogeneity. Heterogeneity was also assessed through the
I2 test, with I2>50 indicating significant
heterogeneity. When no heterogeneity was found, a fixed-effect
model was used to estimate the pooled mean differences and their
corresponding 95% CIs; otherwise, a random-effect model was
applied. The following comparisons were evaluated: Patients with
the CC genotype versus patients with the CT/TT or the CT genotype.
Subgroup analyses were conducted by region and gender. Egger’s
regression test was performed to assess the publication bias. All
statistical tests were two-tailed. P<0.05 indicated a
statistically significant difference. All the analyses were
performed with Comprehensive Meta Analysis V2 software package
(Biostat, Inc., Englewood, NJ, USA).
Results
Characteristics of the eligible
studies
Fig. 1 shows
detailed information on how the studies were selected. There were
15 eligible studies with 3,093 females and 654 males (16,20–33).
Table I shows further detailed
information on the eligible studies. Two studies recruited subjects
in Italy (20,21), one in Japan (30) and 12 in China. Three studies
recruited only male subjects (21,24,26),
one study recruited both male and female subjects (31) and 11 studies recruited only female
subjects. The majority of subjects recruited were postmenopausal
females. BMD measurements in all 15 studies were performed by DXA,
although with different instruments. The BMD values of both the
lumbar spine and femoral neck were measured in 14 of the studies,
with one study measuring only the lumbar spine BMD (30). In three of the 15 eligible studies,
the BMD value was adjusted for age and weight (16,20,21).
Three of the studies had combined CT and TT data (indicated as
CT/TT), without raw CT or TT data (22,24,30).
Genotyping was carried out in a consistent manner across studies
using validated polymerase chain reaction methods. As it is not
possible to introduce substantial bias for BMD values and genotype,
the studies did not specify whether measurements were blinded.
 | Table IDetailed information of the 15
eligible studies. |
Table I
Detailed information of the 15
eligible studies.
| First author, year
(ref.) | Genotype | Gender | Region | n | Age (years) | LS BMD | FN BMD |
|---|
| Braga, 2002 (21) | CC | M | Italy | 45 | 52.64±2.45 | 0.914±0.026 | 0.759±0.017 |
| CT | M | Italy | 111 | 57.41±1.56 | 0.973±0.018 | 0.795±0.010 |
| TT | M | Italy | 97 | 55.55±1.71 | 0.988±0.018 | 0.807±0.011 |
| CT/TT | M | Italy | 208 | 56.54±1.87 | 0.980±0.019 | 0.801±0.012 |
| Braga, 2000
(20) | CC | F | Italy | 77 | 61.09±12.44 | 0.752±0.169 | 0.644±0.110 |
| CT | F | Italy | 296 | 64.35±11.34 | 0.806±0.144 | 0.647±0.109 |
| TT | F | Italy | 342 | 63.42±11.13 | 0.812±0.151 | 0.651±0.111 |
| CT/TT | F | Italy | 638 | 63.85±11.23 | 0.809±0.148 | 0.649±0.110 |
| Tsai, 2003
(16) | CC | F | Taiwan | 123 | 54.17±6.25 | 0.99±0.01 | 0.81±0.01 |
| CT | F | Taiwan | 37 | 54.14±4.44 | 1.04±0.02 | 0.82±0.02 |
| TT | F | Taiwan | 4 | 55.25±6.34 | 0.83±0.07 | 0.68±0.05 |
| CT/TT | F | Taiwan | 41 | 54.25±4.57 | 1.020±0.069 | 0.806±0.048 |
| Zhao, 2003
(22) | CC | F | CHN Shanghai | 321 | 48.42±16.47 | 1.050±0.177 | 0.878±0.152 |
| CT/TT | F | CHN Shanghai | 62 | 46.35±16.46 | 1.072±0.182 | 0.849±0.150 |
| Li, 2005 (23) | CC | F | CHN Guangzhou | 194 | 60±8.3 | 0.6145±0.14 | 0.6468±0.11 |
| CT | F | CHN Guangzhou | 33 | 63±7.8 | 0.6601±0.19 | 0.6750±0.11 |
| TT | F | CHN Guangzhou | 4 | 63±4.3 | 0.5790±0.09 | 0.6387±0.09 |
| CT/TT | F | CHN Guangzhou | 37 | 63±7.46 | 0.6513±0.18 | 0.6711±0.11 |
| Li, 2006 (24) | CC | M | CHN Guangzhou | 205 | 72±6 | 0.65±0.13 | 0.64±0.11 |
| CT | M | CHN Guangzhou | | | | |
| TT | M | CHN Guangzhou | | | | |
| CT/TT | M | CHN Guangzhou | 42 | 70±5 | 0.74±0.23 | 0.67±0.14 |
| Wang, 2008
(25) | CC | F | CHN Anhui | 230 | 61.8±6.5 | 0.773±0.112 | 0.720±0.102 |
| CT | F | CHN Anhui | 10 | 63.6±7.5 | 0.835±0.134 | 0.786±0.086 |
| TT | F | CHN Anhui | 0 | | | |
| CT/TT | F | CHN Anhui | 10 | 63.6±7.5 | 0.835±0.134 | 0.786±0.086 |
| Zhang, 2002
(33) | CC | F | CHN Beijing | 118 | Postmenopause | 0.903±0.015 | 0.734±0.010 |
| CT | F | CHN Beijing | 7 | Postmenopause | 0.807±0.057 | 0.734±0.010 |
| TT | F | CHN Beijing | 2 | Postmenopause | 0.971±0.108 | 0.799±0.075 |
| CT/TT | F | CHN Beijing | 9 | Postmenopause | 0.843±0.096 | 0.748±0.040 |
| Wang, 2007
(26) | CC | M | CHN Shenzhen | 47 | >70 | 0.908±0.115 | 0.668±0.086 |
| CT | M | CHN Shenzhen | 12 | >70 | 0.794±0.119 | 0.628±0.088 |
| TT | M | CHN Shenzhen | 0 | >70 | | |
| CT/TT | M | CHN Shenzhen | 12 | >70 | 0.794±0.119 | 0.628±0.088 |
| Xu, 2005 (27) | CC | F | CHN Hebei | 52 | 53.2±11.8 | 1.021±0.253 | 0.785±0.220 |
| CT | F | CHN Hebei | 7 | | 1.160±0.115 | 0.847±0.127 |
| TT | F | CHN Hebei | 1 | | 0.961±0 | 0.885±0 |
| CT/TT | F | CHN Hebei | 8 | | 1.135±0.128 | 0.852±0.118 |
| Ge, 2010 (28) | CC | F | CHN Fuzhou | 422 | | 0.759±0.125 | 0.807±0.119 |
| CT | F | CHN Fuzhou | 152 | | 0.766±0.119 | 0.821±0.120 |
| TT | F | CHN Fuzhou | 17 | | 0.765±0.122 | 0.809±0.105 |
| CT/TT | F | CHN Fuzhou | 169 | Postmenopause | 0.766±0.119 | 0.820±0.118 |
| Yang, 2012
(29) | CC | F | CHN Shanghai | 102 | Postmenopause | 0.968±0.129 | 0.744±0.105 |
| CT | F | CHN Shanghai | 25 | Postmenopause | 0.927±0.141 | 0.685±0.113 |
| TT | F | CHN Shanghai | 0 | Postmenopause | | |
| CT/TT | F | CHN Shanghai | 25 | Postmenopause | 0.927±0.141 | 0.685±0.113 |
| Hayakawa, 2001
(30) | CC | F | JPN | 113 | Premenopause | 1.16±0.10 | |
| CT | F | JPN | | Premenopause | | |
| TT | F | JPN | | Premenopause | | |
| CT/TT | F | JPN | 27 | Premenopause | 1.12±0.12 | |
| Luan, 2010
(31) | CC | F | CHN Shandong | 171 | 62±8.9 | 1.049±0.16 | 0.910±0.17 |
| CT | F | CHN Shandong | 24 | 62±7.8 | 0.980±0.14 | 0.870±0.10 |
| TT | F | CHN Shandong | 0 | | | |
| CT/TT | F | CHN Shandong | 24 | 62±7.8 | 0.980±0.14 | 0.870±0.10 |
| CC | M | CHN Shandong | 88 | 63±8.9 | 1.104±0.15 | 0.902±0.13 |
| CT | M | CHN Shandong | 7 | 58±5.0 | 1.105±0.07 | 0.873±0.09 |
| TT | M | CHN Shandong | 0 | | | |
| CT/TT | M | CHN Shandong | 7 | 58±5.0 | 1.105±0.07 | 0.873±0.09 |
| Zhao, 2009
(32) | CC | F | CHN Guangzhou | 89 | Postmenopause | 0.742±0.083 | 0.682±0.084 |
| CT | F | CHN Guangzhou | 26 | Postmenopause | 0.741±0.062 | 0.679±0.064 |
| TT | F | CHN Guangzhou | 5 | Postmenopause | 0.752±0.058 | 0.647±0.033 |
| CT/TT | F | CHN Guangzhou | 31 | Postmenopause | 0.743±0.061 | 0.674±0.061 |
Meta-analyses for AluI polymorphism
effects on lumbar spine BMD
As subjects with the TT genotype are rare compared
with those with either CC or CT genotypes, comparisons were only
made between patients with CC and CT genotypes or those with CC and
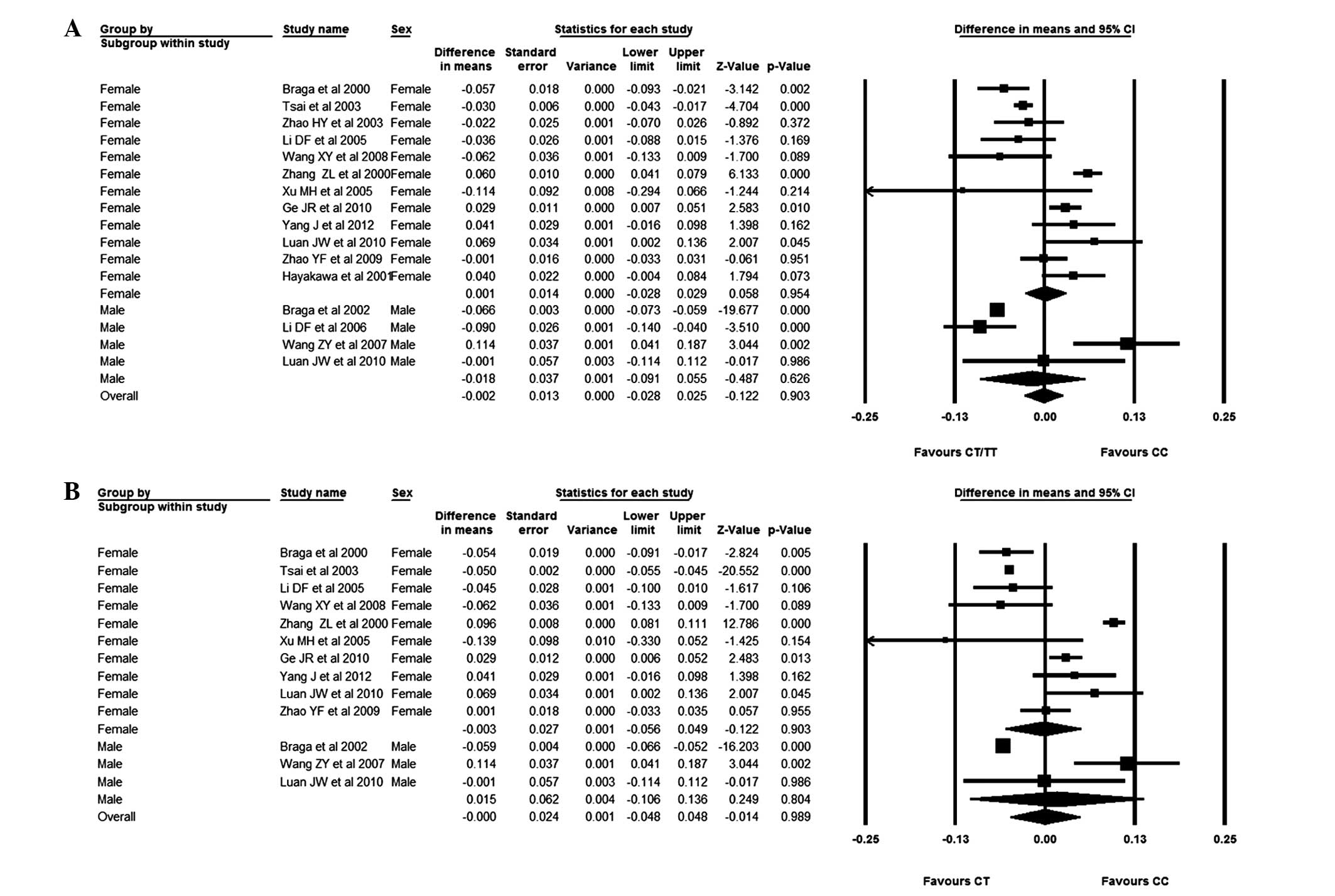
CT/TT genotypes. In male subjects, the weighted mean difference
(WMD) for the CC versus the CT/TT genotypes was −0.018 (95% CI,
−0.091–0.055), and the WMD for the CC versus the CT genotypes was
0.015 (95% CI, −0.106–0.136). Considering the female subjects, the
BMD difference for subjects with the CC genotype versus those with
the CT/TT or CT genotypes was −0.001 (95% CI, −0.028–0.029) and
−0.003 (95% CI, −0.056–0.049), respectively. It was observed that
patients with the CC genotype had a slightly lower BMD than
patients with the CT or CT/TT genotype, although no significant
association between AluI and BMD could be found (Fig. 2).
To clarify whether AluI polymorphisms had an
effect on lumbar spine BMD in a Chinese cohort, the studies
recruiting subjects from countries other than China (20,21,30)
were excluded. In Chinese male and female subjects, those with the
CC genotype had a higher BMD than those with the CT genotype. The
WMD for patients with the CC genotype versus those with the CT
genotype was 0.065 (95% CI, −0.047–0.176) in male subjects and
0.003 (95% CI, −0.055–0.060) in female subjects. The BMD difference
between patients with the CC genotype and those with the CT/TT
genotype was monitored and, similarly, a higher BMD in male and
female subjects with the CC genotype was observed. The WMD for
patients with the CC genotype versus those with the CT/TT genotype
was 0.006 (95% CI, −0.132–0.144) and 0.003 (95% CI, −0.028–0.035)
in male and females, respectively (Fig. 3).
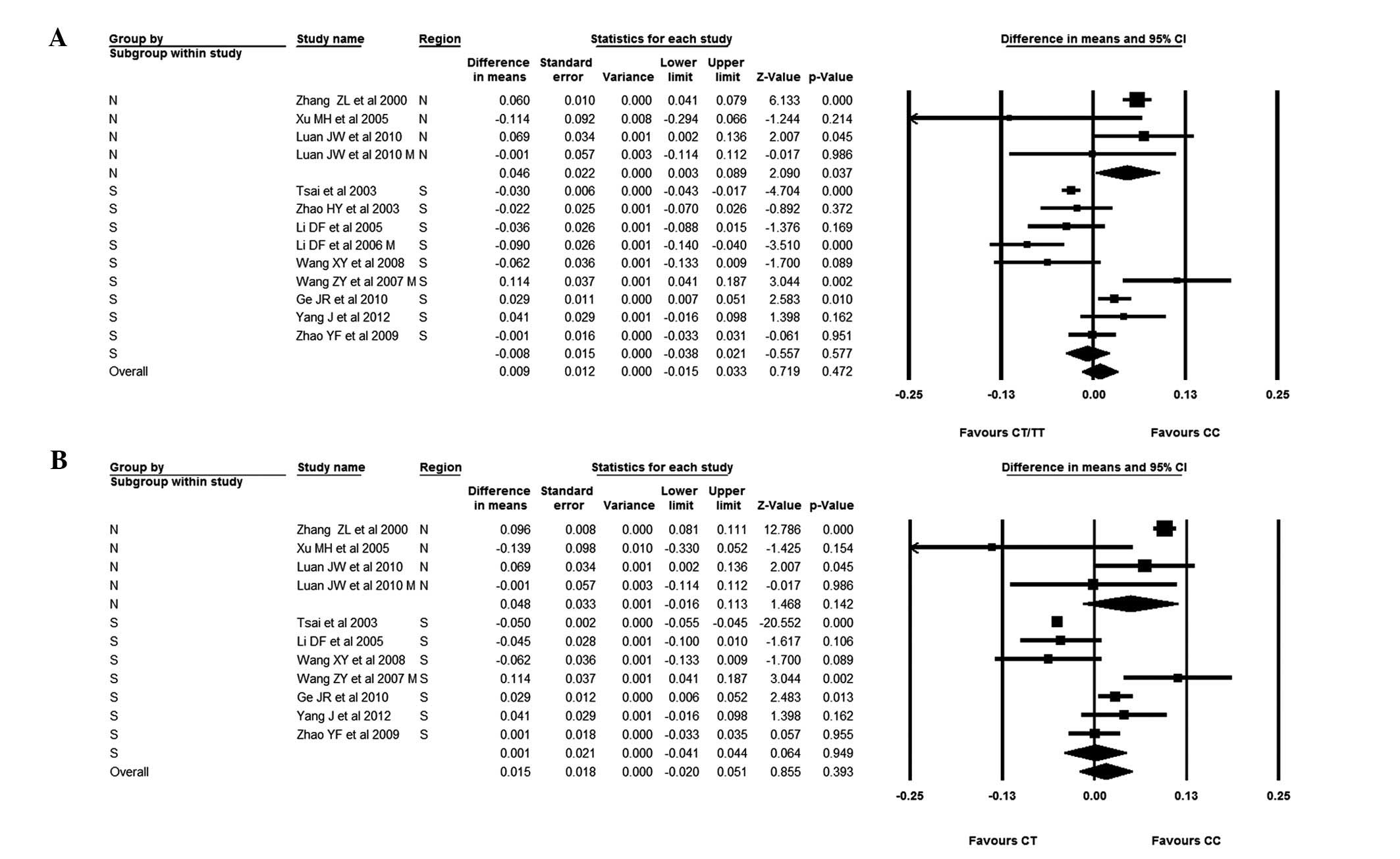
With regard to subgroup analysis for subjects from
Southern and Northern China, it was found that subjects with the CC
genotype from Southern China had a slightly lower BMD than subjects
with the CT genotype; the WMD for the CC versus the CT genotype was
0.001 (95% CI, −0.041–0.044). In subjects from Northern China, it
was observed that those with the CC genotype had a higher BMD than
those with the CT genotype, with a BMD difference of 0.048 (95% CI,
−0.016–0.113). When comparing patients from Northern China with the
CC genotype versus those with the CT/TT genotypes, patients with
the CC genotype were found to have a significantly higher BMD. The
BMD difference was 0.046 (95% CI, 0.003–0.089) (Fig. 4).
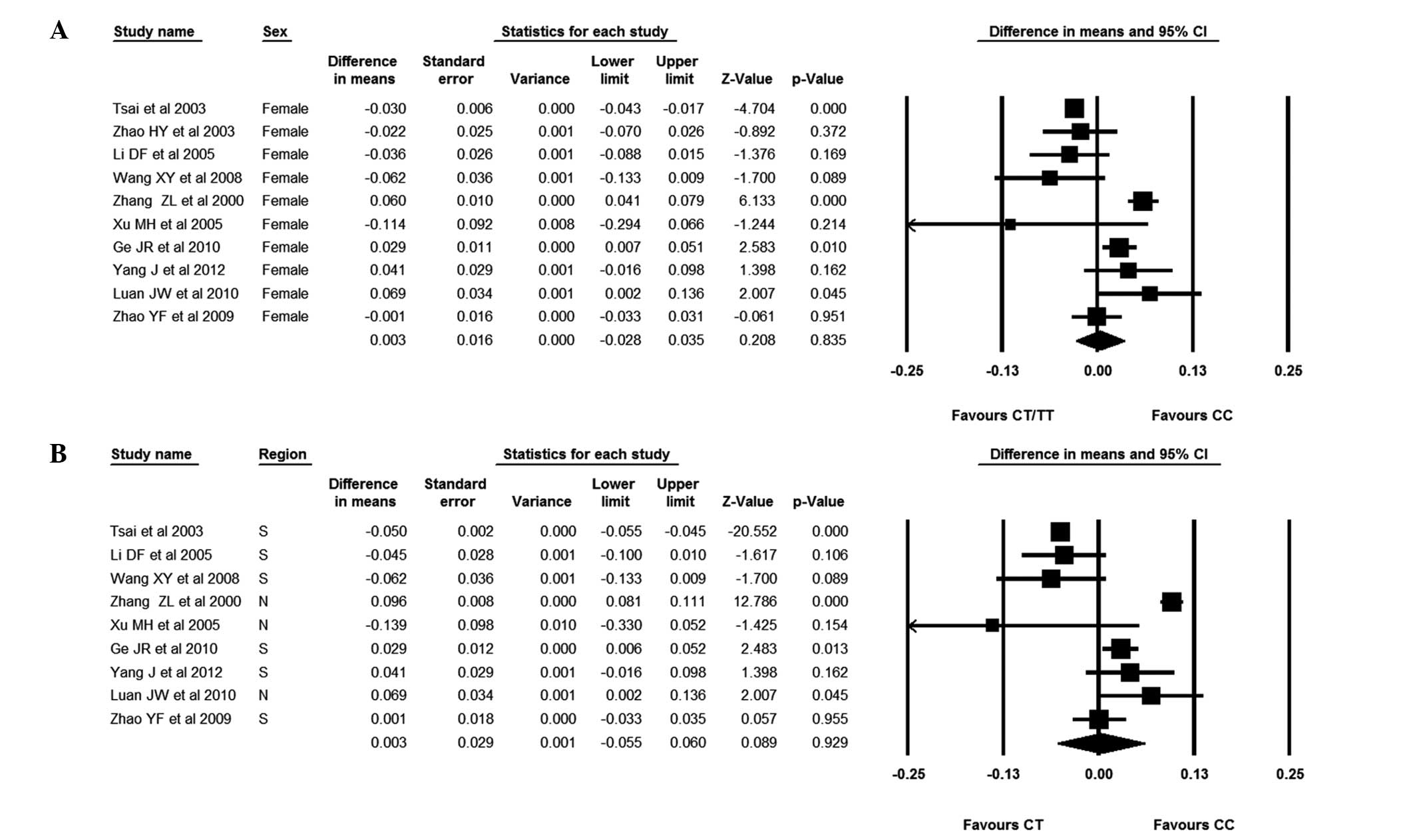
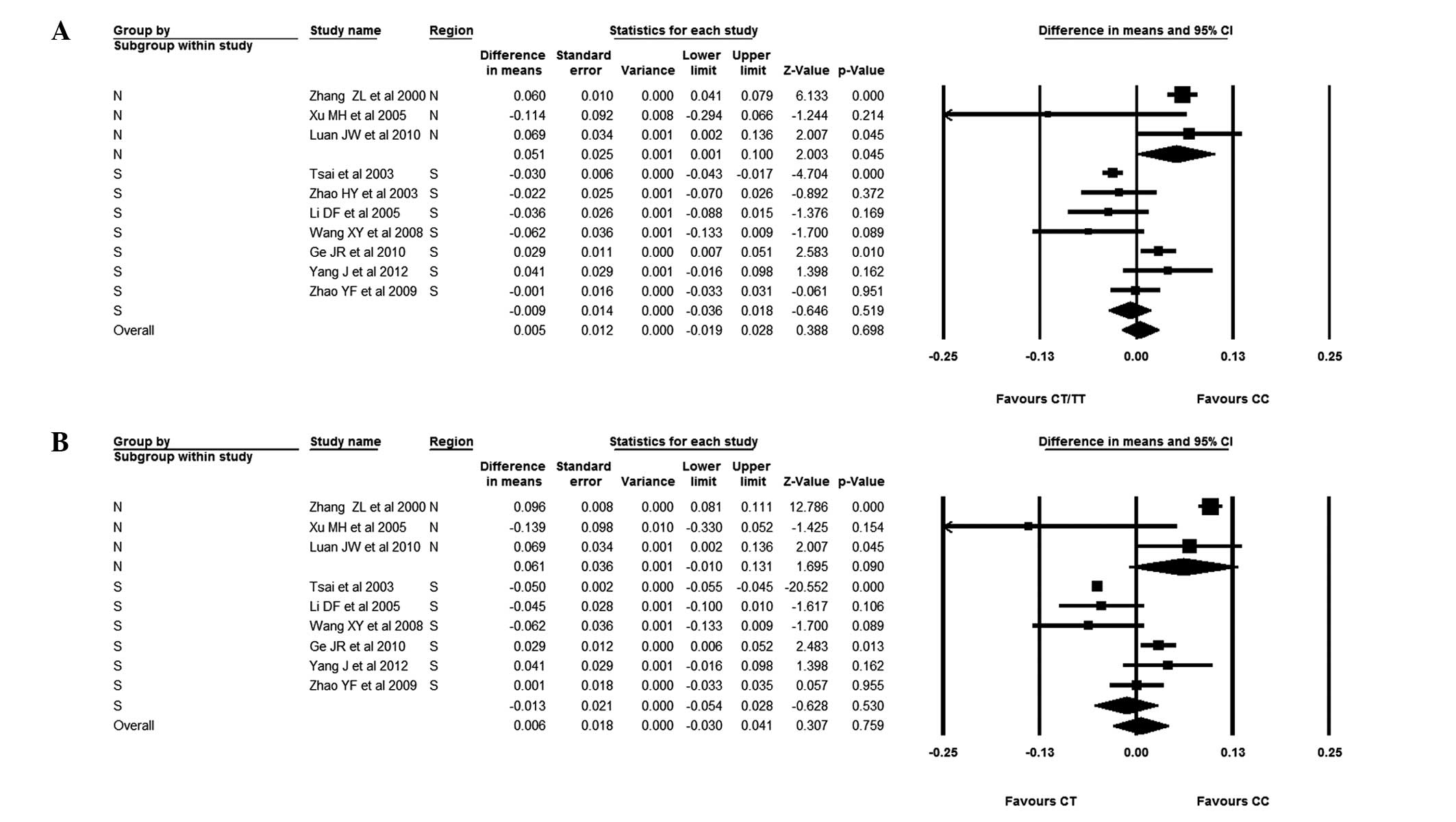
The focus was subsequently changed to the
association between AluI polymorphisms and BMD in Chinese
females. It was observed that patients with the CC genotype had a
slightly higher BMD than those with the CT/TT genotype; the WMD was
0.003 (95% CI, −0.028–0.035). There was, however, no statistical
difference between subjects with the CC and CT/TT genotypes. The
females were also divided into Southern and Northern groups. In the
females from Southern China, those with the CC genotype had a lower
BMD than those with the CT/TT genotype; the WMD was −0.009 (95% CI,
−0.036–0.018). It was evident, however, that females from Northern
China with the CC genotype had a higher BMD than those with the
CT/TT genotype; the WMD was 0.051 (95% CI, 0.001–0.100). Finally,
the studies without BMD values for patients with the CT genotype
were excluded. Chinese females with the CC genotype were compared
with those with the CT genotype; the WMD was 0.003 (95% CI,
−0.055–0.060). It was observed that individuals with the CC
genotype had a higher BMD than those subjects with the CT genotype,
although the difference was not significant. It was also found that
Northern female subjects with the CC genotype had slightly, but not
significantly, higher BMDs than those with the CT genotype; the WMD
was 0.061 (95% CI, −0.010–0.131). In Southern female subjects,
those with the CC genotype had a lower BMD than those with the CT
genotype; the WMD was −0.013 (95% CI, −0.054–0.028) (Figs. 5 and 6).
Meta-analyses for AluI polymorphism
effects on femoral neck BMD
In male subjects, the mean BMD of the femoral neck
was lower in subjects with the CC genotype, although there was no
significant difference between those with the CC and the CT/TT
genotypes. The WMD was −0.013 (95% CI, −0.051–0.024). Similarly,
the mean BMD in female subjects with the CC genotype was lower than
that in subjects with the CT/TT genotype; the WMD was −0.002 (95%
CI, −0.014–0.011), showing no statistical difference between
subjects with the CC and CT/TT genotypes. Subsequently, as for the
lumbar spine evaluation, the mean femoral neck BMD of subjects with
the CC genotype was compared with that of subjects with the CT
genotype. It was observed that male subjects with the CC genotype
had a higher BMD compared with subjects with the CT genotype; the
WMD was 0.004 (95% CI, −0.054–0.062). By contrast, female subjects
with the CC genotype had a lower BMD, with the WMD being −0.005
(95% CI, −0.015–0.005). There was, however, no statistical
difference between those with the CC genotype and those with the CT
genotype in both male and female subjects (Fig. 7).
The Chinese subjects were then considered to confirm
whether there was any association between AluI polymorphism
and BMD. Patients with the CC genotype were compared with patients
with the CT/TT genotype. The results showed that male patients with
the CC genotype had a higher BMD than those with the CT/TT
genotype, while female patients with the CC genotype had a lower
BMD than those with the CT/TT genotype; the WMDs were 0.006
(−0.046–0.058) and −0.001 (−0.015–0.013), respectively. Patients
with the CC genotype were then compared with those with the CT
genotype. There were just two studies that recruited Chinese male
subjects (24,26) and these showed that patients with
the CC genotype had a slightly higher BMD than those with the CT
genotype; the BMD difference was 0.037 (95% CI, −0.010–0.085). In
Chinese female subjects, however, those with the CC genotype had a
lower BMD than subjects with the CT genotype; the WMD was −0.005
(95% CI, −0.016–0.006). No significant BMD difference was observed
between Chinese subjects with the CC and CT or CT/TT genotypes
(Fig. 8).
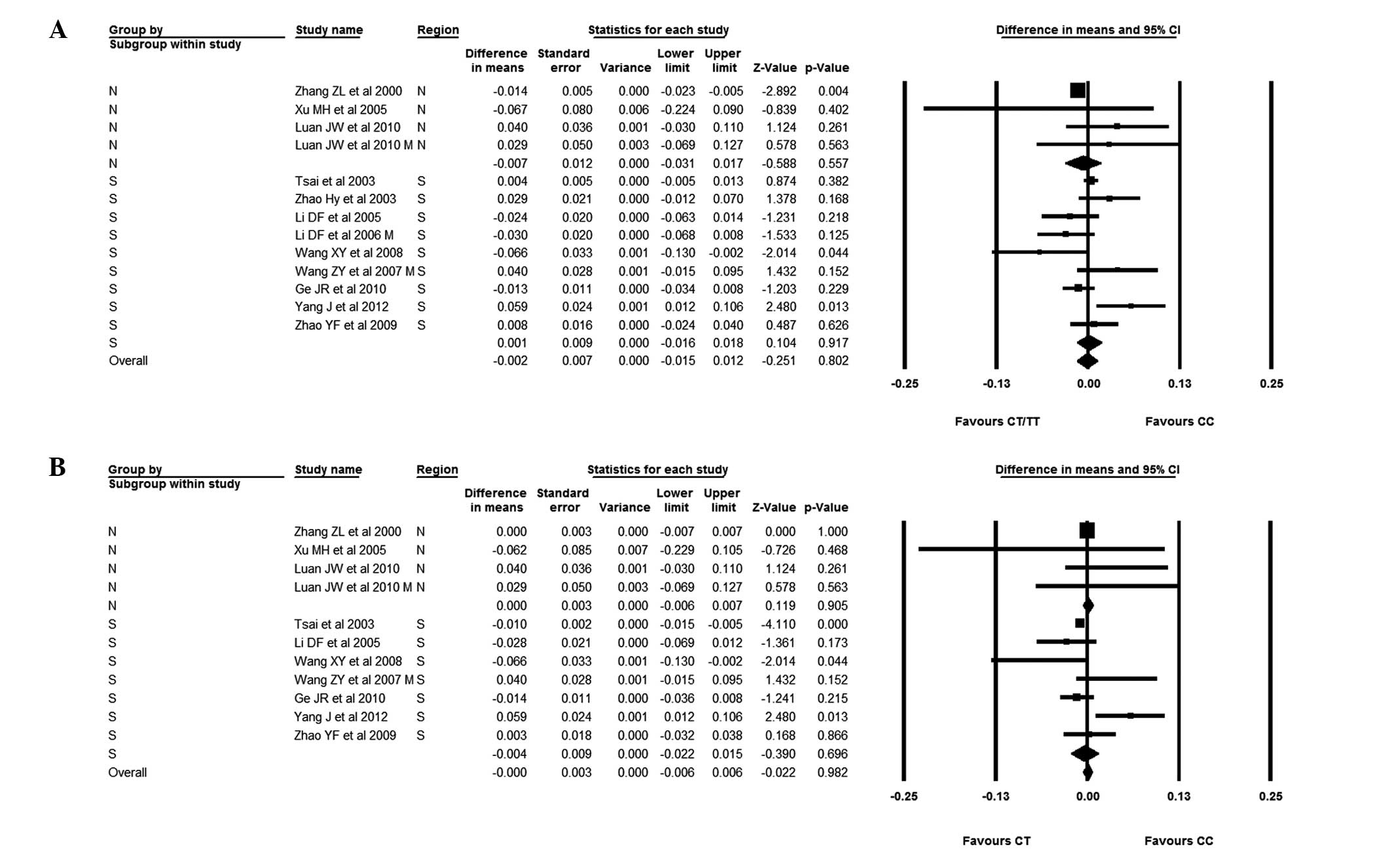
The Chinese subjects were then divided into Southern
and Northern groups; the BMD difference was −0.013 (95% CI,
−0.022--0.003). In Southern subjects, the BMD of those with the CC
genotype was not significantly different from that of subjects with
the CT/TT genotype; the BMD difference was 0.001 (95% CI,
−0.016–0.018). The BMD was similar in Northern Chinese subjects
when considering those with the CC and CT genotypes; the BMD
difference was (95% CI, −0.006–0.007). In Southern Chinese
subjects, however, those with the CC genotype had a slightly lower
BMD than those with the CT genotype. The difference was −0.004 (95%
CI, −0.022–0.015) (Fig. 9).
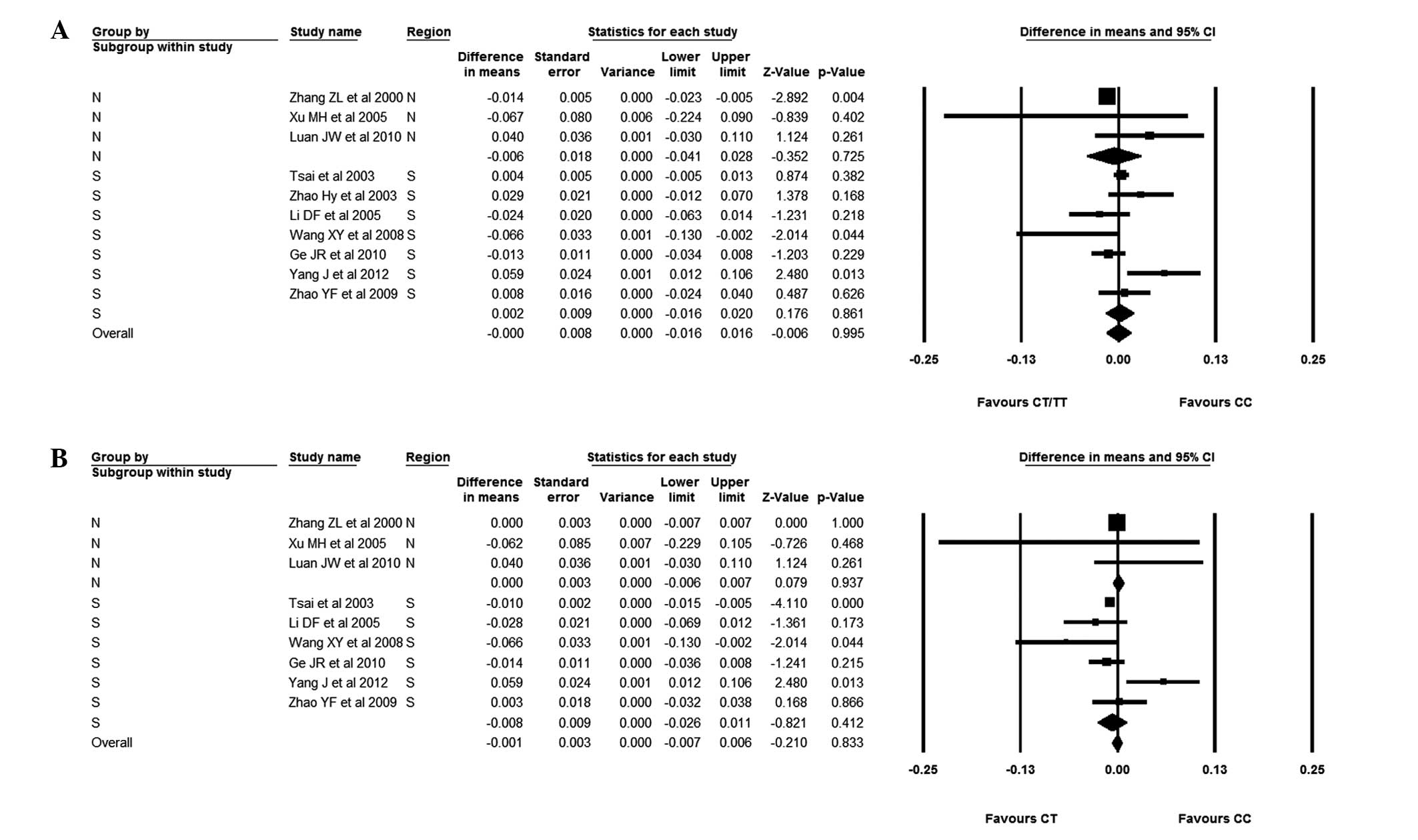
Attention was finally focused on the effect of
polymorphism on femoral neck BMD in Chinese female subjects. No
significant difference was found between subjects with the CC
genotype and subjects with the CT/TT genotype; the BMD difference
was −0.001 (95%CI, −0.015–0.013). This group was then divided into
Chinese female subjects from either the South or the North of
China. It was observed that patients with the CC genotype had
statistically lower BMDs than those with the CT/TT genotype but
only in subjects from Northern China; the BMD difference was −0.013
(95% CI, −0.023--0.004). No significant difference was found,
between subjects with the CC genotype and those with the CT/TT
genotype in Southern Chinese females, although subjects with the CC
genotype had a higher BMD than those with the CT/TT genotype [BMD
difference, 0.002 (95% CI, −0.016–0.020)]. Chinese female subjects
with the CC genotype and those with the CT genotype were also
compared. The results showed that subjects with the CC genotype had
a lower BMD than those with the CT genotype; the BMD difference was
−0.005 (95% CI, −0.016–0.006). The Chinese female subjects with the
CC and CT genotypes were then divided into Northern and Southern
subgroups. In the Northern female subjects, those with the CC
genotype had a similar BMD to those with the CT genotype; the BMD
difference was 0.000 (95% CI, −0.006–0.007). By contrast, those
with the CC genotype had a slightly lower BMD than subjects with
the CT genotype in Southern China; the WMD was −0.008 (95% CI,
−0.026–0.011) (Figs. 10 and
11).
Publication bias assessment
Publication bias was assessed by Egger’s regression
test for all comparisons. Publication bias of subjects with the CC
genotype versus those with the CT/TT genotypes at the lumbar spine
and femoral neck was found (P<0.1). In the other comparisons no
significant publication bias was observed (P>0.1 for comparisons
of the CC and CT genotypes and for the CC and CT/TT genotypes in
Chinese subjects).
Discussion
This meta-analysis was conducted as findings on the
association between AluI polymorphism and BMD are
incongruous. The present study pooled the data on the association
between the AluI polymorphism and BMD at the lumbar spine
and femoral neck in 3,747 subjects. As the frequency of the TT
genotype was rare in the Chinese population, the study compared
patients with the CC genotype with patients with the CT or CT/TT
genotypes. The results demonstrated that, in Asia and Europe,
subjects with the CC genotype had a slightly lower BMD than those
with the CT/TT genotype and a slightly higher BMD than those with
the CT genotype; however, no difference in BMD was found between
male subjects with the CC genotype and those with the CT/TT or CT
genotypes, and this was consistent with previous studies (17,18).
At the femoral neck the results were similar, with no difference
found between patients with the CC genotype and those with the CT
or CT/TT genotypes. In combination, the results suggested that
AluI polymorphism had no effect on lumbar spine and femoral
neck BMD in male subjects, although the CC genotype in males may
have a protective effect at the femoral neck but be a risk factor
in the lumbar spine. When considering the female subjects, those
with the CC genotype had a lower BMD at the lumbar spine and
femoral neck than those with the CT or CT/TT genotypes. The results
suggested that the CC genotype served as a risk factor in female
subjects. Despite this, a statistical difference was not observed
between individuals with the CC genotype and those with the CT or
CT/TT genotypes. Similarly, the implication is that AluI
polymorphism has no effect on BMD.
The subjects of 12 eligible studies were Chinese in
this meta-analysis; therefore, particular attention was focused on
Chinese subjects to explore the association between AluI
polymorphism and BMD. At the lumbar spine, the results showed that
subjects with the CC genotype had a higher BMD than subjects with
the CT and CT/TT genotypes, although the difference was not
significant. These results suggested that the CC genotype may have
a protective effect on the lumbar spine BMD; however, no
significant difference was found between patients with the CC
genotype and those with the CT or CT/TT genotypes. At the femoral
neck, the results were at variance with those at the lumbar spine.
In Chinese female subjects, those with the CC genotype had a lower
BMD than those with the CT/TT genotype, yet the difference was not
significant; this indicated that the CC genotype had a converse
effect on the femoral neck to that on the lumbar spine. No
association was therefore found between AluI polymorphism
and BMD.
Since the Southern and Northern Chinese populations
share a different diet, behavior and environment, subgroup analysis
of Chinese subjects was carried out in accordance with the region.
Notably, in Northern subjects, a significantly lower femoral neck
BMD was observed in subjects with the CC genotype versus that in
subjects with the CT/TT genotype, while those with the CC genotype
had a statistically higher lumbar spine BMD compared with patients
with the CT/TT genotype. These results demonstrated that
AluI polymorphism had an association with BMD in Northern
Chinese patients, with the CC genotype having a protective effect
on the lumbar spine whilst serving as a risk factor at the femoral
neck. The results of the present study were partly consistent with
the results in Korea reported by Lee et al (34). Lee et al also found that
subjects with the CC genotype had a higher BMD at the lumbar spine;
however, the same study also reported that patients with the CC
genotype had a higher BMD at the femoral neck, which was in
contrast to the results revealed here. Furthermore, Bandrés et
al (7) reported a
statistically significant association between the CTR gene
polymorphism and BMD in Spanish females. A common factor among
these findings is that they were all from subjects from Northern
regions; however, the results themselves showed significant
variation. The explanations for this phenomenon remain to be
elucidated, and the mechanism underlying the association requires
clarification.
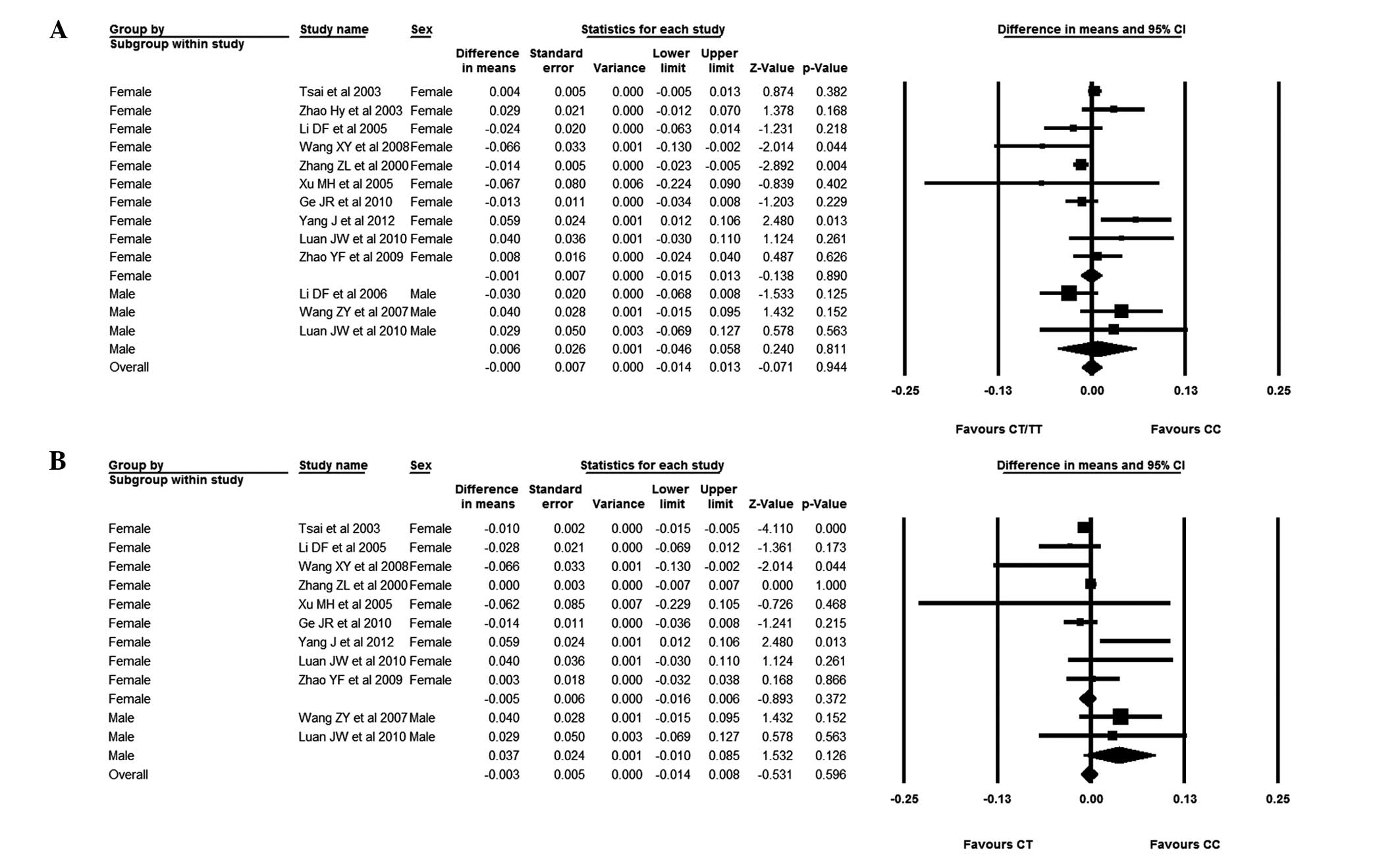
As females are more susceptible to osteoporosis than
males, the association between AluI polymorphism and BMD was
specifically investigated in Chinese female subjects. The results
showed that there was no difference in the BMD of the lumbar spine
and femoral neck between subjects with the CC genotype and those
with the CT/TT or CT genotypes. The Chinese female subjects were
then divided into Southern and Northern groups. The results
suggested that, in the Northern subjects, those with the CC
genotype had a statistically higher lumbar spine BMD than those
with the CT/TT genotype, and subjects with the CC genotype had a
trend of high femoral neck BMD, although this was not significant.
No difference, however, was identified in Southern subjects,
similar to subjects from China as a whole. In combination, it may
be suggested that AluI polymorphism had an association with
the BMD of the lumbar spine in Northern Chinese females.
This meta-analysis had a number of limitations. As
shown in previous studies (22,35,36), the distribution of allelic
frequency is different in Asia and Europe, and the majority of the
individuals included in the present study were Chinese; therefore,
data from different ethnicities is required to identify the exact
association of AluI polymorphism with BMD. In addition, only
published studies were included so publication bias cannot be
absolutely excluded, although no significant publication bias was
observed by Egger’s regression test in the majority of the
comparisons. Furthermore, the small number of subjects with the TT
genotype led to comparisons only of patients with the CC and CT or
combined CT/TT genotypes, which reduced the statistical power of
the study, and insufficient data from male subjects made the
analysis of the association between AluI polymorphism and
BMD in male subjects problematic. Finally, the interaction between
other risk genes and the CTR gene may also contribute to the
pathology of a reduced BMD, which could not be tested due to
insufficient data.
In conclusion, the present study suggested that the
AluI gene polymorphism may have an association with BMD in
Northern Chinese subjects, and the CC genotype may have a
protective effect on BMD at the lumbar spine; however, the CC
genotype may also serve as a risk factor for low femoral neck BMD
in Northern Chinese subjects. Further studies with larger sample
sizes and different ethnicities and genders are required to clarify
the association.
References
|
1
|
NIH Consensus Development Panel on
Osteoporosis Prevention, Diagnosis and Therapy. Osteoporosis
prevention, diagnosis, and therapy. JAMA. 285:785–795. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nguyen T, Sambrook P, Kelly P, et al:
Prediction of osteoporotic fractures by postural instability and
bone density. BMJ. 307:1111–1115. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brown MA, Haughton MA, Grant SF, Gunnell
AS, Henderson NK and Eisman JA: Genetic control of bone density and
turnover: role of the collagen 1alpha1, estrogen receptor, and
vitamin D receptor genes. J Bone Miner Res. 16:758–764. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guéguen R, Jouanny P, Guillemin F, Kuntz
C, Pourel J and Siest G: Segregation analysis and variance
components analysis of bone mineral density in healthy families. J
Bone Miner Res. 10:2017–2022. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pocock NA, Eisman JA, Hopper JL, Yeates
MG, Sambrook PN and Eberl S: Genetic determinants of bone mass in
adults. A twin study. J Clin Invest. 80:706–710. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mizunuma H, Hosoi T, Okano H, et al:
Estrogen receptor gene polymorphism and bone mineral density at the
lumbar spine of pre- and postmenopausal women. Bone. 21:379–383.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bandrés E, Pombo I, González-Huarriz M,
Rebollo A, López G and García-Foncillas J: Association between bone
mineral density and polymorphisms of the VDR, ERalpha, COL1A1 and
CTR genes in Spanish postmenopausal women. J Endocrinol Invest.
28:312–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mosaad YM, Hammad EM, Fawzy Z, et al:
Vitamin D receptor gene polymorphism as possible risk factor in
rheumatoid arthritis and rheumatoid related osteoporosis. Hum
Immunol. 75:452–461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koller DL, Zheng HF, Karasik D, et al:
Meta-analysis of genome-wide studies identifies WNT16 and ESR1 SNPs
associated with bone mineral density in premenopausal women. J Bone
Miner Res. 28:547–558. 2013. View Article : Google Scholar :
|
|
10
|
Wang D, Liu R, Zhu H, Zhou D, Mei Q and Xu
G: Vitamin D receptor Fok I polymorphism is associated with low
bone mineral density in postmenopausal women: a meta-analysis
focused on populations in Asian countries. Eur J Obstet Gynecol
Reprod Biol. 169:380–386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang KJ, Shi DQ, Sun LS, et al:
Association of estrogen receptor alpha gene polymorphisms with bone
mineral density: a meta-analysis. Chin Med J (Engl). 125:2589–2597.
2012.
|
|
12
|
Wallach S, Rousseau G, Martin L and Azria
M: Effects of calcitonin on animal and in vitro models of skeletal
metabolism. Bone. 25:509–516. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Andrade F, Videira M, Ferreira D and
Sarmento B: Nanocarriers for pulmonary administration of peptides
and therapeutic proteins. Nanomedicine (Lond). 6:123–141. 2011.
View Article : Google Scholar
|
|
14
|
Nakamura M, Zhang ZQ, Shan L, et al:
Allelic variants of human calcitonin receptor in the Japanese
population. Hum Genet. 99:38–41. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Masi L, Becherini L, Gennari L, et al:
Allelic variants of human calcitonin receptor: distribution and
association with bone mass in postmenopausal Italian women. Biochem
Biophys Res Commun. 245:622–626. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsai FJ, Chen WC, Chen HY and Tsai CH: The
ALUI calcitonin receptor gene polymorphism (TT) is associated with
low bone mineral density and susceptibility to osteoporosis in
postmenopausal women. Gynecol Obstet Invest. 55:82–87. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Charopoulos I, Trovas G, Stathopoulou M,
et al: Lack of association between vitamin D and calcitonin
receptor gene polymorphisms and forearm bone values of young Greek
males. J Musculoskelet Neuronal Interact. 8:196–203.
2008.PubMed/NCBI
|
|
18
|
Xu J, Gao Y, Yin J, et al: Calcitonin
receptor gene polymorphism in Chinese xinjiang han and uygur women
with primary osteoporosis. J Nutr Health Aging. 18:204–208. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Knobloch K, Yoon U and Vogt PM: Preferred
reporting items for systematic reviews and meta-analyses (PRISMA)
statement and publication bias. J Craniomaxillofac Surg. 39:91–92.
2011. View Article : Google Scholar
|
|
20
|
Braga V, Mottes M, Mirandola S, et al:
Association of CTR and COLIA1 alleles with BMD values in peri- and
postmenopausal women. Calcif Tissue Int. 67:361–366. 2000.
View Article : Google Scholar
|
|
21
|
Braga V, Sangalli A, Malerba G, et al:
Relationship among VDR (BsmI and FokI), COLIA1, and CTR
polymorphisms with bone mass, bone turnover markers, and sex
hormones in men. Calcif Tissue Int. 70:457–462. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao H, Liu J, Ning G, et al: Association
of calcitonin receptor gene polymorphism with bone mineral density
in Shanghai women. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 25:258–261.
2003.(In Chinese). PubMed/NCBI
|
|
23
|
Li D, Wu W, Cai X and Zhi XM: Association
between calcitonin receptor gene polymorphism and bone mineral
density in Guangzhou postmenopausal women. Hua Nan Yu Fang Yi Xue.
12–14. 19:2005.
|
|
24
|
Li D, Cai X, Yang Y, et al: Association
between calcitonin receptor gene polymorphism and bone mineral
density in elderly men. Zhongshan Da Xue Xue Bao. 27:410–413.
2006.
|
|
25
|
Wang X, Shao Y, Zhang Q, Yang Y, Hu H and
Wang Y: Relationship between calcitonin receptor gene polymorphism
and bone mineral density in postmenopausal women in Anhui. Anhui Yi
Ke Da Xue Xue Bao. 82–84. 2008.
|
|
26
|
Wang Z, Wu F, Deng W, et al: A study on
the correlation between calcitonin receptor genotypes and bone
mineral density in male subjects over 70 years old. Re Dai Yi Xue
Za Zhi. 7:691–692. 690:2007.
|
|
27
|
Xu M, Liu H and Tong X: Study of the
relationship between gene polymorphism of vitamin D receptor,
calcitonin receptor and bone mineral density of the Han nationality
woman in Hebei. Zhongguo Kang Fu Li Lun Yu Shi Jian. 11:247–249.
2005.
|
|
28
|
Ge J, Xie L, Chen K, et al: Association
between the AluI polymorphism in the calcitonin receptor gene and
bone mineral density in postmenopausal women. Zhong guo Gu Zhi Shu
Song Za Zhi. 16:829–832. 2010.
|
|
29
|
Yang J, Wang B, Xuan M, Li Y, Chu Y and
Zhang X: Relationship between receptor gene polymorphism and bone
metabolism, glucose metabolism, bone mineral density of
postmenopausal women in Shanghai. Zhonghua Lin Chuang Yi Shi Za
Zhi. 6:7255–7260. 2012.
|
|
30
|
Hayakawa Y, Yanagi H, Hara S, et al:
Genetic and environmental factors affecting peak bone mass in
premenopausal Japanese women. Environ Health Prev Med. 6:177–183.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luan J, Chen X, Yuan B, Zhou Z, et al:
Association between calcitonin receptor gene polymorphism and
primary osteoporosis. Zhongguo Zu Zhi Gong Cheng Yan Jiu Yu Lin
Chuang Kang Fu. 7:1243–1246. 2008.
|
|
32
|
Zhao Y, Chen R and Bai D: Calcitonin
receptor gene polymorphism and traditional Chinese medicine
differentiation type in relation to bone mineral density in female
patients with postmenopausal osteoporosis. Zhongguo Gu Zhi Shu Song
Za Zhi. 15:99–102. 2009.
|
|
33
|
Zhang Z, Meng X, Zhou X, et al:
Association of vitamin D receptor gene and calcitonin receptor gene
polymorphisms with bone mineral density in women of the Han
nationality in Beijing area. Zhonghua Nei Fen Mi Dai Xie Za Zhi.
18:90–94. 2002.
|
|
34
|
Lee HJ, Kim SY, Kim GS, et al: Fracture,
bone mineral density, and the effects of calcitonin receptor gene
in postmenopausal Koreans. Osteoporos Int. 21:1351–1360. 2010.
View Article : Google Scholar
|