Introduction
Gliomas are the most common type of central nervous
system tumors, and the majority of the histological findings of the
gliomas are malignant (1). Gliomas
have obscure boundaries with the surrounding tissue and as a result
the rate of radical resection is lower and the recurrence rate is
higher than other intracranial tumors (2). In recent years with the use of
microsurgical techniques and with the improvement of surgical
skills, as well as radiation and chemotherapy, the overall survival
of patients suffering from gliomas has been improved (3).
High-mobility group box-1 (HMGB1) is a non-histone
DNA-binding protein that is widely present in tissues, including
the heart, liver, lung, lymph, spleen, kidney and brain. In the
liver and brain HMGB1 is primarily found in the cytoplasm; however,
in other tissues it is mostly distributed in the nucleus (4). The functions of HMGB1 include DNA
binding, stabilizing the nucleosome and regulating transcription
(5). A number of studies have
found that higher expression levels of HMGB1 are closely associated
with tumor proliferation, invasion, migration and angiogenesis, as
well as anti-apoptotic effects, and HMGB1 can attenuate the role of
the body in monitoring tumor invasion and metastasis (6,7).
Previous studies have found an increased expression of HMGB1 in
human glioma tissues (8); however,
the associations between expression levels, pathology grades and
the prognostic significance are rarely reported.
At present studies of the molecular biology of
tumors are key issues in order to explore the mechanisms of
neoplasm occurrence and progress. In turn, the development of
molecular biology can guide us in exploring new methods of glioma
therapy, such as targeted therapy, which is a new and effective
treatment method. HMGB1 has been found in most of human tumors and
it is closely related with tumor development. HMGB1 may also play
an important role in human gliomas, however related studies are
rare.
In the present study, the expression of HMGB1 was
examined in 15 samples of normal brain tissue and 65 samples of
different-grade glioma tissue by immunohistochemistry and western
blot analysis, and the associations between the expression level
and pathology grades were analyzed statistically to investigate the
clinical significance. To the best of our knowledge, this is the
first study to investigate the prognostic significance of HMGB1
expression in human gliomas.
Materials and methods
Patients and samples
Tumor tissues were obtained at the first surgery in
65 previously untreated patients with glioma. The patient
population comprised 39 males and 26 females, and the median age
was 43.9±2.4 years (range, 12–78 years). All specimens were
pathologically confirmed referring to the 2007 World Health
Organization classification of tumors of the nervous system and
grading criteria (9). Twenty-seven
cases of low-grade glioma (LGG) were identified, including eight
cases of pilocytic astrocytoma, six cases of diffuse astrocytoma,
eight cases of oligodendroglioma and five cases of ependymoma. In
addition, 38 cases of high-grade glioma (HGG) were identified,
including 16 cases of anaplastic astrocytoma, two cases of
anaplastic ependymoma, three cases of malignant oligodendroglioma,
15 cases of glioblastoma and two cases of medulloblastoma. Fifteen
samples of normal brain tissue were obtained from brain injury
decompression surgery as a control. Consent was received from the
families of the patients to collect and preserve the specimens
using cryopreservation at −80°C. The present study was approved by
the Life Science Ethics committee of Zhengzhou University
(Zhengzhou, China).
Immunohistochemical detection
Immunohistochemistry was performed to detect the
expression of HMGB1. The specimens were embedded, cut into serial
3-μm sections and placed on a slide after pretreatment that was
undertaken by Beijing Bioss. In brief, pretreatment involved
immersion of the slide in cleaning fluid of potassium dichromate
sulfuric acid for 24 h and then rinsing under running water. After
it was rinsed again at least three times with distilled water 95%
ethanol was added and the slide was left to dry and immerse in
poly-L-lysine (0.01%) for about 30 sec. Finally, the slide was
drained and placed in the oven at 45°C for 1 h. Anti-HMGB1
monoclonal antibodies (1:20; Beijing Biosynthesis Biotechnology
Co., Ltd., Beijing, China) were added to the sections for
incubation overnight at 4°C, following by washing with
phosphate-buffered saline (PBS) and incubation with biotin-labeled
secondary antibody (Sigma-Aldrich, St. Louis, MO, USA) at room
temperature for 20 min. Subsequent to further washing with PBS,
horseradish peroxidase-labeled streptavidin working solution
(Beijing Biosynthesis Biotechnology Co., Ltd.,) was added and the
sections were incubated at room temperature for 15 min.
3,3′-diaminobenzidine chromogenic reagent was added for coloration,
followed by rinsing, hematoxylin staining, conventional ethanol
dehydration, xylene clearing, mounting with a neutral gum and
observation under a microscope. Samples in which PBS displaced the
primary antibody staining were classified as negative, while
samples in which the cytoplasm was colored yellow or brown were
classified as positive. An area with a strong immune response was
selected in each slice, and five non-repetitive, high-power fields
of view were observed (magnification, ×400). All controls provided
satisfactory results. The immunohistochemical analysis was
performed by one of the authors, who was blinded to the clinical
data. The HMGB1-positive cells were counted, and the positive rate
was calculated using the following formula: Positive rate = (number
of positive cells/number of total cells) ×100%. The samples were
defined as follows: Negative, 0–5%; weakly positive, 5–25%;
positive, 26–50%; strongly positive, >50%.
Western blot analysis
Frozen tissue samples (250 mg) were cut into
sections, and then homogenized on ice with pre-chilled protein
lysate (1 ml) for 30 min to fully cleave the HMGB1 protein.
Homogenized tissue fluid was placed into 1.5-ml
Eppendorf® tubes (Eppendorf, Hamburg, Germany) and
boiled for 5 min, and then centrifuged for 1 min at 8392 × g.
Following centrifugation, the supernatant was removed into 200-μl
Eppendorf tubes on ice and stored in a refrigerator at −70°C. The
bicinchoninic acid kit (Shanghai GenePharma Co., Ltd., Shanghai,
China) was used in the quantitative testing of HMGB1 protein
levels. Samples were transferred to a polyvinylidene difluoride
membrane following separation by SDS-PAGE and blocked with 5%
skimmed milk overnight at 4°C. Subsequent to adding 1:400 rabbit
anti-human HMGBl antibody (Beijing Biosynthesis Biotechnology Co.,
Ltd.), the membranes were again incubated overnight at 4°C. The
secondary antibody was then added and incubated at 37°C for 1 h. A
gel image analysis system (Media Cybernetics, Rockville, MD, USA)
was used to determine the absorbance value of each band, which
represented the expression of HMGB1 protein.
Statistical analysis
All statistical analyses were performed using SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA), and P<0.05 was
considered to indicate a statistically significant difference.
Measurement data are expressed as the mean ± standard deviation.
Differences between two symmetrical portions of the same group of
patients at different times were analyzed using the paired
Student’s t-test. The correlation between HMGB1 expression and
clinical pathological features was evaluated for statistical
significance by χ2 and Fisher’s exact tests. Survival
curves were calculated using the Kaplan-Meier method. The log-rank
test was used to analyze the survival time for any significant
differences.
Results
Analysis of the expression of HMGB1 in
normal brain tissue and gliomas
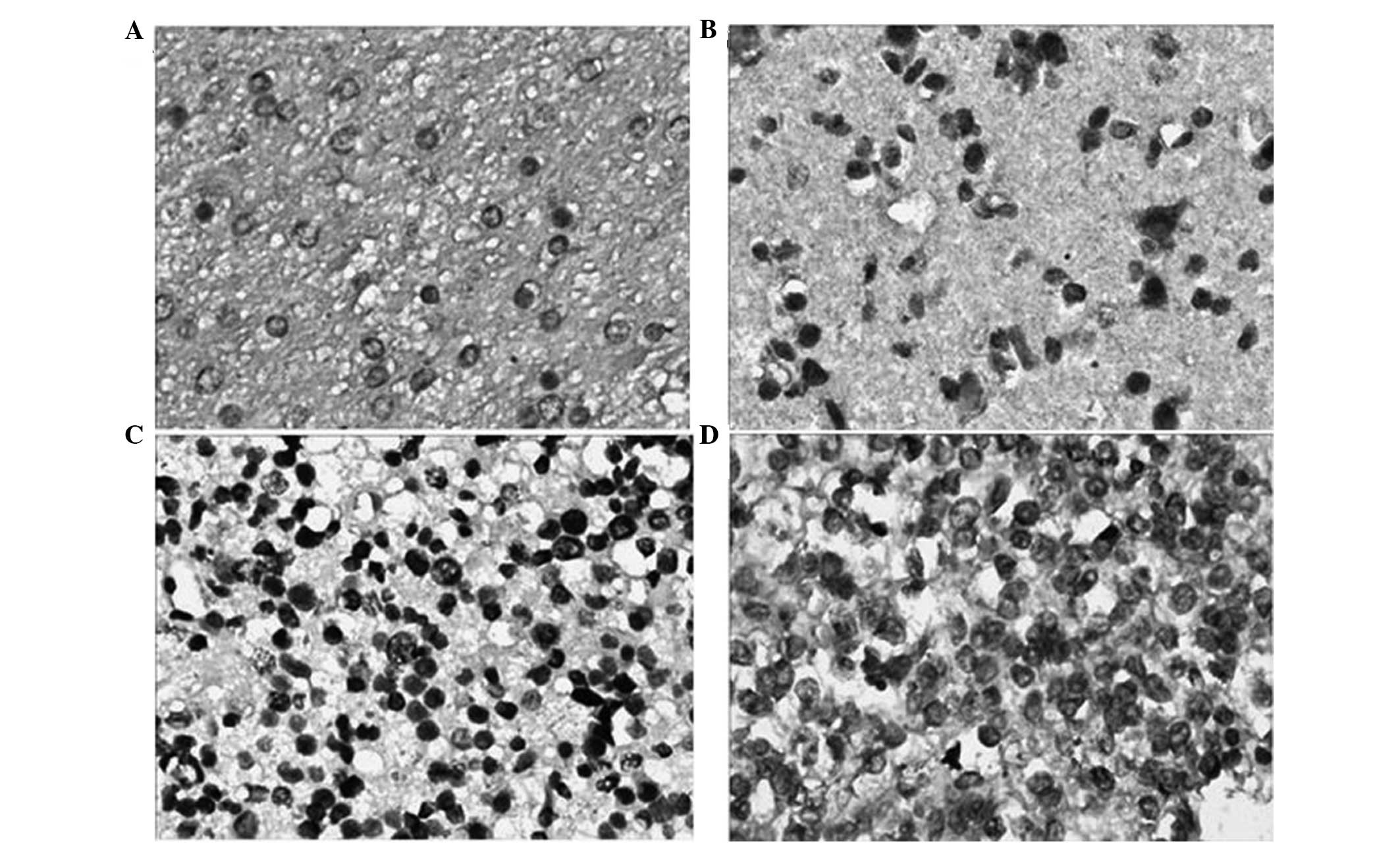
Immunohistochemical staining showed that the colored
areas due to HMGB1 expression were mainly located in the cytoplasm
near the nucleus. The majority of the staining was pale yellow,
while darkly stained areas were brown or tan (Fig. 1). HMGB1 showed lower expression in
the 15 normal brain tissue samples, and the positive expression
rate was 20.0% (3/15). However, in the 65 glioma samples the HMGB1
positive expression rate was 76.9% (50/65), which was higher than
that in the normal brain tissue (P<0.05) (Table I). The difference in HMGB1
expression between the different gender and age groups was not
statistically significant (P>0.05). The positive expression rate
of HMGB1 in the LGG and HGG groups was 63.0% (17/27) and 86.8%
(33/38), respectively, and the difference between the two groups
was statistically significant (χ2=5.070, P=0.024)
(Table II).
 | Table IExpression rates of HMGB1 in normal
brain and glioma tissues. |
Table I
Expression rates of HMGB1 in normal
brain and glioma tissues.
| | HMGB1 expression | | |
|---|
| |
| | |
|---|
| Group | n | Positive, n (%) | Negative, n (%) | χ2 | P-value |
|---|
| NG | 15 | 3 (20.00) | 12 (80.00) | - | - |
| LGG | 27 | 17 (62.96) | 10 (37.04) | 7.136 | 0.008a |
| HGG | 38 | 33 (86.84) | 5 (13.16) | - | <0.001a,b |
 | Table IIAssociation between HMGB1 and the
clinicopathological factors of glioma. |
Table II
Association between HMGB1 and the
clinicopathological factors of glioma.
| | HMGB1 expression | | |
|---|
| |
| | |
|---|
| Variable | n | Positive, n (%) | Negative, n (%) | χ2 | P-value |
|---|
| Gender |
| Male | 39 | 33 (84.62) | 6 (15.38) | 3.250 | 0.071 |
| Female | 26 | 17 (65.38) | 9 (34.62) | | |
| Age in years |
| >45 | 43 | 35 (81.40) | 8 (18.60) | 1.431 | 0.232 |
| ≤45 | 22 | 15 (68.18) | 7 (31.82) | | |
| Pathological
grade |
| LGG | 27 | 17 (62.96) | 10 (37.04) | 5.070 | 0.024 |
| HGG | 38 | 33 (86.84) | 5 (13.16) | | |
Analysis of the expression levels of
HMGB1 by western blotting
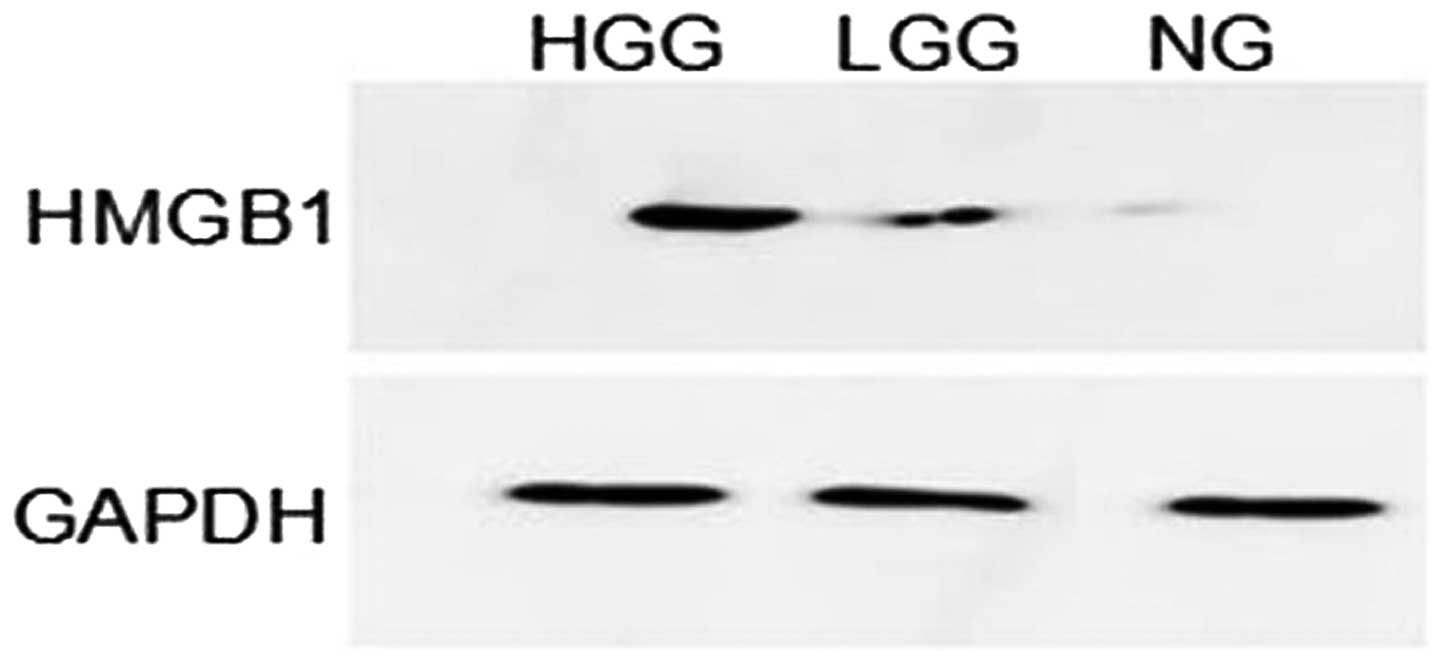
Western blot analysis showed that HMGB1 had lower
levels of expression in normal brain tissue than in glioma tissue.
The HMGB1 band optical density of each specimen was compared with
that of GAPDH and the ratio represented the relative levels of
HMGB1 protein expression. Analysis showed that the data were
consistent with a normal distribution; therefore, analysis of
variance was used for the three sets of data (normal, LGG and HGG).
The difference was found to be statistically significant
(P<0.05). The least significant difference t-test showed that
the expression of HMGB1 in the HGG group was significantly higher
than that in the LGG group (P<0.001) (Table III). A representative western
blot analysis of HMGB1 expression levels from identical cases is
shown in Fig. 2.
 | Table IIIWestern blot analysis detecting HMGB1
expression levels in normal brain and glioma tissues. |
Table III
Western blot analysis detecting HMGB1
expression levels in normal brain and glioma tissues.
| Group | n |
ODHMGB1/GAPDH |
|---|
| NG | 15 | 0.3631±0.1429 |
| LGG | 27 |
0.9115±0.1562a |
| HGG | 38 |
1.7019±0.1581a,b |
Prognostic value of HMGB1 positivity
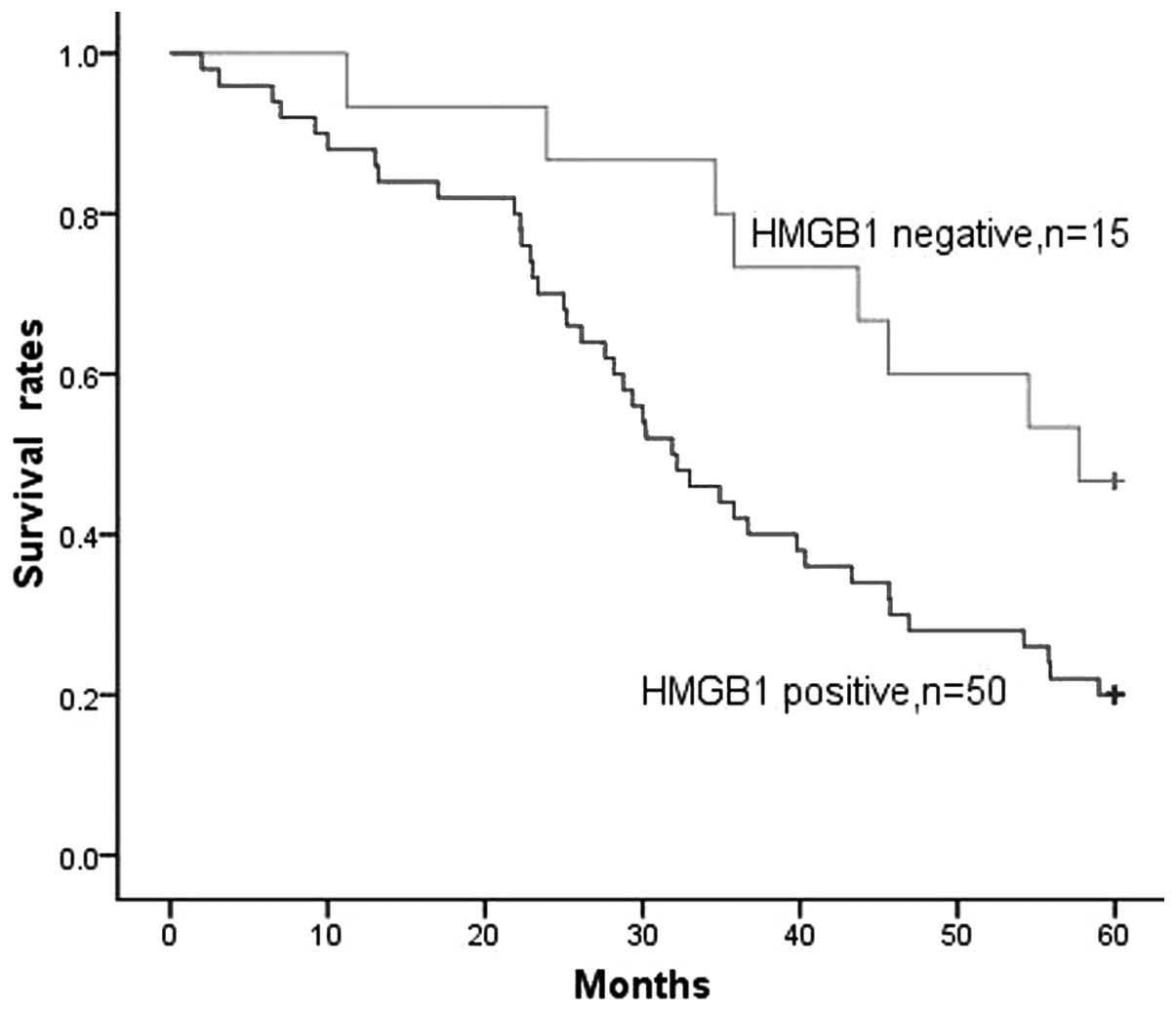
HMGB1 expression was detected in the majority of
patients, with 50/65 (76.9%) of gliomas being HMGB1-positive. When
all patients with glioma were considered together, the survival
rate of patients with HMGB1-negative tumors was significantly
higher than that of patients with HMGB1-positive tumors (P=0.026,
Fig. 3). The survival time of
patients with HMGB1-negative tumors was 48.4±4.0 months, compared
with 35.2±2.6 months for patients with HMGB1-positive tumors.
Prognostic value of HMGB1 expression
levels
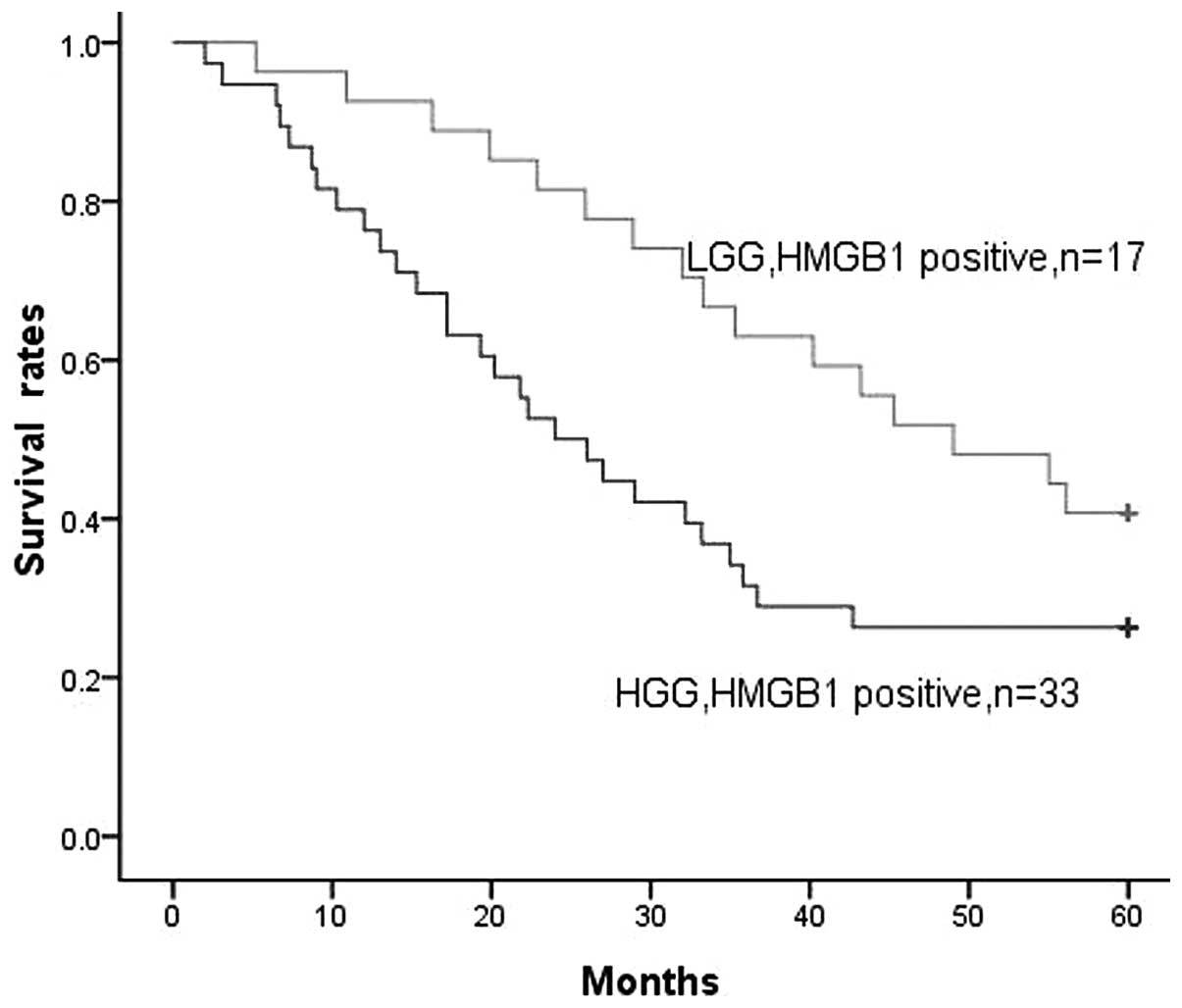
As stated earlier, a positive correlation was
identified between HMGB1 expression levels and the pathological
grades of the gliomas. In the LGG and HGG groups, positive HMGB1
expression was found in 17 and 33 cases, respectively. When all
gliomas were considered, increasing levels of HMGB1 expression were
clearly associated with decreased survival time. There appeared to
be a stronger correlation with reduced survival time for patients
with HGG than for patients with LGG (P=0.045, Fig. 4). HMGB1 expression levels had a
significant association with decreased survival time for patients
with glioma. This suggests that HMGB1 expression at the highest
levels is associated with a more extreme malignant phenotype of
glioma and may also be associated with increased treatment
resistance.
Multivariate analysis of prognostic
factors
In a model that included the presence or absence of
HMGB1 expression, pathological grade (LGG versus HGG), gender and
age, pathological grade (P=0.037) and HMGB1 expression (P=0.021)
were significantly associated with reduced survival times, whereas
age and gender were not. The data are presented in Table IV.
 | Table IVMultivariate analysis of prognostic
factors. |
Table IV
Multivariate analysis of prognostic
factors.
| Variable | B | SE | χ2 | P-value | OR | 95% CI |
|---|
| Gender | 0.124 | 0.562 | 0.049 | 0.835 | 0.394 | 0.135–1.291 |
| Age | −0.521 | 0.669 | 0.918 | 0.376 | 0.608 | 0.197–1.917 |
| HMGB1
expression | −2.961 | 0.864 | 11.558 | 0.021 | 0.061 | 0.019–0.294 |
| Pathological
grades | −1.734 | 0.814 | 4.083 | 0.037 | 0.188 | 0.035–0.957 |
Discussion
Glioma is the most common type of intracranial tumor
and has the highest incidence and mortality. The adult incidence
rate is ~6/100,000 and the five-year survival rate for glioma is
20–30% (10,11). Glioma exhibits the biological
features of infiltrative growth and unclear boundaries; therefore,
total resection is difficult. Additionally, the tumor recurs
easily. Although comprehensive treatments, including radiotherapy
and chemotherapy, are available, the curative effect requires
improvement (12). HMGB1 may be an
important factor involved in the processes of glioma occurrence and
development, and may seriously affect the prognosis of patients
with glioma.
HMGB1, which is a highly conserved nuclear protein,
functions as a chromatin-binding factor. As such, HMGB1 bends DNA
and facilitates access to transcription and protein assembly on
specific DNA targets. The functions of HMGB1 also include
stabilizing the nucleosome and regulating transcription (5). HMGB1 is expressed in the nucleus and
cytoplasm and plays an important role in the chemoresistance of
glioma, in addition to acting as a broad-spectrum tumor biomarker
(13). HMGB1 is passively released
from necrotic cells and actively secreted by inflammatory cells,
and functions as an extracellular signaling molecule during
processes such as inflammation, cell differentiation, tumor cell
proliferation, cell migration and tumor metastasis (14,15).
HMGB1 has been revealed to be constitutively activated in a wide
variety of human tumor tissues and cell lines, including
colorectal, breast, lung, prostate, cervical, stomach and liver
cancer, as well as leukemia (16).
It has been suggested that the overexpression of HMGB1 may promote
certain genes to form a tumor phenotype, rendering the cells immune
to apoptosis and resulting in tumorigenesis (17). The mechanism by which HMGB1 is
involved in tumorigenesis is unclear. However, it is believed to
mainly include the activation of the Janus kinase (JAK)/signal
transducer and activator of transcription (STAT) signaling pathway,
which occurs through HMGB1 binding with high affinity to several
receptors, including the receptor for advanced glycation end
products (RAGE), Toll-like receptor (TLR)-2, TLR-4 and TLR-9. These
interactions trigger the activation of key signaling pathways
involved in the regulation of cell differentiation, growth,
motility and apoptosis. A number of studies have revealed that
HMGB1 can overactivate STAT by activating the JAK/STAT pathway.
Activated STAT, particularly STAT3, inhibits tumor cell apoptosis,
accelerates the cell cycle and thus leads to tumorigenesis
(18–20). Subsequent to HMGB1 being released
into the extracellular environment, it unites with its high
affinity receptor RAGE and upregulates RAGE expression (21). HMGB1-RAGE interactions activate
mitogen-activated protein kinase and protein kinase B signaling
pathways, resulting in extracellular matrix degradation, tumor
invasion and metastasis, leading to tumor development (22). A previous study (23) found that HMGB1 that is released
into the extracellular environment may cause surrounding tumor
cells to undergo constant proliferation and induce the regeneration
of small blood vessels, thus promoting tumor growth. HMGB1 is also
closely associated with tumor drug resistance. A previous study
found that HMGB1 induces autophagy, causing the cells to become
resistant to chemotherapy drugs (24).
As a broad-spectrum tumor marker, the abnormal
expression of HMGB1 contributes to the occurrence and development
of numerous types of tumor (25).
In the present study it was shown that HMGB1 has different levels
of expression in normal brain and glioma tissues. Normal brain
tissues were weakly positive for HMGB1, which could be due to HMBG1
acting as a DNA-binding protein involved in normal physiological
processes of the body (26). In
glioma tissues the expression of HMGB1 was higher, and the degree
of expression in different pathological grade gliomas was
significantly different. HMGB1 is believed to be a pivotal factor
in the association between necrosis and malignancy in glioma due to
its role as an autocrine factor, which can promote the growth and
migration of tumor cells (27).
HMGB1 may cause disordered gene expression, resulting in glial
cells obtaining a tumor phenotype and resistance to apoptosis, and
ultimately leading to tumorigenesis (28). A recent study indicated that
necrotic cells can release HMGB1 into the extracellular environment
(29), and necrosis is a
characteristic feature of malignant gliomas. With the consistent
expression of HMGB1, the glioma grows and progresses continually,
leading to necrosis of certain lesions. The necrotic tumor cells
secrete HMGB1 to cause a cycle of further tumor progression.
Finally, the tumor infiltrates the surrounding brain tissue and
presents a stronger resistance, which makes it difficult to attain
whole resection and causes poor prognosis (30).
In the present study, immunohistochemistry and
western blot analysis were used to analyze the expression rate and
levels of HMGB1 in glioma tissues, and the prognostic significance
of HMGB1 expression in human gliomas was examined for the first
time. It was revealed that the expression of HMGB1 was associated
with pathological grade and poor prognosis. HMGB1 expression
patterns may lend additional insight into the molecular
pathogenesis of these tumors. Furthermore, HMGB1 may be an
important prognostic marker. Therefore, treatments targeting HMGB1
are expected to become a novel therapeutic approach towards the
treatment of patients with glioma.
References
|
1
|
Kong BH, Park NR, Shim JK, et al:
Isolation of glioma cancer stem cells in relation to histological
grades in glioma specimens. Childs Nerv Syst. 29:217–229. 2013.
View Article : Google Scholar
|
|
2
|
Chamberlain MC: Treatment of newly
diagnosed malignant glioma in the elderly people: new trials that
impact therapy. Int J Clin Pract. 67:1225–1227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim SM, Woo JS, Jeong CH, et al: Potential
application of temozolomide in mesenchymal stem cell-based TRAIL
gene therapy against malignant glioma. Stem Cells Transl Med.
3:172–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stros M: HMGB proteins: interactions with
DNA and chromatin. Biochim Biophys Acta. 1799:101–113. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Belgrano FS, de Abreu da Silva IC, Bastos
de Oliveira FM, et al: Role of the acidic tail of high mobility
group protein B1 (HMGB1) in protein stability and DNA bending. PLoS
One. 8:e795722013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fahmueller YN, Nagel D, Hoffmann RT, et
al: Immunogenic cell death biomarkers HMGB1, RAGE, and DNAse
indicate response to radioembolization therapy and prognosis in
colorectal cancer patients. Int J Cancer. 132:2349–2358. 2013.
View Article : Google Scholar
|
|
7
|
Stoetzer OJ, Fersching DM, Salat C, et al:
Circulating immunogenic cell death biomarkers HMGB1 and RAGE in
breast cancer patients during neoadjuvant chemotherapy. Tumour
Biol. 34:81–90. 2013. View Article : Google Scholar
|
|
8
|
Gupta P, Ghosh S, Nagarajan A, et al:
β-defensin-3 negatively regulates TLR4-HMGB1 axis mediated HLA-G
expression in IL-1β treated glioma cells. Cell Signal. 25:682–689.
2013. View Article : Google Scholar
|
|
9
|
Brat DJ, Scheithauer BW, Fuller GN and
Tihan T: Newly codified glial neoplasms of the 2007 WHO
Classification of Tumours of the Central Nervous System:
angiocentric glioma, pilomyxoid astrocytoma and pituicytoma. Brain
Pathol. 17:319–324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sahgal A, Ironside SA, Perry J, et al:
Factors influencing overall survival specific to adult low-grade
astrocytoma: a population-based study. Clin Oncol (R Coll Radiol).
25:394–399. 2013. View Article : Google Scholar
|
|
12
|
Zhang X, Yang H, Gong B, et al: Combined
gene expression and protein interaction analysis of dynamic
modularity in glioma prognosis. J Neurooncol. 107:281–288. 2012.
View Article : Google Scholar
|
|
13
|
Huang J, Ni J, Liu K, et al: HMGB1
promotes drug resistance in osteosarcoma. Cancer Res. 72:230–238.
2012. View Article : Google Scholar
|
|
14
|
Youn JH and Shin JS: Nucleocytoplasmic
shuttling of HMGB1 is regulated by phosphorylation that redirects
it toward secretion. J Immunol. 177:7889–7897. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schlueter C, Weber H, Meyer B, et al:
Angiogenetic signaling through hypoxia: HMGB1: an angiogenetic
switch molecule. Am J Pathol. 166:1259–1263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang D, Kang R, Zeh HJ III and Lotze MT:
High-mobility group box 1 and cancer. Biochim Biophys Acta.
1799:131–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guerriero JL, Ditsworth D, Catanzaro JM,
et al: DNA alkylating therapy induces tumor regression through an
HMGB1-mediated activation of innate immunity. J Immunol.
186:3517–3526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hui L, Yao Y, Wang S, et al: Inhibition of
Janus kinase 2 and signal transduction and activator of
transcription 3 protect against cecal ligation and puncture-induced
multiple organ damage and mortality. J Trauma. 66:859–865. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wagner KU and Schmidt JW: The two faces of
Janus kinases and their respective STATs in mammary gland
development and cancer. J Carcinog. 10:322011. View Article : Google Scholar
|
|
20
|
Diaz T, Navarro A, Ferrer G, et al:
Lestaurtinib inhibition of the Jak/STAT signaling pathway in
hodgkin lymphoma inhibits proliferation and induces apoptosis. PLoS
One. 6:e188562011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rauvala H and Rouhiainen A: Physiological
and pathophysiological outcomes of the interactions of HMGB1 with
cell surface receptors. Biochim Biophys Acta. 1799:164–170. 2010.
View Article : Google Scholar
|
|
22
|
Ohmori H, Luo Y and Kuniyasu H:
Non-histone nuclear factor HMGB1 as a therapeutic target in
colorectal cancer. Expert Opin Ther Targets. 15:183–193. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bassi R, Giussani P, Anelli V, et al:
HMGB1 as an autocrine stimulus in human T98G glioblastoma cells:
role in cell growth and migration. J Neurooncol. 87:23–33. 2008.
View Article : Google Scholar
|
|
24
|
Tang D, Kang R, Cheh CW, et al: HMGB1
release and redox regulates autophagy and apoptosis in cancer
cells. Oncogene. 29:5299–5310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wittwer C, Boeck S, Heinemann V, et al:
Circulating nucleosomes and immunogenic cell death markers HMGB1,
sRAGE and DNAse in patients with advanced pancreatic cancer
undergoing chemotherapy. Int J Cancer. 133:2619–2630.
2013.PubMed/NCBI
|
|
26
|
Naglova H and Bucova M: HMGB1 and its
physiological and pathological roles. Bratisl Lek Listy.
113:163–171. 2012.PubMed/NCBI
|
|
27
|
Kostova N, Zlateva S, Ugrinova I and
Pasheva E: The expression of HMGB1 protein and its receptor RAGE in
human malignant tumors. Mol Cell Biochem. 337:251–258. 2010.
View Article : Google Scholar
|
|
28
|
Jube S, Rivera ZS, Bianchi ME, et al:
Cancer cell secretion of the DAMP protein HMGB1 supports
progression in malignant mesothelioma. Cancer Res. 72:3290–3301.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martins I, Kepp O, Menger L, et al:
Fluorescent biosensors for the detection of HMGB1 release. Methods
Mol Biol. 1004:43–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang GL, Zhang LH, Bo JJ, et al: Increased
expression of HMGB1 is associated with poor prognosis in human
bladder cancer. J Surg Oncol. 106:57–61. 2012. View Article : Google Scholar : PubMed/NCBI
|