Introduction
Idiopathic pulmonary fibrosis (IPF) is the most
common specific form of idiopathic interstitial pneumonia. It is a
chronic, progressive and irreversible lung disease with unknown
cause and is usually fatal. Its histopathological or radiological
appearance is similar to that typically observed for usual
interstitial pneumonia (1–3). The mean survival time for diagnosed
patients is 2.5 to 3.5 years (4);
the survival period is short and the mortality rate is high.
Ischemic heart disease, heart failure, bronchial cancer, infection
and pulmonary embolism are significant causes of mortality in
patients with IPF (5–8).
IPF complicated by infection can cause respiratory
function to decline (4), which may
lead to exacerbations and further reduce the arterial oxygen
pressure. Active control of infection should help to improve lung
function and reduce mortality.
The success of antibiotic therapy not only depends
on the sensitivity of pathogenic microorganisms, but also the drug
concentration in infected tissues, which is particularly important
in the treatment of respiratory tract infections (9). Therefore, improving the concentration
of antimicrobial drugs in the bronchial-pulmonary tissues is likely
to help in controlling infection. According to previous studies,
ambroxol increases the concentration of amoxicillin, erythromycin,
ampicillin and other antibacterial drugs in the normal lung tissues
of animals and humans (9–11). If ambroxol is able to improve
antimicrobial concentrations in the lung tissue of patients with
pulmonary fibrosis, treatment efficacy may be improved. However, to
the best of our knowledge, whether ambroxol has an impact on the
antimicrobial drug concentration in pulmonary interstitial fibrosis
has not previously been reported. Bronchoalveolar lavage techniques
are suitable for detecting the concentrations of antimicrobial
agents in lung tissues (10). In
the present study, the impact of ambroxol on the concentration of
cefotaxime in the lung tissue of rats with pulmonary fibrosis was
tested by a bronchoalveolar lavage technique to evaluate the role
of ambroxol.
Materials and methods
Animal grouping and model
preparation
A total of 54 male Wistar rats (clean grade) with
weights of 180–220 g were purchased from the Animal Center of
Shandong University (Jinan, China). The animals were randomly
divided into three groups. These were a normal control group (group
A), model group (group B) and ambroxol hydrochloride group (group
C). The rats of the B and C groups were administered an
intratracheal injection of bleomycin (5 mg/kg), and those of group
A were administered an infusion of saline via the trachea. In
addition, a daily intraperitoneal injection of ambroxol
hydrochloride 35 mg/kg (7 mg for each rat) (Hebei Aierhaitai
Pharmaceutical Co., Ltd., Shijiazhuang, China) was administered to
the rats in group C. This study was carried out in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals (8th edition, 2011) of the National
Institutes of Health. The animal use protocol was reviewed and
approved by the Institutional Animal Care and Use Committee (IACUC)
of Shandong Provincial Qianfoshan Hospital (Permit Number:
20060828001). On days 7, 14, 28 after the perfusion of bleomycin,
six rats were collected from each group to obtain bronchoalveolar
lavage fluid following the injection of cefotaxime sodium (600
mg/kg; Suzhou Dawnrays Pharmaceutical Co., Ltd., Suzhou, China) via
the tail vein. Liquid chromatography-mass spectrometry (MS) was
used to determine the concentration of cefotaxime in the lavage
fluid. Lung tissues were collected for pathological
observation.
Specimen collecting and handling
A 10% chloral hydrate solution was used to
anesthetize the rats by intraperitoneal injection. Following
fixation, the trachea was isolated from the neck, the trachea was
cut, a catheter was inserted and ligation with silk, and 2 ml
saline was injected into the catheter. Following twice repeated
washing, bronchoalveolar lavage fluid was collected in test tubes
and centrifuged at 444 × g for 10 min. The supernatant was stored
in an EP tube at −20°C. Lungs were removed and fixed with 10%
formaldehyde solution until pathological examination one week
later.
Pathological examination
The right lung was dehydrated, rendered transparent
and embedded in wax. The 7-μm sections were stained with
hematoxylin and eosin and the pathological changes were observed
under a light microscope. The extent of alveolitis and pulmonary
fibrosis was evaluated according to the method of Szapiel (12). Grading standards: (−), no
alveolitis or pulmonary fibrosis; (+), mild alveolitis or pulmonary
fibrosis with an affected area of <20% of the whole lung; (++)
moderate alveolitis or pulmonary fibrosis accounting for 20–50% of
the lung; and (+++), severe alveolitis or pulmonary fibrosis with
an affected area of >50%.
Measurement of cefotaxime
concentration
Chromatography was conducted using a Zorbax SB-C18
analytical column (150×4.6 mm; internal diameter, 5 μm) from
Agilent Technologies (Santa Clara, CA, USA) and a 0.45-μm line
filter (Agilent Technologies). The mobile phase comprised methanol
and water (containing 0.1% acetic acid) in a 30:70 ratio by volume;
the flow rate was 1.0 ml/min and was split to provide a flow rate
of ~0.3 ml/min into a mass spectrometer (Agilent1100Trap VL-ion
trap, Agilent Technologies), with an injection volume of 5 μl.
MS was conducted using an electrospray ionization
(ESI) source, a heated capillary temperature of 350°C, a spray gas
(N2) pressure of 30 psi and a drying gas (N2)
flow of 10 l/min. The positive ion detection mode and multiple
scanning with the multiple reaction monitoring (MRM) mode were
used. The ionic reaction m/z 500→440 was quantitatively analyzed at
a fragmentation voltage of 0.8 V.
Samples were prepared by placing 100 μl
bronchoalveolar lavage fluid in a 5 ml plastic centrifuge tube,
adding 400 μl methanol, vortexing for 0.5 min and centrifuging at
1,776 × g for 10 min. Then, 5 μl supernatant was used for
analysis.
The specificity of the method was determined using a
10 μg/ml methanolic solution of cefotaxime sodium as the control.
An ESI source was used, with a heated capillary temperature of
325°C, spray gas (N2) pressure of 10 psi and drying gas
(N2) flow of 4 l/min. The perfusion injection mode was
used, with a flow rate of 5 μl/min. Cefotaxime sodium mainly
generated [M+Na]+ (m/z 500) in the positive ion mode.
Selective ion scanning was used to analyze the [M+Na]+
peaks by fragmentation product. The most commonly observed ion
fragment of cefotaxime sodium, which had an m/z ratio of 440, was
considered as the product ion in the quantitative analysis.
A standard curve was generated by taking a precise
amount (1.0 mg/ml) of cefotaxime sodium and adding methanol to
prepare solutions of different concentrations (5, 10 and 50 μg/ml).
A 5-μl aliquot of the sample solution was subjected to analysis by
MS. The concentration was plotted as the abscissa and the peak area
as the vertical axis of the linear standard curve to prepare a
standard quantitative curve (in units of μg/ml).
Statistical analysis
The results are expressed as mean ± standard
deviation, using single-factor analysis of variance (one-way ANOVA)
to analyze homogeneity of variance, ANOVA and pairwise comparisons.
Statistical analysis was performed using SPSS statistical software,
version 13.0 (SPSS, Inc., Chicago, IL, USA).
Results
Morphological changes
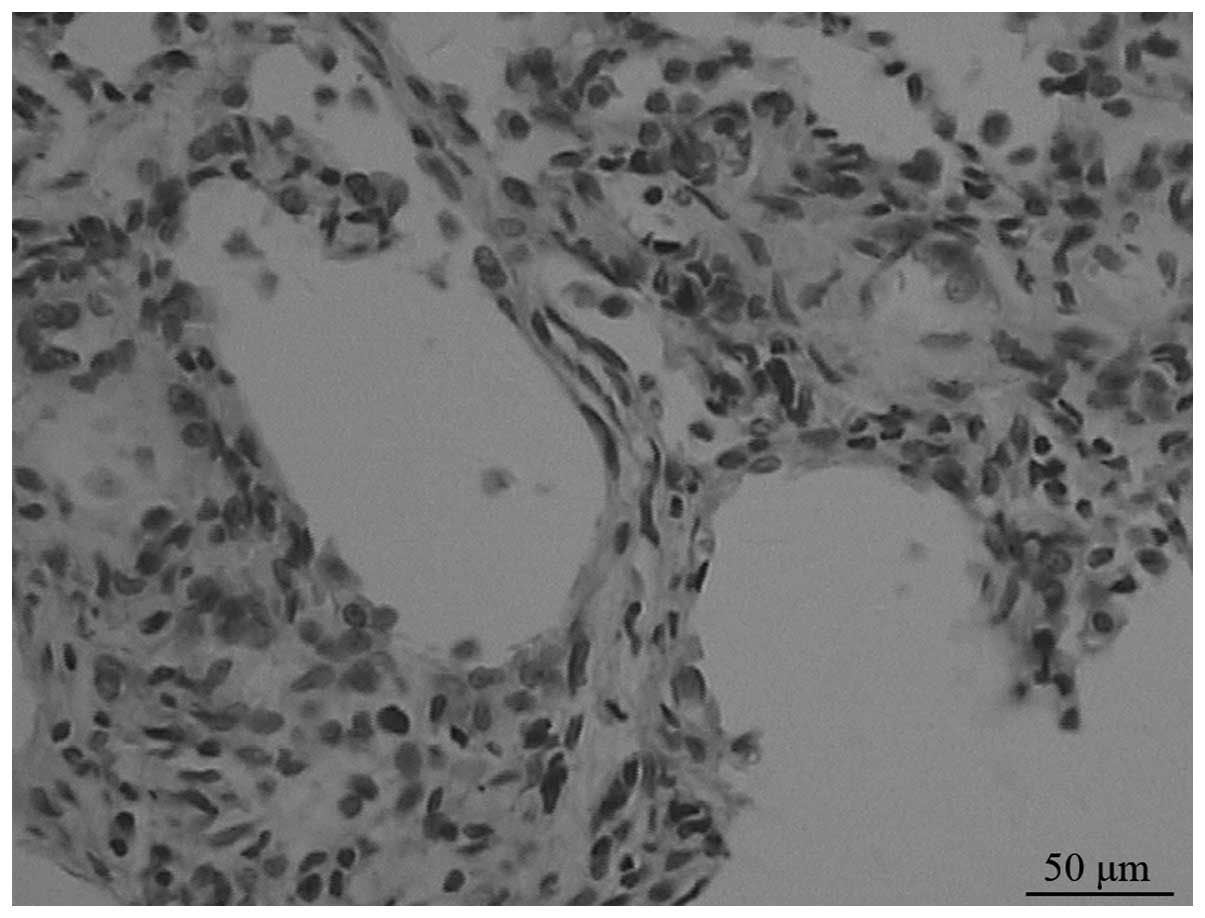
On day 7, under a light microscope, the model group
displayed moderate to severe alveolitis, but only mild alveolitis
was observed on day 28. Alveolitis of the ambroxol group was mild
compared with that of the untreated group. Fibrosis began from day
14 in the model group, and progressively developed until day 28,
when it reached a peak (Fig. 1).
The extent of pulmonary fibrosis in the ambroxol group was reduced
compared with that in the model group (Fig. 2).
Cefotaxime concentration
The cefotaxime concentration in the bronchoalveolar
lavage fluid of the model group was higher than that in the normal
control on day 7, was reduced to a minimum on day 14, and then rose
again by day 28 (Table I, Fig. 3). On day 7, the cefotaxime
concentration in the bronchoalveolar lavage fluid of the ambroxol
group was reduced in comparison with that in the model group;
however, the difference was not statistically significant (P=0.164;
Table II). On day 14, the
cefotaxime concentration in the bronchoalveolar lavage fluid of the
ambroxol group rose and was higher than that of the model group;
the difference between the ambroxol and model groups was
statistically significant (P<0.001; Table III). On day 28, the concentration
in the ambroxol group dropped sharply, and was lower than that in
the model group; however, the difference was not statistically
significant (P=0.126; Table
IV).
 | Table ICefotaxime concentration in the
bronchoalveolar lavage fluid at different time points in the three
groups of rats (μg/ml). |
Table I
Cefotaxime concentration in the
bronchoalveolar lavage fluid at different time points in the three
groups of rats (μg/ml).
| Group | Day 7 | Day 14 | Day 28 |
|---|
| A | 6.64±0.32 | 8.90±2.48 | 6.85±1.08 |
| B | 10.63±2.19 | 8.33±2.34 | 9.11±4.11 |
| C | 9.09±2.14 | 14.39±3.21 | 6.74±1.63 |
 | Table IIPairwise comparison of cefotaxime
concentration in the bronchoalveolar lavage fluid between groups on
day 7. |
Table II
Pairwise comparison of cefotaxime
concentration in the bronchoalveolar lavage fluid between groups on
day 7.
| (I) Group | (J) Group | Mean range (I–J) | Standard error | Significant
level | 95% credibility
interval |
|---|
|
|---|
| Lowest limit | Highest limit |
|---|
| Group B | Group A | 3.9896 | 1.070 | 0.001 | 1.7860 | 6.1932 |
| Group C | 1.5331 | 1.070 | 0.164 | −0.6705 | 3.7368 |
 | Table IIIPairwise comparison of cefotaxime
concentration in the bronchoalveolar lavage fluid between groups on
day 14. |
Table III
Pairwise comparison of cefotaxime
concentration in the bronchoalveolar lavage fluid between groups on
day 14.
| (I) Group | (J) Group | Mean range (I–J) | Standard error | Significant
level | 95% credibility
interval |
|---|
|
|---|
| Lowest limit | Highest limit |
|---|
| Group B | Group A | −0.5794 | 1.479 | 0.698 | −3.6249 | 2.4660 |
| Group C | −6.0588 | 1.479 | 0.000 | −9.1042 | −3.0133 |
 | Table IVPairwise comparison of cefotaxime
concentration in the bronchoalveolar lavage fluid between groups on
day 28. |
Table IV
Pairwise comparison of cefotaxime
concentration in the bronchoalveolar lavage fluid between groups on
day 28.
| (I) Group | (J) Group | Mean range (I–J) | Standard error | Significant
level | 95% credibility
interval |
|---|
|
|---|
| Lowest limit | Highest limit |
|---|
| Group B | Group A | 2.2565 | 1.498 | 0.145 | −0.8290 | 5.3421 |
| Group C | 2.3720 | 1.498 | 0.126 | −0.7136 | 5.4576 |
Discussion
IPF complicated by infection can cause respiratory
function to decline (4),
exacerbate disease and increase mortality. One of the factors that
is key to the success of anti-infective therapy is the
concentration of antimicrobial agent in the infected tissues
(9). Improving the lung tissue
concentrations of antimicrobial drugs should help to control
infection and improve the prognosis.
The present study aimed to determine the impact of
ambroxol on the antimicrobial drug concentration in the lung
tissues of rats with pulmonary fibrosis. Cephalosporins are a
commonly used class of antibacterial drugs, which enter the lung
tissue by diffusion mechanisms (13). In the present study, cefotaxime
sodium was selected as a representative of this drug class. It was
found that the concentration of cefotaxime in the bronchoalveolar
lavage fluid of the model group on day7 (the alveolitis period) was
significantly higher than that of the control group due to an
increase of vascular permeability in the alveolitis period in the
model group, which is consistent with the literature (14). The concentration in the model group
had decreased by day 14 (the initial fibrosis period) and rose
again on day 28 (the fibrosis stage), when no significant
difference was detected when compared with the control group.
It was observed that the salt of ambroxol, in
addition to improving the concentration of cefotaxime in the
bronchoalveolar lavage fluid of the early fibrosis period, also
reduced fibrosis, which is consistent with the literature (15,16).
Previous studies have demonstrated that ambroxol has good
anti-inflammatory and antioxidant effects (17–20),
and can inhibit the synthesis and release of cytokines and
inflammatory mediators (21).
Oxygen free radical damage is an important aspect of interstitial
pulmonary fibrosis. In the present study, ambroxol hydrochloride
reduced alveolitis and pulmonary fibrosis. In the alveolitis and
fibrosis periods, the cefotaxime sodium concentration in the
bronchoalveolar lavage fluid of the ambroxol group was not
significantly different from that of the model group, but was
higher than that of the model group in the initial fibrosis period,
suggesting that the increased impact of ambroxol hydrochloride on
the cefotaxime sodium concentration in the bronchoalveolar lavage
fluid in the early fibrosis period had no significant association
with its anti-inflammatory and antifibrotic effects.
In conclusion, the results revealed that ambroxol
hydrochloride can improve the cefotaxime sodium concentration in
bronchoalveolar lavage fluid. Thus, ambroxol may improve
anti-infection treatment in the early fibrosis period, and these
results may act as a reference for clinical anti-infection
treatment. The mechanism by which ambroxol hydrochloride increases
the concentration of cefotaxime sodium in bronchoalveolar lavage
fluid requires further study.
References
|
1
|
American Thoracic Society and European
Respiratory Society. Idiopathic pulmonary fibrosis: Diagnosis and
treatment. International consensus statement. Am J Respir Crit Care
Med. 161:646–664. 2000. View Article : Google Scholar
|
|
2
|
American Thoracic Society and European
Respiratory Society. International multidisciplinary consensus
classification of the idiopathic interstitial pneumonias. Am J
Respir Crit Care Med. 165:277–304. 2002.
|
|
3
|
Raghu G, Collard HR, Egan JJ, et al:
ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis: An
official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis:
evidence-based guidelines for diagnosis and management. Am J Respir
Crit Care Med. 183:788–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ley B, Collard HR and King TE Jr: Clinical
course and prediction of survival in idiopathic pulmonary fibrosis.
Am J Respir Crit Care Med. 183:431–440. 2011. View Article : Google Scholar
|
|
5
|
Martinez FJ, Safrin S, Weycker D, et al;
IPF Study Group. The clinical course of patients with idiopathic
pulmonary fibrosis. Ann Intern Med. 142:963–967. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olson AL, Swigris JJ, Lezotte DC, Norris
JM, Wilson CG and Brown KK: Mortality from pulmonary fibrosis
increased in the United States from 1992 to 2003. Am J Respir Crit
Care Med. 176:277–284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
King TE Jr, Albera C, Bradford WZ, et al;
INSPIRE Study Group. Effect of interferon gamma-1b on survival in
patients with idiopathic pulmonary fibrosis (INSPIRE): a
multicentre, randomised, placebo-controlled trial. Lancet.
374:222–228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hubbard RB, Smith C, Le Jeune I, Gribbin J
and Fogarty AW: The association between idiopathic pulmonary
fibrosis and vascular disease: a population-based study. Am J
Respir Crit Care Med. 178:1257–1261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spátola J, Poderoso JJ, Wiemeyer JC, et
al: Influence of ambroxol on lung tissue penetration of amoxillin.
Arzneimittelforschung. 37:965–966. 1987.
|
|
10
|
Gené R, Poderoso JJ, Corazza C, Lasala MB,
Wiemeyer JC, Fernández M and Guerreiro RB: Influence of ambroxol on
amoxicillin levels in bronchoalveolar lavage fluid.
Arzneimittelforschung. 37:967–968. 1987.PubMed/NCBI
|
|
11
|
Wiemeyer JC: Influence of ambroxol on the
bronchopulmonary level of antibiotics. Arzneimittelforschung.
31:974–976. 1981.PubMed/NCBI
|
|
12
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis.
120:893–899. 1979.PubMed/NCBI
|
|
13
|
Wang F: Respiratory Tract Infection.
Infectious diseases and antimicrobial therapy. 2nd edition.
Shanghai Medical University Publishing House; Shanghai, China: pp.
216–223. 2000
|
|
14
|
Morgan EJ and Petty TL: Summary of the
National Mucolytic Study. Chest. 97(2 Suppl): 24S–27S. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhi QM, Yang LT and Sun HC: Protective
effect of ambroxol against paraquat-induced pulmonary fibrosis in
rats. Intern Med. 50:1879–1887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pozzi E, Salmona M, Masturzo P, Genghini
M, Scelsi M, Spialtini L and Luisetti M: Role of alveolar
phospholipids in bleomycin-induced pulmonary fibrosis in the rat.
Respiration. 51:23–32. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Winsel K: The antioxidative and
inflammation inhibiting properties of ambroxol. Pneumologie.
46:461–475. 1992.(In German). PubMed/NCBI
|
|
18
|
Beeh KM, Beier J, Esperester A and Paul
LD: Antiinflammatory properties of ambroxol. Eur J Med Res.
13:557–562. 2008.PubMed/NCBI
|
|
19
|
Nowak D, Antczak A, Król M, et al:
Antioxidant properties of Ambroxol. Free Radic Biol Med.
16:517–522. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Piotrowski WJ, Pietras T, Kurmanowska Z,
et al: Effect of paraquat intoxication and ambroxol treatment on
hydrogen peroxide production and lipid peroxidation in selected
organs of rat. J Appl Toxicol. 16:501–507. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stockley RA, Shaw J and Burnett D: Effect
of ambroxol of neutrophil chemotaxis in vitro. Agents Actions.
24:292–296. 1988. View Article : Google Scholar : PubMed/NCBI
|