Introduction
Psoriasis is a chronic inflammatory skin disease
characterized by epidermal hyperproliferation and altered
differentiation, with a prevalence of 2–4% worldwide (1). Chronic plaque psoriasis, or psoriasis
vulgaris, is the most common form of the disease with well a
circumscribed erythematous and indurated plaque scale, accounting
for 85–90% of cases. At present, there is no curative therapy
available to fully treat the disease, and the typical clinical
course is of chronic relapse and remission (2). Previous studies support a pivotal
role for nuclear factor κB (NF-κB) activation in the pathogenesis
of psoriasis (3). The
overactivation of NF-κB in the psoriatic epidermis has been
hypothesized to induce altered keratinocyte proliferation and
differentiation (4). Increased
activation may cause NF-κB to translocate into the nucleus and
subsequently promote the transcription of target gene sequences,
including the keratinocyte differentiation markers of cytokeratin
10 (K10), cytokeratin 16 (K16), loricrin (LOR) and filaggrin (FLG)
(5–8), and also regulate the cell cycle,
which is considered to be significantly accelerated in the
pathogenesis of psoriasis (9).
Various reagents that are capable of regulating the status of the
NF-κB pathway have been used to treat psoriasis. Calcipotriol is
notable among these. A study has demonstrated that calcipotriol may
regulate the NF-κB pathway through inversing the binding activation
of NF-κB to its target gene response elements, including p53 and
interleukin (IL)-8 (10) and
subsequently regulating their transcription and protein expression.
Calcipotriol has shown clear therapeutic effects on psoriasis
vulgaris and is widely used in the majority of countries. However,
clinical studies have reported that calcipotriol may simultaneously
induce clear adverse effects, including impairment of the skin
barrier function and evident irritation to the psoriatic skin,
particularly following long-term topical application (11–13).
Portulaca oleracea L. (purslane) is a green
plant and vegetable consumed mainly in the eastern Mediterranean
region, and is also commonly known as machixian in China and
pursley in the USA. In ancient China, it was medically used as an
effective cure for blasting and burning by gunpowder, and it has
also been used as a folk medicine in a number of other countries to
treat various ailments in humans, including as a cooling diuretic,
refrigerant and tonic, as well as an article of diet used to treat
scurvy, liver complaints, sore nipples, stomach and mouth ulcers,
and for reducing inflammation (14). Modern studies have revealed that
P. oleracea leaves are a rich source of linolenic acid (LNA)
and α-tocopherol (α-TCP) (15,16),
and its extracts are capable of regulating the tumor necrosis
factor-α (TNF-α)-induced NF-κB signaling pathway (17) as well as suppressing the
overexpression of proinflammatory factors, including vascular cell
adhesion molecule-1, intercellular adhesion molecule-1, E-selectin,
matrix metalloproteinase-2 (17)
and transforming growth factor-β1 (18). In dermatology, the fresh crude
extract of P. oleracea has been reported to significantly
stimulate physical wound contraction and accelerate the wound
healing process by decreasing the surface area and increasing the
tensile strength of the skin (19,20),
as well as by inhibiting mushroom tyrosinase, indicating that it
may be used to inhibit tyrosinase in skin, resulting in repression
of the synthesis of melanin pigments and playing a crucial
protective role against skin photocarcinogenesis (21).
In the present study, a clinical right-left
bilateral lesion self-control study was performed to explore the
efficacy of P. oleracea with or without calcipotriol in
psoriatic patients, This involved a comparison between the crude
extracts of P. oleracea (Winona; Kunming Dihon
Pharmaceutical Co., Ltd., Kunming, China) in combination with
0.005% calcipotriol ointment (Daivonex®; LEO
Laboratories Ltd., Dublin, Republic of Ireland) and monotherapy
with 0.005% calcipotriol ointment alone, which was approved in
accordance with the ethical committee approval process of the First
Affiliated Hospital of Chongqing Medical University (Chongqing,
China).
Materials and methods
Human subjects
The present study was conducted as a single-center,
prospective, bilateral comparison study. Written informed consent
was obtained from each patient prior to enrolment in the study. A
total of 11 Chinese patients with plaque psoriasis (seven males and
four females), aged 21.3–47.1 years (mean age 32.4 years), were
recruited. For each individual, it was required that at least two
target lesion pairs on each side of the body were of moderate to
severe severity, and all comparative lesion pairs had to be in
analogous anatomic locations and of approximately equal severity.
Scalp, facial and genital psoriasis was neither treated nor
assessed. Those patients who had received oral, topical, physical
(i.e., ultraviolet or solarium treatment) and systemic
antipsoriatic treatments within the previous six months were
excluded. Other exclusion criteria included a current diagnosis of
unstable psoriasis, pregnancy, benign or malignant tumor, topically
serious skin trauma, uncontrollable systemic disease and a history
of allergy.
Calcipotriol ointment alone was applied twice daily
to the affected areas on one side of the body [monotherapy
(M)group], randomly assigned for each patient. On the other side,
calcipotriol ointment was applied only once in the evening and
humectant containing P. oleracea extract once in the morning
rotationally [combination (C) group]. Due to ethics restrictions,
P. oleracea treatment alone could not be applied during the
study. A fixed dosage was strictly required at a total of 0.5 g (a
fingertip unit (22)) on each
10×10 cm2 area for each application, which was
calculated and strictly instructed by the investigators at the
first visit of the patient. The therapeutic phase of the sides was
equal at four weeks.
Clinical assessments
The severities of the paired psoriatic lesions were
recorded on each visit (weeks 0, 2 and 4).Scales, plaque and
erythema were usually considered as the main complaints of
psoriasis. A nine-value rating scale with 0.5-point increments was
used to evaluate the change in the degree of scales, plaque
elevation and erythema, with the following classifications: 0,
none; 1, mild; 2, moderate; 3, severe; and 4, very severe (23). Total scores were calculated as the
sum of the points. Another assessment of overall efficacy was made
by the patients using the following defined five-point grading
scale on each visit: 1, excellent improvement (>75%); 2, marked
improvement (51–75%); 3, moderate improvement (26–50%); 4, slight
improvement (0–25%); and 5, no improvement or deterioration.
Symptoms including itching, thermalgia, as well as any another
abnormal sensations or adverse events, if any, were documented in
detail on each visit.
Transepidermal water loss (TEWL)
measurement
To evaluate the condition of the skin barrier,
patients were investigated instrumentally for TEWL, using a
Tewameter® TM 300 (Courage + Khazaka electronic GmbH,
Cologne, Germany) under the manufacturer’s instructions. The
condition of all subjects was first stabilized for 15–20 min, in a
climate- and humidity-controlled room, with an ambient temperature
range of 21–25°C and mean relative humidity range of 50–60%. Two
topical comparative plaques of homologous anatomic positions and
severity on each side were measured at each visit at weeks 0, 2 and
4. Additionally, five healthy volunteers were recruited as the
control.
Tissue collection
Skin tissues for downstream analysis were obtained
from each patient at weeks 0 and 4 by a professional operator.
Samples were approximately equally harvested on the bilateral body
each time, and the regions at week 4 were close to those of week 0
while avoiding the scar. The tissues were fixed immediately
according to the different downstream processes. Additionally,
another five skin tissues without substantial lesions were obtained
from the Department of Surgery in The First Affiliated Hospital of
Chongqing Medical University as the normal control.
Hematoxylin and eosin staining (H&E)
and epidermal thickness measurement
Routine H&E staining was conducted following
paraffin sectioning of 5-μm-thick sections. The mean epidermal
thickness was determined on H&E stained sections by measuring
the distance between the outermost surface of the epidermis
excluding the stratum corneum and the dermo-epidermal junction at
five points through the entire length of three examined sections
for each specimen.
Immunohistochemistry (IHC)
To perform IHC, the slides were retrieved in a
high-temperature antigen retrieval solution of citrate buffer and
then incubated overnight at 4°C with primary antibodies (Abs)
against K10, LOR, FLG (Abcam, Cambridge, MA, USA), TNF-α and IL-8
(Cell Signaling Technology, Inc., Danvers, MA, USA). Subsequently,
the appropriate secondary Abs and detection kits were used. The
tissues were visualized with 3,3′-diaminobenzidine substrate and
counterstained with hematoxylin. Images were captured under closely
comparable conditions.
Western blot analysis
Skin tissues from patients were homogenized and
sonicated in lysis buffer (containing 20 mM Tris, 150 mM NaCl, 1 mM
EDTA, 1 mM ethylene glycol tetraacetic acid, 1% Triton, 0.1% sodium
dodecyl sulfate (SDS) and 1% protease inhibitors). Equal amounts of
proteins were separated on 7–12% SDS-polyacrylamide gels in a
minigel apparatus (Bio-Rad, Hercules, CA, USA) and then transferred
electrophoretically to nitrocellulose membranes, followed by
blocking with milk and incubation at 4°C overnight with anti-K10,
LOR, FLG, TNF-α and IL-8 Abs, as well as anti-p65,
phosphorylated-(p-)p65, inhibitor κBα (IκBα) and p-IκBα
(phosphorylated at Ser32) Abs (Cell Signaling Technology, Inc.).
Membranes were incubated for 1 h with horseradish
peroxidase-conjugated secondary Abs. Subsequent to immunoblotting,
the films were scanned and the intensity of the immunoblotting
bands was detected with a Bio-Rad GS-800 Calibrated Densitometer
(Bio-Rad). GAPDH and α-tubulin were used as the loading
controls.
Statistical analysis
Severity scores from the clinical assessments were
compared at the baseline and across three time points using the
Wilcoxon signed-rank test. The comparisons of the various severity
scores between the M group and C group-treated lesion pairs were
evaluated using the Mann-Whitney U test, and comparison of the
adverse effects between the two groups was conducted using the
Pearson’s χ2 test. The results of the readings of TEWL,
epidermis thickness and quantitative western blot images are all
presented as the mean ± standard deviation, and were analyzed using
the two-tailed unpaired Student’s t-test or paired-samples
Student’s t-test. All analyses were performed using SPSS version
13.0 for Windows (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
P. oleracea in combination with
calcipotriol effectively improves the clinical manifestations to
the same extent as calcipotriol monotherapy, but with fewer adverse
effects
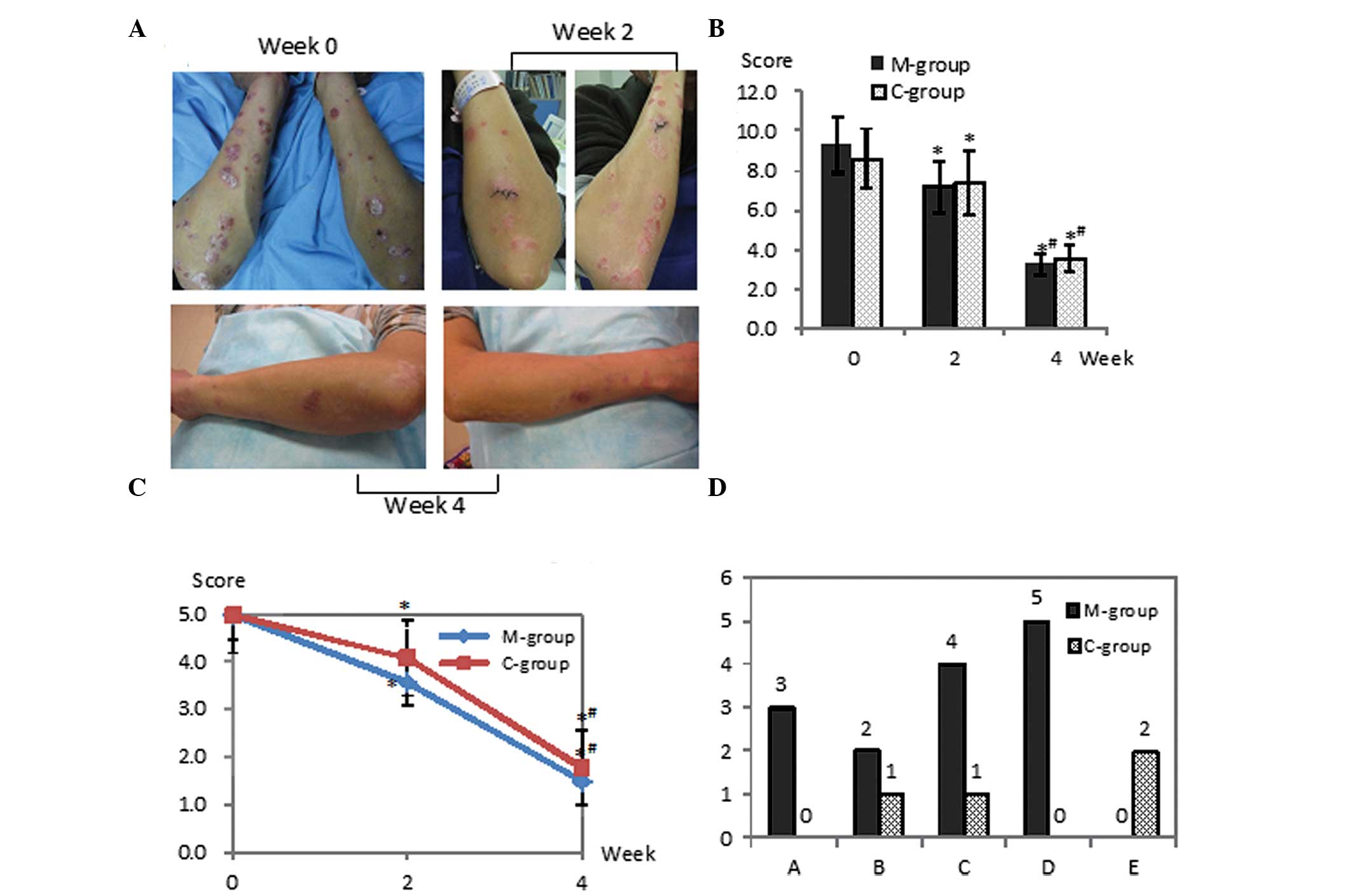
Ten patients (78 target lesion pairs) that completed
the phase of treatment were included in the statistical analysis
for efficacy. The locations of the target lesions were on the legs
(n=37), arms (n=24) and trunk (n=17). One patient dropped out due
to rejection to the second skin biopsy at week 4, when the clinical
manifestation had improved markedly.
At the baseline, the severity of lesions between the
two groups was similar in scaling, plaque elevation, erythema and
overall lesional assessment. Subsequent to treatment, the two
groups showed statistically significant amelioration in scaling,
plaque elevation and erythema, as well as in the overall lesional
severity, which was estimated by the patients themselves at weeks 2
and 4 (Fig. 1A–C). Comparing the
two regimens, no statistically significant difference was
identified at the two visits. However, according to the reports of
the patients, the adverse effects in the M group were of
significantly higher frequency than those in the C group during the
first two weeks (P<0.05), including five individual complaints
of skin dryness and four of transient burning sensation in the
applied area, as well as three and two complaints of immediate
temporary skin redness and/or itching following the application in
the M group. However, all these complaints decreased gradually and
were eventually eliminated in two weeks. By contrast, in the C
group, the main complaints were the slightly aromatic odor of the
humectant, an occasional slight burning sensation and itching
(Fig. 1D).
Combination regimen improves skin barrier
function with greater efficiency than calcipotriol monotherapy
At the beginning of the study, the TEWL of the
patients was significantly higher than that of the normal controls,
which indicated the impaired function of the skin barrier in the
psoriatic lesions. In the M group, the TEWL was slightly lower
following treatment than the initial TEWL at weeks 2 and 4;
however, no statistically significant differences were identified,
indicating that the calcipotriol ointment was not able to protect
against water loss and improve the skin barrier function in the
present study. By contrast, the value of TEWL in the C group was
significantly decreased at the visits at weeks 2 and 4, accompanied
by the normalization of the lesional condition. When compared with
the M group, this clearly indicated that the improvement of skin
barrier function with the combination regimen was more efficient
than that with the monotherapy of calcipotriol (Table I).
 | Table IComparison of transepidermal water
loss between the two treatment groups and healthy controls. |
Table I
Comparison of transepidermal water
loss between the two treatment groups and healthy controls.
| Group | Week 0 | Week 2 | Week 4 |
|---|
| Healthy controls
(n=5) | 9.46±2.27 | | |
| M group (n=10) | 22.17±4.51a | 20.93±4.05a,b | 18.78±3.94a,b |
| C group (n=10) | 21.63±4.85a | 17.17±3.82a–c | 13.46±3.73a–c |
Combination therapy has a higher efficacy
than calcipotriol monotherapy for attenuating the abnormal
differentiation of psoriatic keratinocytes
To evaluate the keratinocyte proliferation status,
epidermal thickness was investigated by routine H&E staining
(Fig. 2). The results clearly
showed that prior to treatment there was a pronounced increase in
the epidermal thickness with the acanthotic appearance and rete
ridges, accompanied by abnormal keratinocyte differentiation with
the loss of the stratum granulosum. The stratum corneum was clearly
thickened with parakeratosis. Subsequent to treatment, however, the
layers of keratinocytes decreased along with the flattened rete
ridges and the stratum granulosum cell layers increased. Comparison
between the two regimens indicated that although the change of
epidermal thickness was slightly greater in the M group than in the
C group, no significant difference was statistically validated.
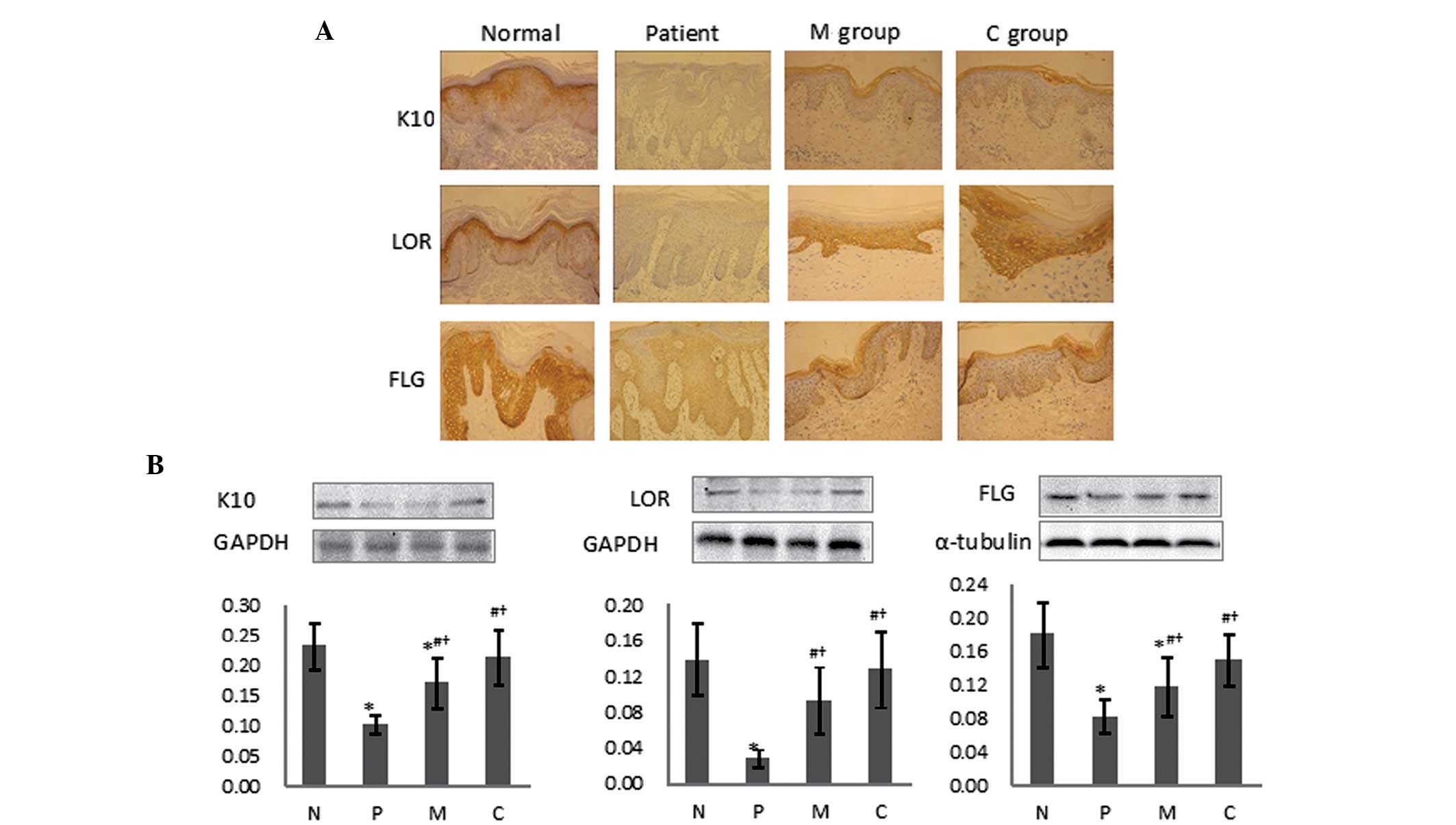
To further explore the keratinocyte differentiation
status, the expression of protein markers reflecting the
differentiation of the psoriatic keratinocytes was then
investigated by IHC (Fig. 3A) and
western blot analysis (Fig. 3B).
These revealed that the K10 protein was mainly expressed in the
stratum spinosum at moderate levels in normal tissue, while no
expression was found in skin psoriatic lesions; however, K10
protein expression was strongly elevated following treatment in the
two treatment groups. A notable quantity of LOR was detected in the
stratum granulosum and lower corneum, which was significantly
downregulated in the psoriasis lesions; however, evident elevation
was detected following treatment. FLG expression of a moderate
level was detected in normal controls and a lower expression was
observed in untreated patients, with upregulation following
treatment. Paralleled with the immunohistological outcomes, western
blotting also revealed the same tendencies for the normal tissue,
psoriatic skin and those following treatment. The protein
expression levels of K10, LOR and FLG were significantly
downregulated in the psoriatic lesions. However, following
treatment, all these changes in protein expression levels were
significantly reversed and accompanied by an improvement in skin
condition. The combination therapy exerted markedly higher
efficacies on inversing the abnormal expression levels of these
differentiation markers for psoriatic keratinocytes.
TNF-α and IL-8 expression is
downregulated significantly more evidently by combination therapy
than by monotherapy
TNF-α and IL-8, two pivotal inflammatory factors
according to previous reports (3,24,25),
are both involved in the pathogenesis of psoriasis, and have been
proposed to be the crucial therapeutic targets for psoriatic
patients. To explore if these factors were differentially regulated
by the two regimens, their expression levels were investigated by
IHC and western blot analysis. Fig.
4A shows that TNF-α and IL-8 expression was at a limited level
and mainly distributed to the basal layers of the epidermis in
normal tissue, but was widely upregulated within the whole
epidermis in the psoriasis tissues, while being significantly
decreased following treatment with the two regimens. Western blot
analysis revealed a comparable tendency, as shown in Fig. 4B. However, following treatment, a
significant difference between the effects of the combination
therapy and monotherapy was statistically concluded for the TNF-α
and IL-8 expression levels by quantitative analysis, thus revealing
that the combination regimen exerted a more potent regulatory
effect on these two inflammatory factors.
P. oleracea and calcipotriol reverse
keratinocyte dysfunction in psoriasis by repression of the NF-κB
signaling pathway via a different pathway than calcipotriol
alone
To further explore the potential mechanism by which
P. oleracea aided calcipotriol in reversing the psoriatic
condition, the status of the NF-κB pathway was investigated by
western blot analysis. Since p65 is the main functional subunit of
NF-κB, and its activation is mainly regulated by its inhibitory
protein, IκBα (26,27), the expression levels of p65, p-p65,
IκBα and p-IκBα were investigated. Fig. 5 shows that the expression levels of
p65 were not significantly different between either the psoriatic
lesions and normal controls, or prior and subsequent to treatment.
However, the expression levels of p-p65 and p-IκBα were markedly
increased, and IκBα levels were decreased in the psoriatic patients
compared with those in normal tissue. Following treatment, the
p-p65 and p-IκBα levels were decreased significantly, and
subsequently the level of IκBα was reversed in the C group, but not
in the calcipotriol M group. For the calcipotriol M group, no
changes in the expression of p-p65, IκBα and p-IκBα were identified
following treatment, which was consistent with a previous study
(9), and therefore it was proposed
that the combination regimen but not the calcipotriol directly
suppressed the degradation of IκBα in the present study, which
subsequently resulted in the accumulation of IκBα and inhibited the
NF-κB activation and nuclear translocation.
Discussion
Calcipotriol, a vitamin D analog, is one of the most
common therapies for topical psoriasis lesions. It has been
reported to effectively attenuate the abnormal differentiation and
proliferation of psoriatic keratinocytes (28–31),
but simultaneously arouse clear adverse effects, including
impairment of the skin barrier function and the induction of
irritating erythema and itching, particularly following long-term
topical application (11–13). Therefore it is of great
significance to explore novel potential adjuvants or combinative
regimens that are capable of not only enhancing the efficacy of
treatment but also reducing the adverse reactions. P.
oleracea L., a traditional Chinese herb once used to treat
certain dermatological diseases in traditional Chinese medicine,
was used in combination with calcipotriol in the present study to
treat topical psoriasis. The findings showed that extracts of P.
oleracea clearly aided calcipotriol in improving the condition
of psoriasis lesions, together with markedly alleviating adverse
effects compared with calcipotriol monotherapy, and thus revealed a
potential therapeutic efficiency in psoriatic patients by the lower
dosage of calcipotriol required and its slighter irritation to the
skin.
In the combination regimen, the skin barrier
dysfunction was effectively reversed. TEWL, one of the most
important parameters reflecting the skin barrier function, was
verified to be clearly impaired in the psoriatic lesions prior to
treatment and significantly improved in the C group but not the
calcipotriol M group, demonstrating that the combination regimen
with P. oleracea could further aid the reversal of the skin
barrier dysfunction compared with the calcipotriol monotherapy. The
skin barrier is mainly dependent on the integrity and normal
differentiation of keratinocytes, the intercellular matrix and
superficial cuticles on the epidermis (32). To evaluate the condition of
psoriatic keratinocyte proliferation and differentiation, the
epidermal thickness and the differential markers K10, LOR and FLG
were investigated. It was shown that the combination and
monotherapy regimens evidently repressed the proliferation of
psoriatic keratinocytes. Although the reduction of epidermal
thickness was slightly higher in the monotherapy than the
combination regimen, no statistical significant difference was
revealed. The expression levels of K10, LOR and FLG indicated by
immunohistochemistry, paralleled with the western blot analysis,
revealed that all the differentiation markers were significantly
downregulated in psoriatic compared with normal tissue, which
revealed the impaired differentiation properties of keratinocytes
were involved in the pathophysiology of psoriasis. Furthermore,
following treatment, the reversion of K10, LOR and FLG was more
effective in the combination regimen than in the monotherapy, which
clearly demonstrated that P. oleracea was capable of aiding
calcipotriol in attenuating the severity and extent of psoriatic
lesions, and that the underlying mechanism may partially be through
regulation of the differentiation dysfunction of keratinocytes.
Furthermore, to explore the potential mechanism by
which P. oleracea regulated the psoriatic keratinocyte
differentiation, the levels of the inflammatory factors TNF-α and
IL-8, which have been reported to play crucial roles in psoriatic
pathogenesis, were investigated (24,25).
The results showed that TNF-α and IL-8 were significantly
upregulated in psoriatic compared with normal tissue, and markedly
decreased following the treatments. However, the reversion
efficiency for the combination therapy was higher than that for the
calcipotriol monotherapy. This not only indicated that these
inflammatory factors were involved in the psoriatic
pathophysiology, but also that P. oleracea exhibits
properties that aid calcipotriol in regressing the inflammation in
psoriasis lesions, which is consistent with a previous study of the
effect of P. oleracea on human disease (33).
Ongoing studies have disclosed that calcipotriol
regulates the target gene transcription through the NF-κB signal
pathway by adjusting the binding activation of NF-κB to its target
gene promoters, including TNF, IL-8 and IL-1α (24,25),
but without the property of affecting the phosphorylation of IκB
(9). The conditions of the pivotal
proteins within the NF-κB pathway were investigated by western blot
analysis. No expression level changes were revealed for p65 between
the psoriatic lesions and normal controls. However, the expression
level of IκBα was decreased and those of p-IκBα and p-p65 increased
markedly in the psoriatic lesions, disclosing that activation of
the NF-κB pathway was involved in the pathogenesis of psoriasis and
that the hyperfunction of the NF-κB signal was induced, at least
partially, by the decreased phosphorylation of IκB but not the
increased levels of p65. Furthermore, following treatment, the
expression level of p65 was not significantly changed by the
treatment regimens, but the expression levels of p-p65 and p-IκBα
were significantly decreased, and subsequently the level of IκBα
increased in the C group but not in the calcipotriol M group,
indicating that the NF-κB pathway was not repressed by calcipotriol
but by P. oleracea (however, due to the ethics
restrictions, P. oleracea treatment alone could not be
administered). This finding is in accordance with an earlier study
in which Johansen et al (10) showed that the application of
calcipotriol was not capable of regulating the phosphorylation of
IκBα and IκBβ, but normalized the abnormal NF-κB binding activity
to certain gene promoter sites including p53 and IL-8. This lead to
the hypothesis that P. oleracea may aid calcipotriol in
repressing inflammatory factors and reversing the impaired
differentiation of psoriatic keratinocytes through different
pathways within the NF-κB signaling pathway, but with a reciprocal
synergy.
In conclusion, the combination of calcipotriol and
P. oleracea extracts may not only decrease the required
dosage of calcipotriol and reduce its adverse effects, but also
protect the skin barrier function and benefit the remedy of the
impaired proliferation and differentiation of the psoriatic
keratinocytes. It is speculated that the potential mechanism may
lie in the mutual complementarity of the pharmacological effects of
the two components within the same NF-κB pathway, in which
calcipotriol inhibited NF-κB binding to the relevant gene promoter
while P. oleracea decreased the phosphorylation of IκB and
subsequently inhibited its degradation. As a rich source of LNA and
α-TCP, P. oleracea is capable of further remedying the
impaired intercellular matrix and decreasing the water loss and
TEWL. Therefore, it is feasible and advisable that P.
oleracea extracts may be administered as an efficient adjuvant
reagent with calcipotriol application for psoriasis, which may lead
to a novel auxiliary treatment for this chronic inflammatory skin
disease.
Acknowledgements
This study was funded by the Sanitary Science
Foundation of Chongqing Municipality, China (to Hengguang Zhao).
The project number is 2010-2-014.
References
|
1
|
Armstrong AW, Harskamp CT and Armstrong
EJ: The association between psoriasis and obesity: a systematic
review and meta-analysis of observational studies. Nutr Diabetes.
2:e542012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fadzil MH, Ihtatho D, Affandi AM and
Hussein SH: Area assessment of psoriasis lesions for PASI scoring.
J Med Eng Technol. 33:426–436. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldminz AM, Au SC, Kim N, et al: NF-κB:
an essential transcription factor in psoriasis. J Dermatol Sci.
69:89–94. 2013. View Article : Google Scholar
|
|
4
|
Andrés RM, Payá M, Montesinos MC, et al:
Potential antipsoriatic effect of chondroitin sulfate through
inhibition of NF-κB and STAT3 in human keratinocytes. Pharmacol
Res. 70:20–26. 2013. View Article : Google Scholar
|
|
5
|
Sugawara T, Gallucci RM, Simeonova PP and
Luster MI: Regulation and role of interleukin 6 in wounded human
epithelial keratinocytes. Cytokine. 15:328–336. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sayama K, Hanakawa Y, Nagai H, et al:
Transforming growth factor-beta-activated kinase 1 is essential for
differentiation and the prevention of apoptosis in epidermis. J
Biol Chem. 281:22013–22020. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakayama H, Ikebe T and Shirasuna K:
Effects of IkappaB kinase alpha on the differentiation of squamous
carcinoma cells. Oral Oncol. 41:729–737. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ying S, Kojima T, Kawada A, et al: An
intronic enhancer driven by NF-κB contributes to transcriptional
regulation of peptidylarginine deiminase type I gene in human
keratinocytes. J Invest Dermatol. 130:2543–2552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dajee M, Lazarov M, Zhang JY, et al:
NF-kappaB blockade and oncogenic Ras trigger invasive human
epidermal neoplasia. Nature. 421:639–643. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johansen C, Flindt E, Kragballe K, et al:
Inverse regulation of the nuclear factor-kappaB binding to the p53
and interleukin-8 kappaB response elements in lesional psoriatic
skin. J Invest Dermatol. 124:1284–1292. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanghetti EA: The role of topical vitamin
D modulators in psoriasis therapy. J Drugs Dermatol. 8(8 Suppl):
S4–S8. 2009.PubMed/NCBI
|
|
12
|
Effendy I, Kwangsukstith C, Chiappe M and
Maibach HI: Effects of calcipotriol on stratum corneum barrier
function, hydration and cell renewal in humans. Br J Dermatol.
135:545–549. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
von Brenken S, Jensen JM, Fartasch M and
Proksch E: Topical vitamin D3 derivatives impair the epidermal
permeability barrier in normal mouse skin. Dermatology.
194:151–156. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan K, Islam MW, Kamil M, et al: The
analgesic and anti-inflammatory effects of Portulaca oleracea L.
subp. sativa (Haw.) Celak. J Ethnopharmacol. 73:445–451. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Teixeira MC, Carvalho IS and Brodelius M:
Omega-3 fatty acid desaturase genes isolated from purslane
(Portulaca oleracea L.): expression in different tissues and
response to cold and wound stress. J Agric Food Chem. 58:1870–1877.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szalai G, Dai N, Danin A, et al: Effect of
nitrogen source in the fertilizing solution on nutritional quality
of three members of the Portulaca oleracea aggregate. J Sci Food
Agric. 90:2039–2045. 2010.PubMed/NCBI
|
|
17
|
Lee AS, Lee YJ, Lee SM, et al: Portulaca
oleracea ameliorates diabetic vascular inflammation and endothelial
dysfunction in db/db mice. Evid Based Complement Alternat Med.
2012:7418242012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee AS, Lee YJ, Lee SM, et al: An aqueous
extract of Portulaca oleracea ameliorates diabetic nephropathy
through suppression of renal fibrosis and inflammation in diabetic
db/db mice. Am J Chin Med. 40:495–510. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rashed AN, Afifi FU and Disi AM: Simple
evaluation of the wound healing activity of a crude extract of
Portulaca oleracea L. (growing in Jordan) in Mus musculus JVI-1. J
Ethnopharmacol. 88:131–136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laitiff AA, Teoh SL and Das S: Wound
healing in diabetes mellitus: traditional treatment modalities.
Clin Ter. 161:359–364. 2010.PubMed/NCBI
|
|
21
|
Baurin N, Arnoult E, Scior T, et al:
Preliminary screening of some tropical plants for anti-tyrosinase
activity. J Ethnopharmacol. 82:155–158. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Long CC, Finlay AY and Averill RW: The
rule of hand: 4 hand areas = 2 FTU = 1 g. Arch Dermatol.
128:1129–1130. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tzung TY, Wu JC, Hsu NJ, et al: Comparison
of tazarotene 0.1% gel plus petrolatum once daily versus
calcipotriol 0.005% ointment twice daily in the treatment of plaque
psoriasis. Acta Derm Venereol. 85:236–239. 2005.
|
|
24
|
Doger FK, Dikicioglu E, Ergin F, et al:
Nature of cell kinetics in psoriatic epidermis. J Cutan Pathol.
34:257–263. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu X, Du J, Liang J, et al:
Transcriptional regulatory network for psoriasis. J Dermatol.
40:48–53. 2013. View Article : Google Scholar
|
|
26
|
Okamoto K, Iwai Y, Oh-Hora M, et al:
IkappaBzeta regulates T(H)17 development by cooperating with ROR
nuclear receptors. Nature. 464:1381–1385. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rao P, Hayden MS, Long M, et al:
IkappaBbeta acts to inhibit and activate gene expression during the
inflammatory response. Nature. 466:1115–1119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feldman SR, Mills M, Brundage T and
Eastman WJ: A multicenter, randomized, double-blind study of the
efficacy and safety of calcipotriene foam, 0.005%, vs vehicle foam
in the treatment of plaque-type psoriasis of the scalp. J Drugs
Dermatol. 12:300–306. 2013.PubMed/NCBI
|
|
29
|
Takahashi H, Ibe M, Kinouchi M, et al:
Similarly potent action of 1,25-dihydroxyvitamin D3 and its
analogues, tacalcitol, calcipotriol, and maxacalcitol on normal
human keratinocyte proliferation and differentiation. J Dermatol
Sci. 31:21–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang RC and Levine B: Calcipotriol induces
autophagy in HeLa cells and keratinocytes. J Invest Dermatol.
131:990–993. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tiberio R, Bozzo C, Pertusi G, et al:
Calcipotriol induces apoptosis in psoriatic keratinocytes. Clin Exp
Dermatol. 34:e972–e974. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Elias PM: Structure and function of the
stratum corneum extracellular matrix. J Invest Dermatol.
132:2131–2133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee AS, Kim JS, Lee YJ, et al: Anti-TNF-α
activity of Portulaca oleracea in vascular endothelial cells. Int J
Mol Sci. 13:5628–5644. 2012. View Article : Google Scholar
|