Introduction
Melasma is a common, acquired hyperpigmentary
disorder predominantly affecting dark-skinned populations, which
has a severe impact on the quality of life of a patient (1). Its long-term management remains a
significant therapeutic challenge for dermatologists (2). Melanin is a durable compound that is
hard to destroy (3). Therefore,
most available treatments target the formation of melanin by
blocking its biosynthesis, which is a slow and inefficient process.
Skin lightening actives with new mechanisms are now becoming of
interest (1,4). Lignin peroxidase (LIP) is a purified
active enzyme derived from the fermented fungus Phanerochaete
chrysosporium under controlled sterility conditions. The
molecular structure of lignin is similar to that of melanin, and a
previous study confirmed that LIP has the potential to improve skin
tone by reducing eumelanin. This enzyme is enabled by the pulse
feeding of H2O2 and breaks down melanin,
creating a fast-acting melanin-eliminating effect (5). The present study aimed to investigate
the efficacy and tolerability of a novel whitening lotion
containing LIP in the treatment of melasma.
Materials and methods
Study design
This was an 8-week, single-center, open label,
self-controlled prospective study. The subjects that participated
in the study were healthy Chinese women, 25–55 years of age (mean ±
SD, 42.12 ± 8.37 years), who had melasma involving the face. Only
patients who had not undergone topical treatment with hydroquinone
cream, corticosteroids, or any other de-pigmenting medication or
any other photosensitizing medication within 3 months prior to
enrollment or who had not undergone light-to-medium peels or
microdermabrasion within 6 months prior to study enrollment were
included. Pregnant women or women taking birth control medication,
hormone replacement therapy or any other hormone-altering
medication 6 months prior to study recruitment were further
excluded. This study was conducted at Beijing, China, in March to
May 2012 in accordance with the WHO guidelines for good clinical
practice (GCP) for trials on pharmaceutical products (6). The protocol was reviewed and approved
by Ethics Committee of Peking University First Hospital (Beijing,
China). Each subject provided signed informed consent.
Treatment
Following a washout period of 2 weeks with a gentle
cleanser and a sun protection factor 30+ (SPF30+) sunscreen, all
subjects were given a study products set, which included a
cleanser, whitening lotion, activator lotion and sunscreen. All
test products were provided by Syneron Medical Inc. (Irvine, CA,
USA). The subjects were instructed to clean their face using the
cleanser twice a day. After cleaning, the subjects were instructed
to cover the whole face with the whitening lotion and wait 1 min
before applying the activator lotion, which allowed the enzyme to
attain a balance at the appropriate pH value. Subjects applied the
study products twice daily for 8 weeks. Subjects were permitted to
continue their usual facial treatment regimen that did not contain
active ingredients (e.g., α-hydroxy acid, salicylic acid, vitamin A
or arbutin). In addition, the patients were instructed to use
SPF30+ sunscreen during the day for the duration of the study
course.
Assessment regimen and instrumental
measurements
The subjects were photographed and measurements
taken four times during the study course: prior to the treatment
(day 0), and one week (day 7), 4 weeks (day 28) and 8 weeks (day
56; end of treatment period) after the first treatment.
Test environment
Prior to each measurement, subjects washed their
face with the assigned facial cleanser and underwent equilibration
for 30 min in a room with controlled temperature (21–24°C) and
relative humidity (30–50%).
Spectrophotometric analysis
The forehead and cheeks were assessed for pigment
lightening using the CM-2500d Spectrophotometer (Minolta, Tokyo,
Japan) as a chromameter as described previously (7). The L* value (luminance) defines the
relative lightness ranging from total black (L*=0) to total white
(L*=100); the a* value represents the balance between red (positive
value) and green (negative value). Three consecutive measurements
were taken at each site and the average of three measured values
was calculated and considered to be the absolute value.
Facial imaging
As shown in Fig. 2,
standardized images of each side of the face of all subjects were
captured under the same visible light and UV light conditions with
the Visia® (Canfield Imaging Systems, Fairfield, NJ,
USA) complexion analysis system at different time points, using a
previously described method (8).
Evaluation of changes in
pigmentation
Evaluations of melasma area severity index (MASI)
scores were performed at baseline and during the test period by two
dermatologists independently according to the method described
previously (9). The average of the
two assessments was used for analysis.
Assessment of adverse effects
During the test period, skin irritation findings and
adverse effects including erythema, desquamation, burning/stinging
sensation and dryness were evaluated by dermatologists using a 0–3
Likert scale: 0, none; 1, mild; 2, moderate; and 3, severe.
Statistical analysis
Statistical analysis was conducted using SPSS
software, version 12.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± SD of triplicate measurements. Data were
analyzed and compared prior to and following the treatment using
analysis of variance (ANOVA) or Dunnett’s comparison for parametric
data and/or the Friedman or Wilcoxon test for non-parametric data.
For all analyses, P<0.05 was considered to indicate a
statistically significant difference.
Results
Study completion
The study included 33 melasma patients in total and
31 patients completed the study. Two patients were lost at day 7
due to the adverse effects not being following up, and so were
excluded from the data analysis.
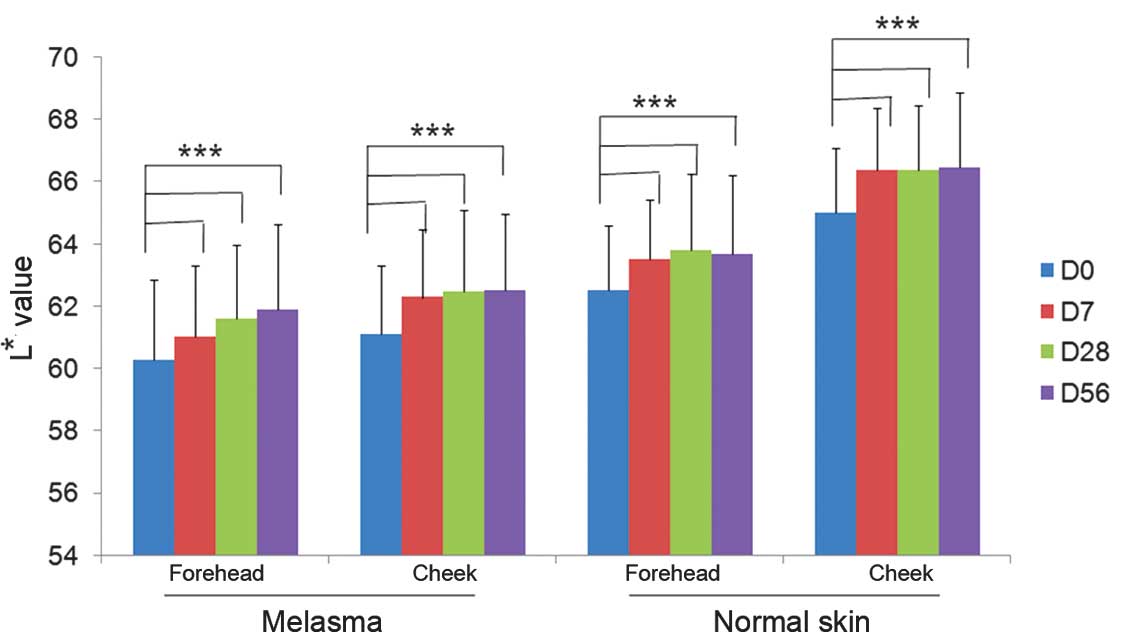
Skin lightness measurements
At baseline, the mean L* value representing the skin
lightness of facial subareas with melasma was 60.57±2.39 while the
respective value of facial subareas without melasma was 64.16±2.73
(P<0.05; ANOVA). Following 7 days of product application, the
mean values increased significantly with mean L* values of
61.42±2.46 and 65.41±2.62 for melasma and non-melasma subareas,
respectively (P<0.05; ANOVA). The mean values representing skin
lightness at different subareas of the face continued to increase
progressively at day 28 and further on day 56. The difference in L*
values was statistically significant between all time points
(Fig. 1).
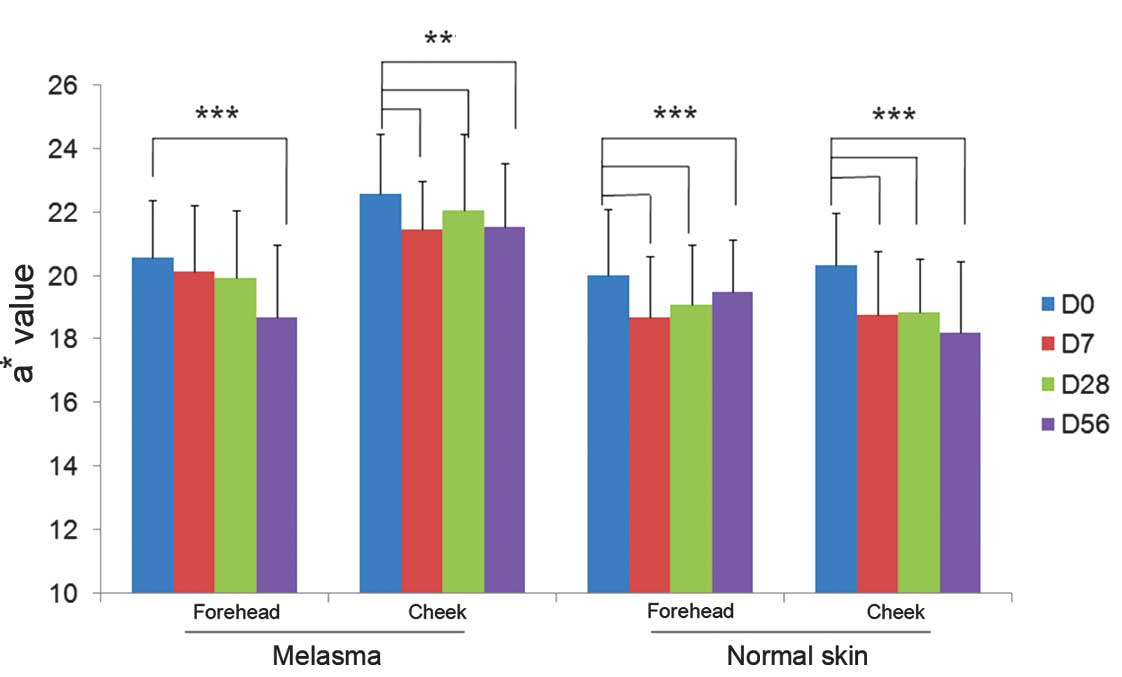
Skin redness measurements
At baseline, the mean values representing the
redness of the skin (a*) were higher at all facial subareas
affected by melasma as compared with those without melasma.
Following 7 days of study product application, all mean values
representing skin redness decreased significantly compared with
their respective baseline values (P<0.05; ANOVA). The mean
values of subareas with and without melasma were also significantly
reduced compared with baseline values at days 28 and 56 (Fig. 3).
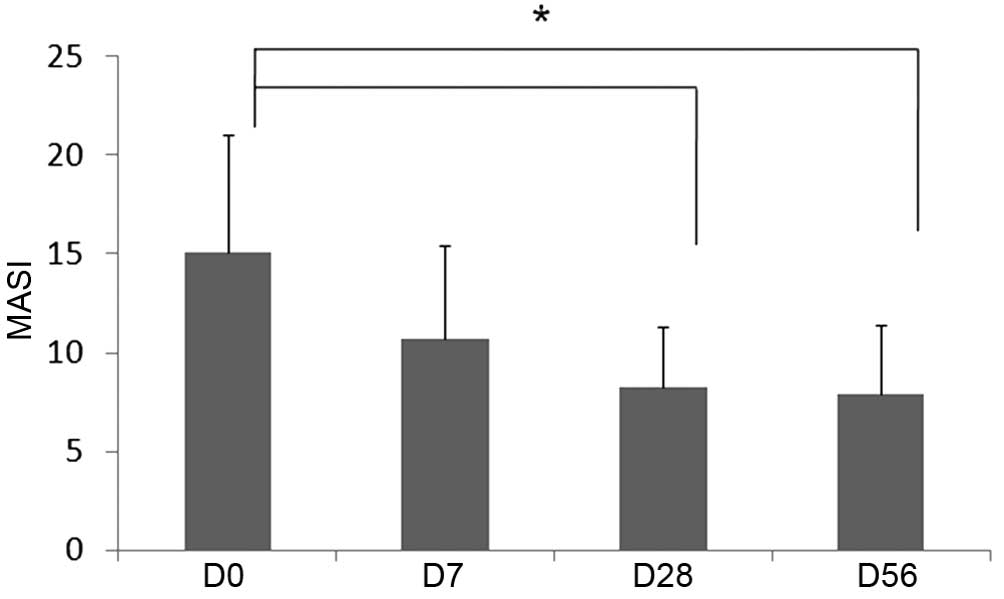
Clinical evaluation of melasma
severity
Physician assessment of melasma severity
demonstrated improvement following 1, 4 and 8 weeks of product
application. The difference in severity from the baseline value was
statistically significant at 4 and 8 weeks after the initiation of
treatment (Fig. 4).
Safety evaluation
The treatment was tolerated well by all subjects. No
adverse events and/or complications occurred during the period of
product application.
Discussion
Melasma is a common skin pigment disorder that
sometimes has a severe emotional effect on the patient. The
etiology of melasma remains unclear and current treatments have
varying effectiveness on depigmentation (10). The gold standard of medical therapy
is 4% hydroquinone, as a monotherapy or in combination with other
depigmenting agents such as corticosteroids and/or retinoids
(2). Over-the-counter (OTC)
hydroquinone has been banned in Europe and Asian countries, since
oxidized hydroquinone is toxic to melanocytes. In the USA, the Food
and Drug Administration is currently evaluating the status of OTC
hydroquinone and prescription products that are sold without
approval (11).
This regulatory arena has created a requirement for
pigment lightening alternatives with high tolerability and efficacy
for worldwide use.
Melanin is composed of covalently linked indoles; it
is a heterogeneous polymer formed from dihydroxyindole units
(12). Its structure is similar to
that of lignin or coal, in which polymers are composed of indolic
or phenolic subunits. White-rot fungus, Phanerochaete
chrysosporium, causes decolorization and depolymerization of
low-grade coal under culture conditions that facilitate the
mineralization of lignin. These actions of lignin-degrading enzymes
make it possible to use them to decolorize melanin, which possesses
a structure similar to those of coal or lignin (13). A previous study confirmed that the
enzyme LIP has the potential to improve skin tone by reducing
eumelanin (5). LIP is produced
extracellularly during submerged fermentation of the fungus
Phanerochaete chrysosporium and may be purified from the
fermented liquid medium (5).
Following final formulation and packaging, the product contains an
active enzyme component and an activator component
H2O2. The combination of the two formulations
creates a temporary reaction that results in a targeted and
time-limited catalysis of eumelanin degradation in the
epidermis.
The present clinical study demonstrated that the
active enzyme LIP, when formulated and used together with activator
H2O2 is effective in improving skin
pigmentation. Eight weeks of the twice daily regimen increased skin
lightness and markedly decreased dyspigmentation in the facial
subareas affected by melasma and those without melasma. Most
importantly, effective results were observed as early as 7 days
after the start of product application, suggesting a rapid response
to treatment. Skin brightness at all areas increased gradually over
the treatment period and became most significant 56 days after the
initiation of treatment. It should be emphasized that the product
was tolerated well by all subjects and no associated adverse events
occurred during the treatment period, which should allow its
continued use over a prolonged period of time.
It is concluded that LIP represents a novel
skin-lightening product that provides a completely innovative
advanced approach to achieve a rapid-acting skin-whitening effect.
The results suggest that the skin-brightening complex, when
appropriately formulated for topical use and used together with
daily sun protection, is a valuable alternative to existing
whitening products. However, the present study was a preliminary
clinical study with limited subject numbers. The superiority of
this depigmenting effect would be better assessed in a parallel
group, double-blind, vehicle and active comparator-controlled
clinical study with a higher number of volunteers (14).
Acknowledgements
This study was sponsored by Syneron Medical Inc.
References
|
1
|
Sheth VM and Pandya AG: Melasma: a
comprehensive update: part I. J Am Acad Dermatol. 65:689–697. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rajaratnam R, Halpern J, Salim A and
Emmett C: Interventions for melasma. Cochrane Database Syst Rev.
7:CD0035832010.PubMed/NCBI
|
|
3
|
Borovansky J and Elleder M: Melanosome
degradation: fact or fiction. Pigment Cell Res. 16:280–286. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gillbro JM and Olsson MJ: The
melanogenesis and mechanisms of skin-lightening agents - existing
and new approaches. Int J Cosmet Sci. 33:210–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Woo SH, Cho JS, Lee BS and Kim EK:
Decolorization of melanin by lignin peroxidase from Phanerochaete
chrysosporium. Biotechnol Bioprocess Eng. 9:256–260. 2004.
View Article : Google Scholar
|
|
6
|
Idänpään-Heikkilä JE: WHO guidelines for
good clinical practice (GCP) for trials on pharmaceutical products:
responsibilities of the investigator. Ann Med. 26:89–94. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park SB, Huh CH, Choe YB and Youn JI: Time
course of ultraviolet-induced skin reactions evaluated by two
different reflectance spectrophotometers: DermaSpectrophotometer
and Minolta spectrophotometer CM-2002. Photodermatol Photoimmunol
Photomed. 18:23–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Costa A, Moisés TA, Cordero T, Alves CR
and Marmirori J: Association of emblica, licorice and belides as an
alternative to hydroquinone in the clinical treatment of melasma.
An Bras Dermatol. 85:613–620. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pandya AG, Hynan LS, Bhore R, et al:
Reliability assessment and validation of the Melasma Area and
Severity Index (MASI) and a new modified MASI scoring method. J Am
Acad Dermatol. 64:78–83. 2011. View Article : Google Scholar
|
|
10
|
Sehgal VN, Verma P, Srivastava G, Aggarwal
AK and Verma S: Melasma: treatment strategy. J Cosmet Laser Ther.
13:265–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Department of Health and Human Services,
Food and Drug Administration. Skin bleaching drug products for
over-the-counter human use: proposed rule. Federal Register.
71:51146–51155. 2006.
|
|
12
|
Riley PA: Melanin. Int J Biochem Cell
Biol. 29:1235–1239. 1997. View Article : Google Scholar
|
|
13
|
Ollikka P, Alhonmaki K, Leppanen VM, et
al: Decolorization of azo, triphenyl methane, heterocyclic, and
polymeric dyes by lignin peroxidase isoenzymes from Phanerochaete
chrysosporium. Appl Environ Microbiol. 59:4010–4016.
1993.PubMed/NCBI
|
|
14
|
Pandya A, Berneburg M, Ortonne JP and
Picardo M: Guidelines for clinical trials in melasma. Pigmentation
Disorders Academy. Br J Dermatol. 1:21–28. 2006. View Article : Google Scholar
|