Introduction
Urinary tract infections (UTIs) are among the most
common infections in both outpatient and inpatient settings.
Pseudomonas aeruginosa is a common pathogenic bacteria
isolated from UTIs, particularly catheter-associated UTIs (1,2).
With the development of medical technology, urinary catheters are
applied to greater numbers of people and the time of application is
longer. Regarding hospitalized patients, 25% of patients undergo
short-term urinary catheterization (<7 days), which increases
the risk of developing an infection. Moreover, the UTI rate could
reach 100% in hospitalized patients with long-term catheterization
(≥30 days) (3). Once the bacterial
biofilm develops, the bacterial cells are able to withstand host
immune responses, and they are much less susceptible to antibiotics
than their nonattached individual planktonic counterparts (4). Due to multiple resistance mechanisms,
the higher resistance is more challenging to the clinician.
Therefore, numerous researchers have studied the formation,
regulation and resistance of biofilms (5–7).
Macrolides have been shown to have a good effect on
P. aeruginosa infection in the airway tract. However, they
are not a good choice for UTI infections (8), and there is little research
concerning the effect of macrolides on UTIs. Therefore, in the
present study, P. aeruginosa isolates were collected from
the urinary catheters of hospitalized patients and the P.
aeruginosa isolates from the urinary catheters was
characterized. In addition, azithromycin (AZM) was selected as a
representative macrolide to investigate its effect in treating
UTIs.
Materials and methods
Bacterial strains
Urinary catheters that were applied in hospitalized
patients for more than one week were carefully collected under
aseptic conditions. The catheters used longer than 7 days were
collected and cut open, and a cotton swab was used to scrape the
inner wall of possible biofilm infection. The cotton swab was sent
immediately to the laboratory for analysis. Isolates were
identified by biochemical assays and using the Vitek system
(bioMérieux Vitek, Hazelwood, MO, USA). P. aeruginosa PAO1
(American Type Culture Collection, Manassas, VA, USA) was used as
the control strain and preserved in the laboratory. The P.
aeruginosa isolates were examined in the following experiments.
The present study was conducted according to the principles of the
Declaration of Helsinki (2008), and the experimental protocol was
approved by the Ethics Committee of Chongqing Red Cross Hospital
(Chongqing, China) (approval number, KY201411). Informed consent
was obtained from all patients and their families prior to the
collection of urinary catheter samples.
Antimicrobial susceptibility testing
Antimicrobial susceptibility was determined
according to the standards of the Clinical and Laboratory Standards
Institute (CLSI, 2011) (9) using
the Kirby-Bauer disk diffusion assay on freshly prepared P.
aeruginosa test medium [Müller-Hinton (MH)]. P.
aeruginosa ATCC27853 and Escherichia coli ATCC25922
(American Type Culture Collection) were used as control strains for
minimum inhibitory concentration (MIC) testing. The resistance rate
was calculated as the number of resistant strains divided by the
total number of strains. The susceptible strains included those
with susceptibility and medium sensitivity according to the CLSI
standards.
Biofilm assay
The static biofilm assay was performed as outlined
by Wang et al and Naik et al (10,11).
In brief, the P. aeruginosa strains were grown overnight in
Luria-Bertani (LB) broth and diluted to 1×106 CFU/ml
with fresh LB. The inoculated culture (150 μl) was transferred to a
96-well polystyrene microtiter plate and incubated at 37°C for 24
h. The planktonic cells were removed from the wells after
incubation and the wells were washed three times with sterile
water. This was followed by staining of the wells with 0.1% crystal
violet for 10 min and washing the unbound stain thrice with sterile
water. The cell-bound dye was extracted with 300 μl 95% ethanol,
and the absorbance of the solution was measured using a Multiskan
Spectrum microplate reader at 595 nm (Thermo Fisher Scientific,
Vantaa, Finland).
Adhesion on urinary catheters
The adhesion on urinary catheters was assayed using
the standard crystal violet staining method with a few revisions
(12). In brief, a 3-cm urinary
catheter was placed into a 6-well board with 4.5 ml LB broth, and
50 μl bacterial culture (1×106 CFU/ml) was added to the
well. After incubation for 6 h, unattached cells were removed with
sterile water, and attached cells were stained with 1% crystal
violet for 20 min. The dye bound to the adherent cells was then
solubilized with ethanol-acetone (75:15, v/v) and the optical
density of the solution was measured at 570 nm.
Swimming motility
The swimming motility was assayed using the method
of Bala et al (13).
Bacterial strains were incubated at 37°C overnight. Swimming plates
containing 1% tryptone, 0.5% NaCl and 0.3% agar were prepared for
the assay. The plates with and without 1/4 MIC AZM were
point-inoculated with a sterile toothpick and incubated at 37°C for
24 h. The zone diameter was measured to assess the swimming
motility.
Quantitative analysis of virulence factor
production
Determination of elastase
activity
Elastase activity was determined with elastin-Congo
red (ECR) and the steps were as follows: P. aeruginosa
strains were inoculated onto MH agar plates, and incubated at 37°C
for 18 h. A single colony was transferred into 2 ml peptone tryptic
soy broth (PTSB) medium, and then the bacterial cultures were
transferred into 18 ml PTSB medium with and without 1/4 MIC AZM
once the OD540 nm reached 0.5. After incubation for 16 h
at 37°C with shaking at 250 rpm, the cultures were centrifuged at
12,100 × g for 15 min at 4°C and the supernatant was filtered with
0.45 μm syringe filter. Then, 1 ml ECR reaction buffer (ECR 20 mg,
0.1 M Tris-HCl/1 mM CaCl2, pH 7.2) was added to 1 ml
filtered supernatant. When the mixture had been incubated at 37°C
for 18 h with shaking at 250 rpm, 0.1 ml 0.12 M EDTA was added to
stop the reaction. The reactant was placed on ice and the insoluble
ECR was removed by centrifugation at 3,000 × g, 4°C. The elastase
activity was determined at 495 nm. Three samples of each type were
examined and the experiment was repeated three times.
Determination of rhamnolipids
The concentration of rhamnolipid was determined
spectrophotometrically by the orcinol reaction using rhamnose as a
standard. The orcinol reagent [0.19% orcinol in 53% (v/v) sulfuric
acid] was prepared immediately prior to use. The reaction mixture,
composed of 100 μl sample (0, 50, 100, 200 and 300 μg/ml) and 900
μl reagent, was well stirred, warmed for 30 min at 80°C, and then
kept for 15 min at room temperature. The absorbance was measured at
421 nm. A standard curve was constructed according to the
absorbance and concentration of rhamnose.
P. aeruginosa strains were grown on LB agar
plates at 37°C for 18 h. A single colony was transferred into LB
broth and incubated overnight at 37°C with shaking. Then, the
OD600nm of the bacterial culture was adjusted to 0.05
with proteose peptone glucose ammonium salts (PPGAS) medium (0.02 M
NH4Cl, 0.02 M KCl, 0.12 M Tris-HCl, 0.0016 M
MgSO4, 1% peptone and 0.5% glucose). Bacterial cultures
(20 ml) with and without 1/4 MIC AZM were incubated for 48–72 h at
37°C with shaking at 200 rpm. Rhamnolipids were purified by first
separating the cells from the supernatant by centrifugation at
6,000 × g for 10 min. The supernatant was then acidified using 12 M
hydrochloric acid to pH 2.0, and the precipitated rhamnolipids were
collected by centrifugation at 12,100 × g for 5 min. Rhamnolipids
were extracted twice with ethyl acetate, which was then evaporated
away leaving behind relatively pure rhamnolipids. The rhamnolipids
were dissolved in 0.5 ml ddH2O and stored at 4°C. For
each sample, 100 μl rhamnolipids were determined
spectrophotometrically using this method. The content of rhamnose
was calculated on the basis of the standard curve. The
concentration of rhamnolipid was calculated based on the assumption
that 1 μg rhamnose corresponds to 2.5 μg rhamnolipid.
Results
Bacterial strains and susceptibility
A total of 159 urinary catheters were collected and
32 showed positive bacterial cultures. Six urinary catheters had
more than two kinds of bacteria. Eight P. aeruginosa
isolates were collected from the urinary catheters. The resistance
rates of the eight P. aeruginosa isolates to amikacin,
ciprofloxacin, levofloxacin, minocycline, ceftazidime, cefotaxime,
piperacillin, meropenem, netilmicin, tetracycline and cefepime were
87.5, 87.5, 75.0, 62.5, 87.5, 75.0, 100.0, 62.5, 75.0, 87.5 and
75.0%, respectively. The 1/4 MIC values of AZM on the isolates of
P. aeruginosa are presented in Table I. The 1/4 MICs ranged from 32 to
256 μg/ml.
 | Table IEffect of 1/4 MIC azithromycin on the
biofilm formation and adhesion of P. aeruginosa. |
Table I
Effect of 1/4 MIC azithromycin on the
biofilm formation and adhesion of P. aeruginosa.
| Variable | PAO1 | PA1 | PA2 | PA3 | PA4 | PA5 | PA6 | PA7 | PA8 |
|---|
| 1/4MIC | 64 | 128 | 64 | 64 | 128 | 64 | 256 | 32 | 64 |
|
ODbiofilm | 0.46 | 0.33 | 0.48 | 0.32 | 0.66 | 0.27 | 0.68 | 0.37 | 0.39 |
|
ODadhesion | 0.43 | 0.52 | 0.47 | 0.44 | 0.36 | 0.36 | 0.31 | 0.61 | 0.47 |
Biofilm formation and adhesion
The effects of AZM on the biofilm formation and
adhesion of the P. aeruginosa isolates are shown in Table I. The strains from urinary
catheters had stronger biofilm formation capability, and the
biofilms of eight isolates were thicker than those of P.
aeruginosa PAO1 (data not shown) The 1/4 MIC AZM may reduce the
biofilm formation capability and the adhesion to urinary
catheters.
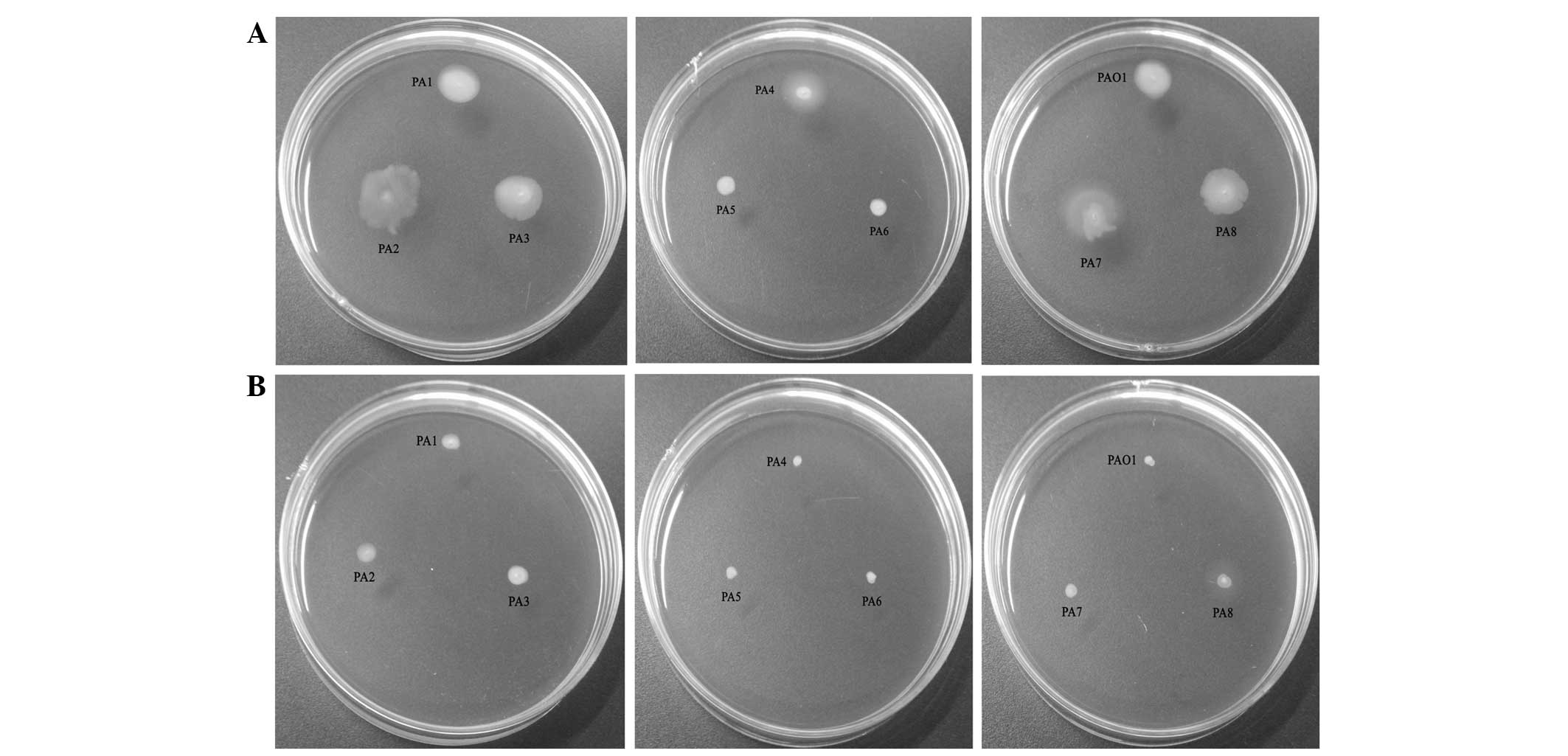
Swimming motility
The results of the assay investigating the effects
of AZM on swimming motility are presented in Fig. 1. It was observed that the presence
of AZM significantly reduced the motility of all P.
aeruginosa strains.
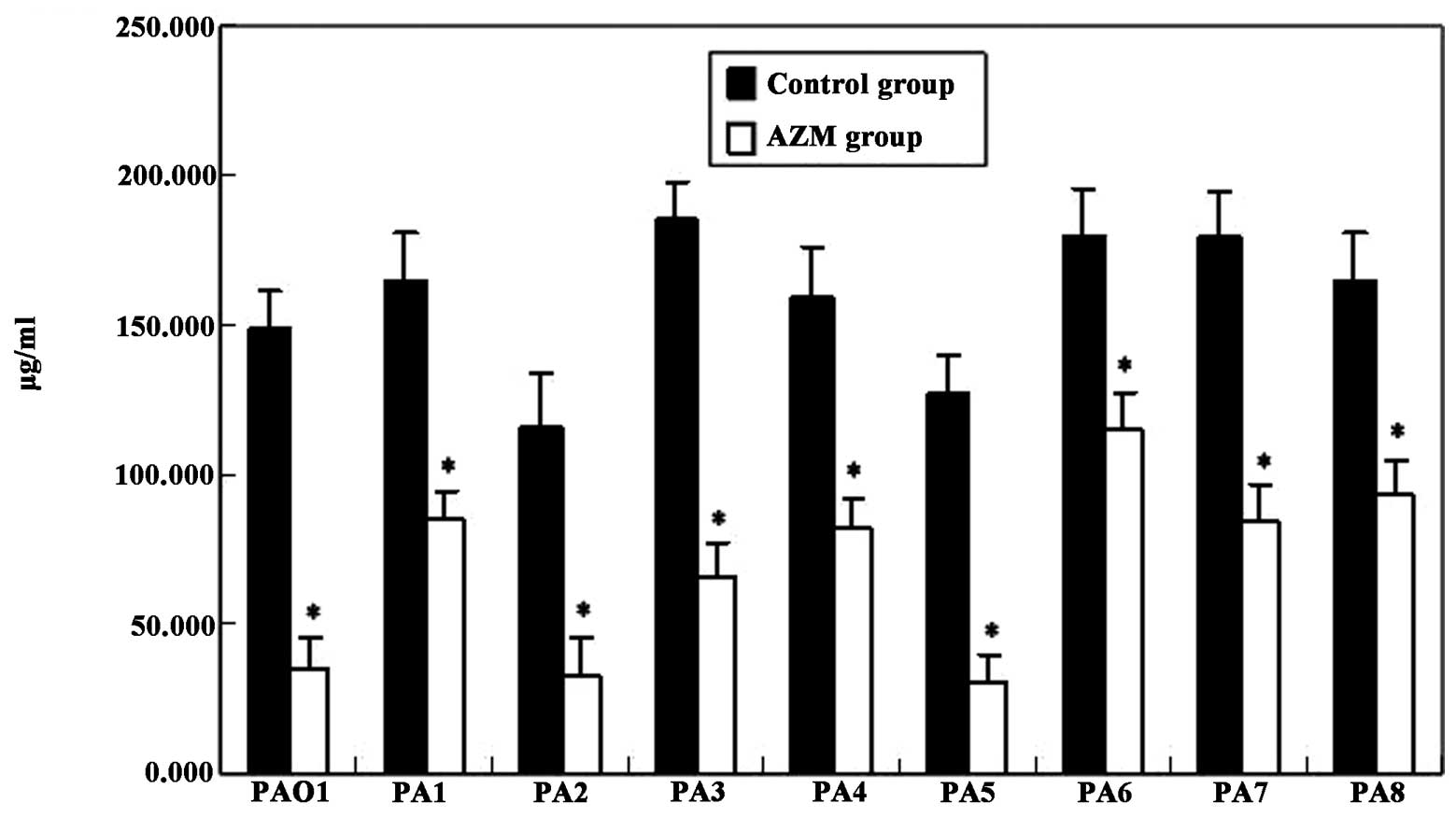
Virulence factors
The elastase activity of all P. aeruginosa
clinical isolates was higher than that of PAO1 in the control and
AZM groups, respectively (Fig. 2).
The production of rhamnolipids by the P. aeruginosa clinical
isolates was higher than that by PAO1, with the exception of
isolates PA2 and PA5 (Fig. 3).
Moreover, the presence of AZM in the culture medium greatly
decreased the expression of these virulence factors (P<0.01;
Figs. 2 and 3).
Discussion
With the development of medical technology, urinary
catheterization is increasingly common in hospitalized patients,
which is a risk factor of bacteriuria and symptomatic UTI (14). In the present study, the rate of
isolation of positive strains was 20%, and the P.
aeruginosa-positive rate was 5%. Among the eight obtained
isolates, the biofilms were thicker than those of P.
aeruginosa PAO1, which suggested that the strains isolated from
the urinary catheters had stronger biofilm formation capability.
Therefore, for P. aeruginosa in UTI, the possibility of
biofilm formation during therapy should be considered. Swimming
motility is a significant etiological factor. In the presence of
1/4 MIC AZM, the swimming motility was depressed greatly. Moreover,
AZM not only decreased biofilm formation capability, but also
decreased the adhesion to urinary catheters, which is similar to
the findings of previous studies (13,15).
P. aeruginosa elastase (PE) is a 39.5-kDa
metalloproteinase and one of the strongest virulence factors of
P. aeruginosa, which can degrade the elastin of human matrix
proteins including laminin and collagen types III and IV (16–18).
PE has also been found to be an immunosuppressive factor (19,20).
In biofilm formation, rhamnolipids have important actions, which
are involved in modulating the swarming and colonization of
incipient biofilm forming (21).
The inhibitory effect of AZM on the production of rhamnolipids was
less significant than its inhibitory effect on elastin. However,
the rhamnolipid levels were markedly decreased. The results
demonstrated that AZM decreased the elastase activity greatly,
indicating that AZM might relieve the pathogenicity of P.
aeruginosa from UTIs.
AZM has been shown to exert a good therapeutic
effect on cystic fibrosis (22).
However, to the best of our knowledge, there are few reports
concerning the use of AZM in the treatment of UTIs. Administration
of prophylactic antibiotics following catheter application
decreases UTI rates (23).
However, prophylactic antibiotics are usually
trimethoprim-sulfamethoxazole and quinolones (23). Macrolides are not a good choice for
UTIs according to the clinical application principles of
antibiotics of China (8). As AZM
is a new macrolide antibiotic, it is inferred from the present
study results that macrolides may be a good choice in the treatment
of UTIs involving P. aeruginosa. However, there are many
factors affecting the clinical treatment effect, such as the
immunity condition of patients, the original disease and resistance
of P. aeruginosa. Therefore, it is necessary to conduct
further clinical studies.
References
|
1
|
Ronald A: The etiology of urinary tract
infection: traditional and emerging pathogens. Am J Med. 113(Suppl
1A): 14S–19S. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jarvis WR and Martone WJ: Predominant
pathogens in hospital infections. J Antimicrob Chemother. 29(Suppl
A): 19–24. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stickler DJ: Bacterial biofilms and the
encrustation of urethral catheters. Biofouling. 9:293–305. 1996.
View Article : Google Scholar
|
|
4
|
Nickel JC, Ruseska I, Wright JB and
Costerton JW: Tobramycin resistance of Pseudomonas aeruginosa cells
growing as a biofilm on urinary catheter material. Antimicrob
Agents Chemother. 27:619–624. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cole SJ, Records AR, Orr MW, Linden SB and
Lee VT: Catheter-associated urinary tract infection by Pseudomonas
aeruginosa is mediated by exopolysaccharide-independent biofilms.
Infect Immun. 82:2048–2058. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tielen P, Rosin N, Meyer AK, et al:
Regulatory and metabolic networks for the adaptation of Pseudomonas
aeruginosa biofilms to urinary tract-like conditions. PLoS One.
8:e718452013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Narten M, Rosin N, Schobert M and Tielen
P: Susceptibility of Pseudomonas aeruginosa urinary tract isolates
and influence of urinary tract conditions on antibiotic tolerance.
Curr Microbiol. 64:7–16. 2012. View Article : Google Scholar
|
|
8
|
Ministry of Health, Administration of
Traditional Chinese Medicine and General Logistics Department of
Health. Guidelines for Clinical Use of Antibiotics. Chinese Medical
Association; Beijing, China: 2004
|
|
9
|
Clinical Laboratory Standards Institute.
Performance standards for antimicrobial susceptibility testing;
21st informational supplement. M100-S21. 31. Clinical Laboratory
Standards Institute; Wayne, PA, USA: 2011
|
|
10
|
Wang Q, Sun FJ, Liu Y, Xiong LR, Xie LL
and Xia PY: Enhancement of biofilm formation by subinhibitory
concentrations of macrolides in icaADBC-positive and -negative
clinical isolates of Staphylococcus epidermidis. Antimicrob Agents
Chemother. 54:2707–2711. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naik DN, Wahidullah S and Meena RM:
Attenuation of Pseudomonas aeruginosa virulence by marine
invertebrate-derived Streptomyces sp. Lett Appl Microbiol.
56:197–207. 2013. View Article : Google Scholar
|
|
12
|
Tomaras AP, Dorsey CW, Edelmann RE and
Actis LA: Attachment to and biofilm formation on abiotic surfaces
by Acinetobacter baumannii: involvement of a novel chaperone-usher
pili assembly system. Microbiology. 149:3473–3484. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bala A, Kumar R and Harjai K: Inhibition
of quorum sensing in Pseudomonas aeruginosa by azithromycin and its
effectiveness in urinary tract infections. J Med Microbiol.
60:300–306. 2011. View Article : Google Scholar
|
|
14
|
Saint S and Lipsky BA: Preventing
catheter-related bacteriuria: should we? Can we? How? Arch Intern
Med. 159:800–808. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vranes J: Effect of subminimal inhibitory
concentrations of azithromycin on adherence of Pseudomonas
aeruginosa to polystyrene. J Chemother. 12:280–285. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wretlind B and Pavlovskis OR: Pseudomonas
aeruginosa elastase and its role in pseudomonas infections. Rev
Infect Dis. 5(Suppl 5): S998–S1004. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saulnier JM, Curtil FM, Duclos MC and
Wallach JM: Elastolytic activity of Pseudomonas aeruginosa
elastase. Biochim Biophys Acta. 995:285–290. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bejarano PA, Langeveld JP, Hudson BG and
Noelken ME: Degradation of basement membranes by Pseudomonas
aeruginosa elastase. Infect Immun. 57:3783–3787. 1989.PubMed/NCBI
|
|
19
|
Kharazmi A, Döring G, Høiby N and Valerius
NH: Interaction of Pseudomonas aeruginosa alkaline protease and
elastase with human polymorphonuclear leukocytes in vitro. Infect
Immun. 43:161–165. 1984.PubMed/NCBI
|
|
20
|
Jacquot J, Tournier JM and Puchelle E: In
vitro evidence that human airway lysozyme is cleaved and
inactivated by Pseudomonas aeruginosa elastase and not by human
leukocyte elastase. Infect Immun. 47:555–560. 1985.PubMed/NCBI
|
|
21
|
Caiazza NC, Shanks RM and O’Toole GA:
Rhamnolipids modulate swarming motility patterns of Pseudomonas
aeruginosa. J Bacteriol. 187:7351–7361. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Delden C, Köhler T, Brunner-Ferber F,
François B, Carlet J and Pechère JC: Azithromycin to prevent
Pseudomonas aeruginosa ventilator-associated pneumonia by
inhibition of quorum sensing: a randomized controlled trial.
Intensive Care Med. 38:1118–1125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marschall J, Carpenter CR, Fowler S and
Trautner BW: CDC Prevevention Epicenters Program: Antibiotic
prophylaxis for urinary tract infections after removal of urinary
catheter: meta-analysis. BMJ. 346:f31472013. View Article : Google Scholar
|