Introduction
Limbic encephalitis is a rare neurological syndrome
that selectively affects the structures of the limbic system,
including the hippocampus, amygdala and hypothalamus. Since the
initial cases of this disease were often accompanied by cancer,
such as small cell lung cancer (SCLC), the disease was subsequently
referred to as paraneoplastic limbic encephalitis (PLE) (1–6). The
main clinical manifestations of PLE are seizures associated with
progressive short-term memory loss, which may develop into
dementia. In addition, there may be different degrees of
involvement in such extra-limbic-system tissues as the cerebellum,
brainstem and thalamus. Electroencephalography (EEG) typically
exhibits epileptic activity in the unilateral or bilateral temporal
lobes, with focal or global slow waves; magnetic resonance imaging
(MRI) T2 or flair images show high-signal, abnormal
lesions in the interior sides of the unilateral or bilateral
temporal lobes. In the majority of cases, temporal lobe atrophy
develops. Cerebrospinal fluid examination typically exhibits
inflammatory changes, with mildly to moderately increased
lymphocytes, as well as increased protein levels; glucose levels
would be normal and the immunoglobulin G (IgG) index would most
likely be increased. Furthermore, oligoclonal bands would be
apparent (7–9).
A number of studies have revealed that the
pathogenesis of PLE is an immune-mediated response, primarily
effected by cytotoxic T cells and antibodies that act on neuronal
antigens, such as anti-Hu (10,11),
anti-Ma2 (12), anti-amphiphysin
(13) and anti-Yo (14) antibodies. Treatments for PLE
include anti-cancer therapy and immunotherapy; the effects of the
former are more marked, but the overall prognosis is typically poor
(8,15). Research into PLE has made progress
over the past decade (16), and
certain clinical manifestations and imaging appearances have been
found to be consistent with PLE. Furthermore, since the generation
of antibodies targets neuronal cell membrane antigens, the
development of specific immunotherapies could lead to improvements
in prognosis for patients with PLE (15,17).
To date, few studies have focused on PLE in patients of Chinese Han
nationality; therefore, the present study described two cases of
PLE in patients of Chinese Han nationality and summarized four
cases in the literature.
Materials and methods
Clinical data
Two male patients with PLE associated with SCLC were
hospitalized in the Department of Neurology, the First Affiliated
Hospital of Bengbu Medical College (Bengbu, China) between October
1999 and July 2013. The patients were aged 69 and 83 years,
respectively, and exhibited serious memory impairment, which
prevented the patients from recalling the five designated objects
presented on admission 5 min later. One of the patients complained
of headache. One patient suffered from generalized tonic-clonic
seizures (GTCSs), while the other suffered from complex partial
seizures. The serum sodium levels of the two patients were as low
as 115 and 130 mmol/l, and one patient had intractable
hyponatremia. Cranial MRI was performed prior to treatment in both
cases. This study was conducted in accordance with the Declaration
of Helsinki and with approval from the Ethics Committee of Bengbu
Medical College. Written informed consent was obtained from all
participants.
Wechsler Adult Intelligence Scale (WAIS)
determination
A psychiatric doctor performed the test on the two
patients prior to treatment (18).
The language assessment included six aspects (knowledge test,
comprehension, arithmetic, similarities, digit span and vocabulary)
and the operation test consisted of five parts (number signs,
picture filling, block design, picture arrangement and object
assembly). The scores were obtained from the coarse score scale,
and added to obtain the language IQ score, the operation IQ score
and the total IQ points.
Immunohistochemistry
The cerebral cortex and cerebellum were obtained
from a neurologically normal individual within 6 h after mortality
(the brain tissue was provided by Professor Zhou Jiangning from the
School of Life Sciences, University of Sciences and Technology of
China, Hefei, China). Consent was provided by the family of the
deceased. Pieces were embedded in optimal cutting temperature
compound and snap-frozen in isopentane cooled by liquid nitrogen,
prior to being stored at −80°C. Tissue sections measuring 6 μm were
sequentially incubated with 0.3% hydrogen peroxide (to block
endogenous peroxidase activity) for 10 min and 10% normal goat
serum (Organon Teknika-Cappel, West Chester, PA, USA) was then
added as the blocking serum, prior to incubation for 15 min. The
sera of the patients were serially diluted overnight at 4°C and
then incubated with biotinylated goat anti-human IgG (Vector
Laboratories, Burlingame, CA, USA) for 1 h and the
Vectastain® avidin-biotin complex (Vector Laboratories)
for 30 min at room temperature. The substrate staining was
developed with 0.05% diaminobenzidine tetraahydrochloride (Sigma,
St Louis, MO, USA) and 0.01% hydrogen peroxide in
phosphate-buffered saline (PBS).
Western blotting
Western blotting was performed using the Euroline
Neuronal Antigens Profile 2 IgG kit (DL1111-1601-2 G; Euroimmun AG,
Lübeck, Germany). The film strip was removed and placed in the
incubation tank. Sample buffer (1.5 ml) was added and incubated in
a shaker (Euroimmun AG, Lübeck, Germany) at room temperature for 5
min, prior to the absorption of the liquid into the tank. Diluted
serum sample (1.5 ml) was then added into the incubation vessel and
agitated at room temperature (18–25°C) for 30 min incubation.
Following incubation, the liquid was absorbed into the tank and the
film strip was washed three times in a shaker with 1.5 ml washing
buffer for 5 min/time. A total of 1.5 ml diluted enzyme conjugate
(alkaline phosphatase-labeled anti-human IgG) was added into the
incubation tank and then agitated in the shaker at room temperature
for 30 min. The liquid was subsequently absorbed into the tank, and
the film strip was washed in a shaker a further three times with
1.5 ml washing buffer for 5 min/time. Following washing, 1.5 ml
substrate solution was added for another incubation at room
temperature (18–25°C) for 10 min, prior to the liquid being
absorbed into the tank and the film strip being washed three times
with distilled water for 1 min/time. The film strip was then
rotated in the result detecting template (Neuronal Antigens Profile
2; Euroimmun AG) for the analysis of the results following
air-drying.
Purified recombinant HuD
Purified recombinant HuD fusion protein, provided by
Professor J.B. Posner (Department of Neurology, Memorial
Sloan-Kettering Cancer Center, New York, NY, USA), was determined
using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Inc.,
Richmond, CA, USA). Samples were boiled in 0.0625 M Tris-HCl (pH
6.8), 2% sodium dodecyl sufate, 0.001% bromophenol blue and 5%
2-mercaptoethanol for 10 min. Aliquots (1 mg) were subsequently
electrophoresed on preparative 10% sodium dodecyl
sulfate-polyacrylamide gel, and the proteins were transferred to
nitrocellulose membranes using the methodology described by Towbin
et al (19). The membranes
were then blocked with 5% Blotto (Carnation Company, Glendale, CA,
USA) in PBS. The nitrocellulose membranes were cut into strips and
incubated with the indicated amount of serum, which was diluted in
buffer containing 1% bovine serum albumin, 10 mM Tris-HCl (pH 7.4),
0.9% sodium chloride and 0.5% Triton X-100 overnight at room
temperature. Following incubation, the samples were washed four
times for 15 min in the aforementioned buffer and incubated with
biotinylated goat anti-human IgG (Vector Laboratories) for 1 h and
the Vectastain avidin-biotin complex (Vector Labs) for 30 min at
room temperature. The substrate staining was developed with 0.05%
diaminobenzidine tetrahydrochloride (Sigma), 0.5% Triton X-100 and
0.01% hydrogen peroxide in PBS.
Results
WAIS determination
Prior to the treatment, the language IQ scores of
the two patients were 45 and 57 points, the operation IQ scores
were 42 and 43 points and the total IQ scores were 40 and 48
points. The patients scored poorly in the arithmetic, picture
filling, block design, picture arrangement and object assembly.
EEG
EEG revealed abnormalities in both cases, including
focal or sharp slow waves in the bilateral frontotemporal lobes
(Table I).
 | Table IClinical features of paraneoplastic
limbic encephalitis. |
Table I
Clinical features of paraneoplastic
limbic encephalitis.
| Patient no.
(ref.) | Gender, age in
years | Tumor
type/location | Symptoms and
signs | EEG | CSF protein
(mg/day) | Brain MRI and
18F-FDG/PET-CT | Neuronal
antibodies | Prognosis |
|---|
| 1 (present
study) | M, 69 | SCLC | Disorientation and
GTCS for 90 days, Na+ 115 mmol/l | Bilateral frontal
slow wave, right temporal lobe focal sharp-wave | Normal | Brain MRI: Atrophy in
the bilateral temporal lobe and hippocampal area |
Anti-Hu+ | Mortality |
| 2 (present
study) | M, 83 | SCLC | Progressive
short-term memory loss, partial complex seizure Na+ 130
mmol/l | Bilateral frontal,
right temporal lobe slow wave | Normal | MRI: High signal
intensity on the flair and T2-weighted image in the
bilateral amygdala and hippocampal area |
Anti-amphiphysin+ | Mortality |
| 3 (31) | M, 49 | Pancreatic
cancer | Clumsy, apathy,
echoing speech, memory loss, partial complex seizure; sucking,
groping and grasping reflexes and a diffuse, brisk, deep tendon
reflex; disorganization | Bilateral frontal,
right temporal lobe, focal slow wave | Protein: 900
mg/l | MRI: Bilateral
frontal lobe, temporal lobe, left parietal lobe, occipital lobe,
cerebellar hemisphere, right parietal lobe. PET-CT: Bilateral
frontal temporal lobe, right parietal lobe, occipital lobe,
decreased metabolism | Negative | Mortality |
| 4 (33) | F, 22 | Ovarian teratoma | Apathy, babbing,
conscious disturbance, GTCS status, Na+ 130 mmol/l | Bilateral diffuse
slow wave | WBC:
16×106/l
Protein: 50 mg/l | Brain MRI:
Normal | Negative | Recovery |
| 5 (30) | F, 17 | Ovarian
teratoma | Short-term memory
loss, emotional disturbance, GTCS status | Bilateral diffuse
height amplitude δ waves | Intracranial
hypertension, 220 mm H2O; WBC: 16×106/l,
Protein: 580 mg/l | Brain MRI:
Normal |
NMDAR+ | Recovery |
| 6 (32) | M, 52 | SCLC | Short-term memory
loss, partial complex seizure, Lambert-Eaton syndrome | - | - | MRI: Brain
atrophy | Negative | Mortality |
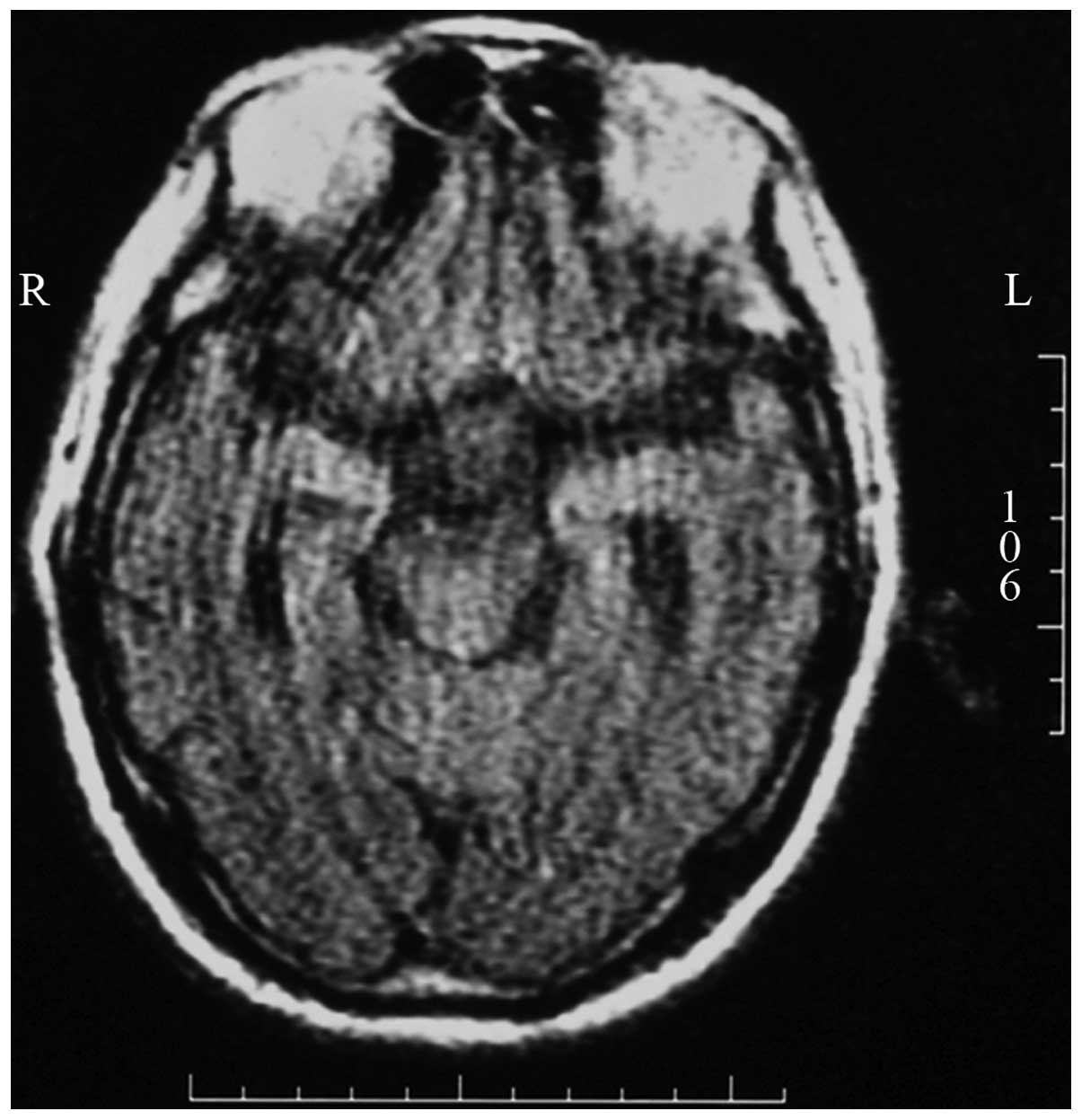
Cranial MRI
One case had atrophy in the bilateral temporal lobe
and hippocampal area and one case had high signal intensity on the
flair and T2-weighted images in the bilateral amygdala
and hippocampal area (Fig. 1).
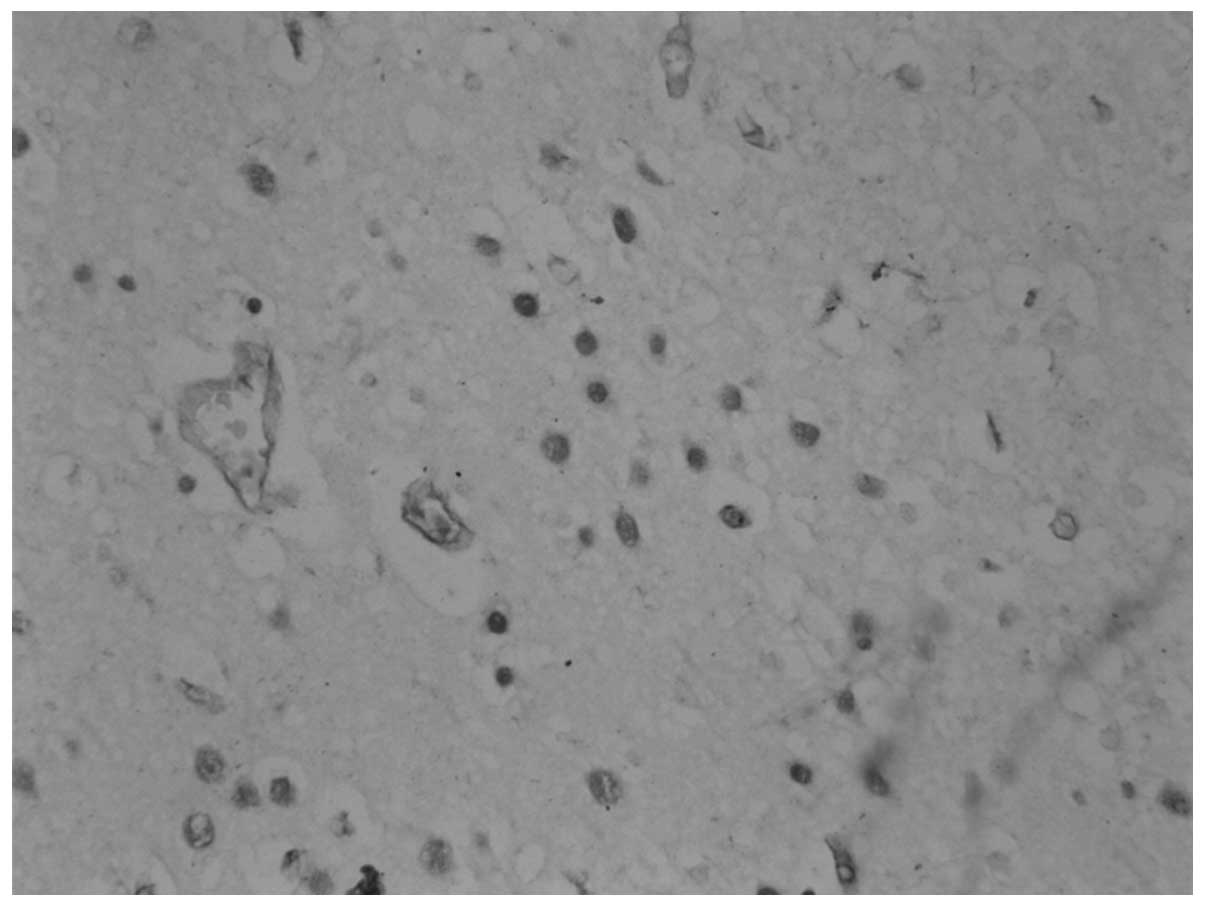
Immunohistochemistry
The 69-year-old patient with PLE and SCLC was found
to have anti-Hu antibodies in the serum. Following incubation of a
section of the frontal cortex for 60 min with the patient’s serum
[serum dilution, 1:1,000–1:16,000; cerebrospinal fluid (CSF)
dilution, 1:100–1:800], positive staining of the neuronal nuclei
was observed in a homogeneous pattern. No staining of the nucleoli
was observed, and negative results were obtained for anti-Yo and
-Ri antibodies (Fig. 2). The
83-year-old patient was found to have anti-amphiphysin antibodies
in the serum. Anti-amphiphysin antibodies belong to the antibodies
targeting the synaptic vesicle. Immunohistochemical tests are
unable to show positive staining even in the presence of
anti-amphiphysin antibodies.
Western blotting
The paraneoplastic neuronal antibody spectrum
examination included six well-characterized onconeuronal
antibodies: Anti-Hu, anti-Yo, anti-Ri, anti-CV2,
anti-paraneoplastic antigen Ma2 (PNMA2) and anti-amphiphysin. One
of the patients with PLE and SCLC was positive for anti-amphiphysin
antibodies (Fig. 3).
Purified recombinant HuD
Immunoblots of purified recombinant HuD reacted with
the serum of one of the patients with PLE and SCLC. The serum of
the patient was positive for anti-Hu antibody (dilution,
1:50–1:200) (Fig. 4).
Discussion
PLE is considered a rare manifestation that is
characterized by the development of the neuropsychiatric symptoms
of a paraneoplastic neurological disorder, but additionally
associated with cancer in the absence of invasion of the nervous
system by tumor cells. The tumor most commonly found in association
with PLE is SCLC. The disease can affect individuals aged 10–85
years, with the females being less susceptible than males (5,20).
Rarer malignancies associated with PLE are thymoma (21), ovarian teratoma (22,23),
esophagastric squamous cell carcinoma (24), adenocarcinoma of the colon
(14), prostate cancer (25), testicular neoplasm (26,27),
Hodgkin’s lymphoma (20),
non-Hodgkin’s lymphoma (28),
leukemia, lymphoma (29) and acute
myeloid leukemia (17). A
literature search identified four studies containing data on
cancer-related PLE in patients of Chinese Han nationality (30–33).
Through the clinical manifestations, psychology, WAIS
determination, CSF analysis, electrophysiology, imaging,
immunological determination and anti-immunotherapy in the patients
with PLE, it was found that the patients had two types of clinical
manifestations, simple and complex, and that the lesions could also
be divided into focal and scalable types. Of the four patients
identified in the literature and the present two cases, three
patients had PLE with SCLC (32),
one case had PLE with pancreatic cancer (31) and two patients had PLE with ovarian
teratoma (30,33). Four of the cases presented as an
isolated neurological syndrome (progressive short-term memory loss,
GTCSs) (32,33). One of the patients MRI showed the
involvement of the bilateral frontal, right temporal and occipital
lobes, as well as the left cerebellar hemisphere (31). PET-CT confirmed that the metabolism
in the bilateral frontal, temporal, right parietal and occipital
lobe was reduced. The patient had sucking, groping and grasping
reflexes and a diffuse, brisk, deep tendon reflex (31,32).
One case with PLE and SCLC had Lambert-Eaton myasthenic syndrome,
and the lesion involved voltage-sensitive calcium channels of the
presynaptic membrane in neuromuscular transmission (Table I).
The clinical diagnosis of PLE is problematic;
however, the identification of a number of specific circulating
autoantibodies, such as anti-Hu (34,1),
anti-PNMA2 (12), anti-Yo
(14), anti-N-methyl-D-aspartate
receptor (NMDAR) (35) and
anti-voltage-gated potassium channel (17) antibodies, in these patients has
revolutionized the diagnosis and understanding of these syndromes
and demonstrated a role for the immune system in such neurological
disorders. We have speculated that the clinical manifestations and
lesions scopes are associated with certain types of tumors and
antibodies. In cases of autoantibodies targeting intracellular
antigens (anti-Hu, anti-PNMA2, anti-Yo and anti-amphiphysin), an
associated malignancy can nearly always be observed. These
neurological disorders are predominantly associated with neuronal
death, and patients are rarely sensitive to immunomodulatory
treatments; cellular immunity appears to play a major role in this
lack of sensitivity. By contrast, patients with autoantibodies
targeting membrane antigens (receptors, channels or receptors
associated with proteins) almost always have ovarian teratoma
(21,35,33,30),
and the neurological disorders are associated with a reversible
neuronal dysfunction. These patients are mostly sensitive to
immunomodulatory treatments, and it appears that humoral immunity
and autoantibodies play a major role.
Using avidin-biotin immunoperoxidase methods, it was
found in the present study that the serum of one patient with PLE
and SCLC reacted with a section of frontal cortex (positive
staining of the neuronal nuclei in a homogeneous pattern, but
negative in the nucleoli). In purified recombinant HuD western
blotting, the serum of one patient reacted with a band of 40-kDa
purified recombinant HuD, a human neuronal RNA-binding protein
(36), while Euroline Neuronal
Antigens Profile 2 IgG western blotting showed the serum of the
other patient to be positive for anti-amphiphysin antibodies.
Anti-Hu and anti-amphiphysin are considered to be
well-characterized onconeuronal antibodies, and their presence
leads to the diagnosis of the PLE as classical paraneoplastic
syndrome. Based on these considerations, the two patients in the
present study developed definite paraneoplastic neurological
syndrome (PNS) (37).
Anti-amphiphysin antibodies have been identified in a few patients
with PNS and SCLC, whose manifestations included
encephalomyelitis/sensory neuropathy, cerebellar degeneration and
opsoclonus (13). In the present
study, the serum of one patient with PLE was positive for
anti-amphiphysin antibody, which was rare. Despite the lack of
evidence that these antibodies are the causative agents of the
neuropathological process, they have proven to be useful markers of
the autoimmune inflammation of structures in the limbic system.
Dalmau et al (22) first
described a number of cases of encephalitis with ovarian teratoma
that were associated with the NMDAR antibodies, and subsequently
analyzed the characteristics of 100 patients (35): 56% had ovarian teratoma, and the
patients that received early tumor treatment and immunotherapy had
an improved outcome. In a study by Xu et al, two cases had
ovarian teratoma and one case had serum NMDAR antibodies (30). Tumor resection and immunotherapy
resulted in a full recovery.
Patients with PLE of Chinese Han nationality had two
types of clinical manifestation: simple and complex. Furthermore,
the lesions could also be divided into focal and scalable lesions.
The clinical manifestations and lesion scopes were associated with
certain types of cancer and antibodies. Compared with patients with
PLE with autoantibodies targeting intracellular antigens, the
prognosis for patients with PLE with autoantibodies targeting
membrane antigens is improved as a result of immunomodulatory
treatments and anti-cancer therapy.’
Acknowledgements
The authors would like to thank Professor J.B.
Posner (Department of Neurology, Memorial Sloan-Kettering Cancer
Center) for providing the purified recombinant HuD fusion protein
and Professor Yinbao Guo (Department of Psychiatry, Bengbu Medical
College) for the assistance with the WAIS determination. This study
was supported by the Anhui Provincial Natural Science Research
Foundation Major Project (no. 03023049); the Anhui Provincial
Natural Science Foundation Project (no. 03043715); the Anhui
Provincial Personnel Department Trans-Century Talents Major Project
(no. 041218); the Anhui Provincial Health Bureau Fifth Scientific
Foundation (2002) No. 391; and the Anhui college and university
provincial level Natural Science Foundation Project (no.
2000jl164).
References
|
1
|
Alamowitch S, Graus F, Uchuya M, Rene R,
Bescansa E and Delattre JY: Limbic encephalitis and small cell lung
cancer Clinical and imunological features. Brain. 120:923–928.
1997. View Article : Google Scholar
|
|
2
|
Ryu JY, Lee SH, Lee EJ, et al: A case of
paraneoplastic limbic encephalitis associated with small cell lung
cancer. Tuberc Respir Dis (Seoul). 73:273–277. 2012. View Article : Google Scholar :
|
|
3
|
Bowyer S, Webb S, Millward M, Jasas K,
Blacker D and Nowa A: Small cell lung cancer presenting with
paraneoplastic limbic encephalitis. Asia Pac J Clin Oncol.
7:180–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosenfeld MR and Dalmau J: Paraneoplastic
limbic encephalitis associated with small-cell lung cancer. Comm
Oncol. 4:491–494. 2007. View Article : Google Scholar
|
|
5
|
White D and Beringer T: Paraneoplastic
limbic encephalitis in an elderly patient with small cell lung
carcinoma. Ulster Med J. 79:22–24. 2010.PubMed/NCBI
|
|
6
|
Said S, Cooper CJ, Reyna E, Alkhateeb H,
Diaz J and Nahleh Z: Paraneoplastic limbic encephalitis, an
uncommon presentation of a common cancer: Case report and
discussion. Am J Case Rep. 14:391–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Urbach H, Soeder BM, Jeub M, Klockgether
T, Meyer B and Bien CG: Serial MRI of limbic encephalitis.
Neuroradiology. 48:380–386. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bien CG and Elger CE: Limbic encephalitis:
a cause of temporal lobe epilepsy with onset in adult life.
Epilepsy Behav. 10:529–538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dreessen J, Jeanjean AP and Sindic CJ:
Paraneoplastic limbic encephalitis: Diagnostic relevance of CSF
analysis and total body PET seanning. Acta Neurol Belg. 104:57–63.
2004.PubMed/NCBI
|
|
10
|
Dalmau J, Furneaux HM, et al: Detection of
the anti-Hu antibody in the serum of patients with small cell lung
cancer - a quantitative western blot analysis. Ann Neurol.
27:544–552. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Langer JE, Lopes MB, et al: An unusual
presentation of anti-Hu-associated paraneoplastic limbic
encephalitis. Dev Med Child Neurol. 54:863–866. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leyhe T, Schüle R, Schwarzler F, Gasser T
and Haarmeier T: Second primary tumor in anti-Mal/2-positive
paraneoplastic limbic encephalitis. J Neurooncol. 78:49–51. 2006.
View Article : Google Scholar
|
|
13
|
Saiz A, Dalmau J, Butler MH, Chen Q,
Delattre JY, De Camilli P and Graus F: Anti-amphiphysin I
antibodies in patients with paraneoplastic neurological disorders
associated with small cell lung carcinoma. J Neurol Neurosurg
Psychiatry. 66:214–217. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adam VN, Budincevic H, Mrsic V, Stojcic
EG, Matolic M and Markic A: Paraneoplastic limbic encephalitis in a
patient with adenocarcinoma of the colon: a case report. J Clin
Anesth. 25:491–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dalmau J and Rosenfeld MR: Paraneoplastic
syndromes of the CNS. Lancet Neurol. 7:327–340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Honnorat J and Viaccoz A: New concepts in
paraneoplastic neruological syndromes. Rev Neurol (Paris).
167:729–736. 2011. View Article : Google Scholar
|
|
17
|
Alcantara M, Bennani O, Verdure P,
Leprêtre S, Tilly H and Jardin F: Voltage-gated potassium channel
antibody paraneoplastic limbic encephalitis associated with acute
myeloid leukemia. Case Rep Oncol. 6:289–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaufman AS and Lichtenberger E: Assessing
Adolescent and Adult Intelligence. 3rd edition. Wiley; Hoboken, NJ:
pp. 32006
|
|
19
|
Towbin H, Staehelin T and Gordon J:
Electrophoretic transfer of proteins from polyacrylamide gels to
nitrocellulose sheets: procedure and some applications. Proc Natl
Acad Sci USA. 76:4350–4354. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mollier-Saliner J, Thouvenin S, Darteyre
S, Jaziri F, Vasselon C, Convers P and Stephan JL: Paraneoplastic
limbic encephalitis: 2 pediatric cases. Arch Pediatr. 20:386–390.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ingenito GG, Berger JR, David NJ and
Norenberg MD: Limbic encephalitis associated with thymoma.
Neurology. 40:3821990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dalmau J, Tüzün E, Wu HY, et al:
Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis
associated with ovarian teratoma. Ann Neurol. 61:25–36. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawano H, Hamaguchi E, Kawahito S,
Tsutsumi YM, Tanaka K, Kitahata H and Oshita S: Anaesthesia for a
patient with paraneoplastic limbic encephalitis with ovarian
teratoma: relationship to anti-N-methyl-D-aspartate receptor
antibodies. Anaesthesia. 66:515–518. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McCormack O, Cooney JM, Doherty CP,
Muldoon C and Reynolds JV: Paraneoplastic limbic encephalitis from
esophagogastric squamous cell carcinoma successfully managed by
radical gastrectomy. Surgery. 154:638–640. 2013. View Article : Google Scholar
|
|
25
|
Jakobsen JK, Zakharia ER, Boysen AK,
Andersen H, Schlesinger FE and Lund L: Prostate cancer may trigger
paraneoplastic limbic encephalitis: A case report and a review of
the literature. Int J Urol. 20:734–737. 2013. View Article : Google Scholar
|
|
26
|
Ahern GL, O’Connor M, Dalmau J, et al:
Paraneoplastic temporal lobe epilepsy with testicular neoplasm and
atypical amnesia. Neurology. 44:1270–1274. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Burton GV, Bullard DE, Walther PJ and
Burger PC: Paraneoplastic limbic encephalopathy with testicular
carcinoma a reversible neurologic syndrome. Cancer. 62:2248–2251.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Semnic M, Jovanovic D, Petrovic D, Nad I
and Semnic R: Paraneoplastic limbic encephalitis in a patient with
non-Hodgkin’s lymphoma. Arch Oncol. 12:71–73. 2004. View Article : Google Scholar
|
|
29
|
Laffon M, Giordana C, Almairac F,
Benchetrit M and Thomas P: Anti-Hu-associated paraneoplastic limbic
encephalitis in Hodgkin lymphoma. Leuk Lymphoma. 53:1433–1434.
2012. View Article : Google Scholar
|
|
30
|
Xu CL, Zhao WQ, Li JM, et al:
Anti-N-methyl-D-aspartate receptor encephalitis: an adolescent with
ovarian teratoma. Zhong Hua Shen Jing Ke Za Zhi. 43:781–783.
2010.(In Chinese).
|
|
31
|
Zhang KZ, Wang Z, Chen WX and Song CJ:
Paraneoplastic limbic encephalitis in one patient with pancreatic
cancer. Zhong Hua Shen Jing Ke Za Zhi. 41:6652008.(In Chinese).
|
|
32
|
Zhao CP, Xie ZH, Peng CL, Wang Y and Sun
L: Paraneoplastic limbic encephalitis associated with Lamber-Eaton
syndrome in one patient with small cell lung cancer. Zhong Guo Shen
Jing Jing Shen Bing Xue Za Zhi. 36:5162010.(In Chinese).
|
|
33
|
Zhou SN, Fu XZ, Liu YM, et al:
Paraneoplastic limbic encephalitis in one patient with ovarian
teratoma. Zhong Hua Shen Jing Ke Za Zhi. 42:686–688. 2009.(In
Chinese).
|
|
34
|
Graus F, Cordon-Cardo C and Posner JB:
Neuronal antinuclear antibody in sensory neuronopathy from lung
cancer. Neurology. 35:538–543. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dalmau J, Gleichman AJ, Hughes EG, et al:
Anti-NMDA-receptor encephalitis: case series and analysis of the
effects of antibodies. Lancet Neurol. 7:1091–1098. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Manley GT, Smitt PS, Dalmau J and Posner
JB: Hu antigens: reactivity with Hu antibodies, tumor expression,
and major immunogenic sites. Ann Neurol. 38:102–110. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Graus F, Delattre JY, Antoine JC, et al:
Recommended diagnostic criteria for paraneoplastic neurological
syndromes. J Neurol Neurosurg Psychiatry. 75:1135–1140. 2004.
View Article : Google Scholar : PubMed/NCBI
|