Introduction
Hepatocellular carcinoma (HCC) is a common type of
malignant tumor in China. The distinguishing characteristics of HCC
are its rapid progression and high mortality rate (1). Epidemiological investigation has
shown that the morbidity of males with HCC is significantly higher
than that of females. According to worldwide statistics, liver
cancer in males is the fifth most frequently diagnosed cancer and
the second most frequent cause of cancer-related mortality. In
females, it is the seventh most commonly diagnosed cancer and the
sixth leading cause of cancer mortality (2). The ratio of morbidity in males to
that in females is 2–4:1 (3–5).
These observations suggest that the occurrence of HCC may be
related to the cancer microenvironment, and provide evidence that
the androgen receptor (AR) and matrix metalloproteinases (MMPs) may
play roles in cancer invasion and potentially lead to the
progression of cancer infiltration. The present study was designed
to evaluate the possibility that these proteins are significant in
cancer invasion and staging by comparing the expression of AR and
MMP in HCC tissues with that in tissues adjacent to the HCC.
Materials and methods
Origins of specimens
A total of 30 tissue specimens were acquired from
the surgical resections of patients with HCC during 2006–2011 in
Hai’an Hospital (Nantong, China), which including 26 male cases and
4 female cases. The age range was from 26 to 73 years, with a mean
age of 53 years; the median age was 45 years. Of these patients, 22
had undergone palliative resections due to severe hepatic
disorders. In 20 cases, the patients had more than one tumor focus,
including intrahepatic and distant metastases and/or portal venous
tumor emboli (T3-4). The remaining 10 cases had only one
intrahepatic primary focus (T1-2). There were 25 cases
(83.33%) that were infected with hepatitis B virus and 17 cases
(56.67%) had alcohol-related lesions (Table I). All specimens were confirmed by
pathological assay for primary HCC. All cases were selected at the
initial diagnosis, and had not been treated with chemotherapy or
radiation therapy. All of the patients had never received treatment
with sex hormones or steroid hormone drugs. This study was approved
by the ethics committee of Hai’an Hospital (Nantong, China). All
patients included in the present study provided written informed
consent prior to participation.
 | Table IClinical characteristics of 30
patients with HCC. |
Table I
Clinical characteristics of 30
patients with HCC.
| Clinical feature | No. of cases |
|---|
| Gender |
| Male | 26 |
| Female | 4 |
| Age (years) |
| <50 | 23 |
| ≥50 | 7 |
| Tumor number |
| One | 10 |
| Multiple | 20 |
| Size of tumor |
| <5 cm | 10 |
| ≥5 cm | 20 |
| Hepatitis B
virus |
| Yes | 25 |
| No | 5 |
| Alcohol drinker |
| Yes | 17 |
| No | 13 |
Immunohistochemical (IHC) staining
Paraffinized sections (6 μm thick) were prepared
from the HCC tissue and peritumoral tissues of every paraffin block
specimen. All paraffin sections were analyzed by
immunohistochemical methods using AR, MMP-2 and MMP-9 assay kits
(Boster Biological Engineering Co., Ltd., Wuhan, China). A prostate
tissue section was used as the positive control for AR expression,
while phosphate-buffered saline (PBS) was instead of the primary
antibody to treat the section used as an internal negative
control.
Judgment standards
Every immunostained section was analyzed by two
independent pathologists. Cytoplasmic and/or or nuclear staining
(brown reaction products) was regarded as a positive result. The
sections were viewed under a microscope and 10 high power field
were randomly selected. ARs were located in the nuclei of HCC cells
and the tissue adjacent to the carcinoma. Points were awarded
according to the percentage of stained cells in the tumor and
peritumoral tissue, and were divided into following categories: 0%,
0 points; 1–9%, 1 point and >10%, 2 points. Points were also
awarded for staining intensity, and were divided into the following
categories: No staining, 0 points; weak staining (buff in color), 1
point; medium staining (brown-yellow), 2 points; and strong
staining (brown), 3 points. The two types of points were added
together, and a score >2 was recorded as AR expression positive;
otherwise, AR expression was regarded as negative. MMP-2 and MMP-9
were located in the cytoplasm of HCC cells and the peritumoral
cells. Points were awarded according to the percentage of stained
cells in the tumor and peritumoral tissue as follows: 0%, 0 points;
<25%, 1 point; 25–49%, 2 points; and >50%, 3 points. Points
were also awarded for staining intensity as follows: No staining, 0
points; weak staining (buff in color), 1 point; medium staining
(brown-yellow), 2 points; and strong staining (brown), 3 points.
The two types of points were added together, and a score >3 was
recorded as MMP-2 or MMP-9 expression positive; otherwise, the
expression was regarded as negative.
Statistical analysis
Data analysis was performed using the χ2
test with SPSS software, version 13.0 (SPSS Inc, Chicago, IL, USA)
for Windows. P<0.05 was considered to indicate a statistically
significant result.
Results
AR, MMP-2 and MMP-9 expression
The expression levels of AR, MMP-2 and MMP-9 in the
HCC tissues and adjacent tissues are presented in Table II. The positive expression rates
of AR, MMP-2 and MMP-9 in the HCC tissues were higher than those in
the tissues adjacent to the HCC (P<0.05). The expression levels
of AR, MMP-2 and MMP-9 were significantly different between the
T3/T4 and T1/T2 stages
of HCC (P<0.05, Table
III).
 | Table IIExpression of AR, MMP-2 and MMP-9 in
HCC tissues and tissues adjacent to HCC. |
Table II
Expression of AR, MMP-2 and MMP-9 in
HCC tissues and tissues adjacent to HCC.
| Tissue | n | AR (+) | MMP-2 (+) | MMP-9 (+) |
|---|
| HCC | 30 | 23 | 22 | 23 |
| Adjacent to
carcinoma | 30 | 8 | 13 | 15 |
| χ2 | | 15.017 | 5.554 | 4.593 |
| P-value | | 0.000 | 0.018 | 0.032 |
 | Table IIIExpression of AR, MMP-2 and MMP-9 in
different stages of HCC. |
Table III
Expression of AR, MMP-2 and MMP-9 in
different stages of HCC.
| Stage | n | AR (+) | MMP-2 (+) | MMP-9 (+) |
|---|
|
T3/T4 | 20 | 18 | 17 | 19 |
|
T1/T2 | 10 | 5 | 5 | 4 |
| χ2 | | 5.963 | 4.176 | 11.273 |
| P-value | | 0.015 | 0.041 | 0.001 |
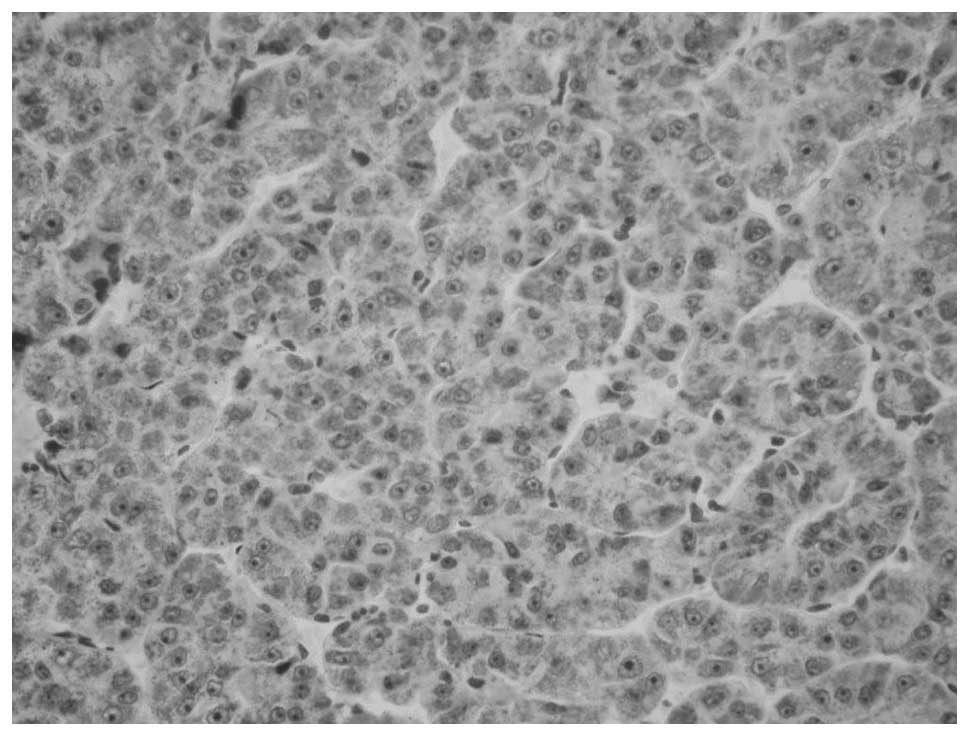
Immunostained sections: Figs. 1 shows the representative
immunostained sections of peritumoral tissue and Fig. 2 shows the AR(+) HCC tissue from the
specimens analyzed in the present study. The MMP-2 or MMP-9
expression in the immunohistochemical staining sections was similar
to the AR expression results.
Discussion
HCC is a kind of highly invasive malignant tumor,
which is always diagnosed according to clinic symptoms and signs,
imaging data and specific markers such as α-fetoprotein (6). Once it occurs, HCC always progress
rapidly and only a few cases can be diagnosed at an early stage.
Factors associated with the prognosis index of HCC include
diagnosis stage, tumor size, the completeness of the tumor capsule,
vascular invasion, pathological type and cell proliferation
(7,8). The majority of studies have shown AR
expression in HCC tissues (9,10,11).
However, due to the specimens being from different sources, such as
fresh specimens and older paraffin-embedded specimens, differences
in the detection methods, variations in the sample sizes and racial
differences in the patients, the positive rate of the AR in
different reports is variable. The present study found that the
positive rate of AR was 76.67%, which was similar to that in the
majority of the reports. The study also confirmed that the positive
rate of AR expression in primary liver cancer cells is
significantly higher than that in adjacent cells (P<0.05).
Subgroup analysis according to stage also indicated that the
positive rate of AR expression in HCC patients with intrahepatic
metastasis, distant metastasis or portal vein invasion was higher
than that in patients without any metastasis, and the difference
was significant (P<0.05). This finding indicated that the
positive expression of AR in HCC is associated with vascular
invasion of the liver and metastasis. Males under 50 years of age
comprised 76.7% of the study group (n=30); the data shows that
young male patients were in the majority in this group of HCC
patients at the first diagnosis, and that their tumors invade and
transfer earlier. The present study demonstrated that androgens and
the androgen-AR signaling pathway in patients may play a very
important role in the biological behavior of HCC, such as in its
occurrence, development, invasiveness and metastasis. A previous
study found that ARs are able to adjust hepatitis B virus
transcription and promote the formation of HCC associated with
hepatitis B (9); however, the
mechanism by which androgens and the androgen-AR signaling pathway
regulate and control hepatocarcinogenesis and its progress are
unclear. The majority of primary liver cancer patients have
hepatitis B virus infection or lesions due to chronic alcohol use.
The metabolism of androgens has been observed to be decreased or
disordered in patients when chronic hepatic lesions are present
(12). It is unclear whether
hepatocarcinogenesis and its progress are associated with long-term
and continued high levels of androgens. Further studies concerning
the association between the androgen-AR signaling pathway and HCC
are required.
Invasion and metastasis of a tumor are multiple and
complicated processes. Cancer cells break away from the primary
lesion and initially break through the extracellular matrix and
basement membrane. Degradation of the extracellular matrix and the
basement membrane is one pivotal step. Extracellular matrix
degradation occurs due to the actions of matrix metalloproteinases,
hyaluronidase and a large number of phagocytic cells in liver, and
MMP-2 and MMP-9 enzymes play important roles in the degradation of
the extracellular matrix and basement membrane. MMP-2 and MMP-9 are
not only involved in the enzymolysis of intercellular matrix
components, but also are the key enzymes in the degradation of
extracellular matrix collagen IV (13). MMP-2 and MMP-9 exist extensively in
HCC tissues. The results of the present study indicate that MMP-2
and MMP-9 are expressed at higher levels in HCC tissue than in
peritumoral tissues. Subgroup analysis according to stage suggests
that the rate of distant metastases or portal vein invasion in
patients that are MMP-2 or/and MMP-9 positive is significantly
higher than that of patients who are negative for these enzymes
(P<0.05), which is consistent with previous studies (14,15).
The results of the present study confirmed that the overexpression
of MMP-2 and MMP-9 is likely to increase tumor invasion and
metastasis.
In summary, ARs are associated with local cancer
cell infiltration, in which MMP-2 and MMP-9 may play important
roles in the HCC microenvironment to increase invasion and
metastasis (16). AR, MMP-2 and
MMP-9 may be predictive markers for HCC and could be regarded as
candidates for indicating the cancer stage.
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu MW and Chen CJ: Elevated serum
testosterone levels and risk of hepatocellular carcinoma. Cancer
Res. 53:790–794. 1993.PubMed/NCBI
|
|
4
|
Yu MW, Cheng SW, Lin MW, et al:
Androgen-receptor gene CAG repeats, plasma testosterone levels, and
risk of hepatitis B-related hepatocellular carcinoma. J Natl Cancer
Inst. 92:2023–2028. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee CM, Lu SN, Changchien CS, et al: Age,
gender, and local geographic variations of viral etiology of
hepatocellular carcinoma in a hyperendemic area for hepatitis B
virus infection. Cancer. 86:1143–1150. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hashemi M and Ghavami S: Hepatocellular
recurrence after orthotopic liver transplantation: Is combination
of α-fetoprotein and glypican-3 a reliable marker?: Hepatocellular
recurrence after orthotopic liver trasplantation. Hepat Mon.
11:155–156. 2011.PubMed/NCBI
|
|
7
|
Bruix J and Llovet JM: Major achievements
in hepatocellular carcinoma. Lancet. 373:614–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma WL, Hsu CL, Wu MH, Wu CT, Wu CC, Lai
JJ, Jou YS, Chen CW, Yeh S and Chang C: Androgen receptor is a new
potential therapeutic target for the treatment of hepatocellular
carcinoma. Gastroenterology. 135:947–955. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tavian D, De Petro G, Pitozzi A, et al:
Androgen receptor mRNA under-expression in poorly differentiated
human hepatocellular carcinoma. Histol Histopathol. 17:1113–1119.
2002.PubMed/NCBI
|
|
11
|
Feng H, Cheng AS, Tsang DP, et al: Cell
cycle-related kinase is a direct androgen receptor-regulated gene
that drives β-catenin/T cell factor-dependent hepatocarcinogenesis.
J Clin Invest. 121:3159–3175. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boonyaratanakornkit V and Edwards DP:
Receptor mechanisms mediating non-genomic actions of sex steroids.
Semin Reprod Med. 25:139–153. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lelongt B, Trugnan G, Murphy G and Ronco
PM: Matrix metalloproteinases MMP2 and MMP9 are produced in early
stages of kidney morphogenesis but only MMP9 is required for renal
organogenesis in vitro. J Cell Biol. 136:1363–1373. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Lau GK, Chen L, Dong SS, et al:
Interleukin 17A promotes hepatocellular carcinoma metastasis via
NF-kB induced matrix metalloproteinases 2 and 9 expression. PLoS
One. 6:e218162011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang P, Yuan W, He J, Wang J, et al:
Overexpression of EphA2, MMP-9, and MVD-CD34 in hepatocellular
carcinoma. Implications for tumor progression and prognosis.
Hepatol Res. 39:1169–1177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|