Introduction
Gossypol is a phenolic aldehyde extracted from
cotton and tropical plants that is able to permeate cells. It forms
an extensive network of hydrogen bonding with residues Arg146 and
Asn143 in Bcl-2 throughout the aldehyde group and the adjacent
hydroxyl group on the right naphthalene ring (1). Gossypol is as a Bcl-2 homology domain
3 (BH3)-mimetic inhibitor of antiapoptotic Bcl-2 family members,
including Bcl-2, Bcl-xL and Mcl-1, and induces apoptosis in various
types of cancer (2–4). Gossypol also mediates a number of
signaling pathways, including inhibiting the growth of prostate
cancer cells by modulation of the TGF-β/Akt signaling pathway
(5) and activation of TP53
(6), and enhancement of
radiation-induced apoptosis through the SAPK/JNK pathway (7).
(-)-Gossypol, an optical isomer of gossypol, was
found to significantly inhibit the growth of various tumor cells.
For example, (-)-gossypol has been shown to inhibit the expression
of antiapoptotic proteins, including Bcl-2, Bcl-xL and Mcl-1, and
further induce the expression of apoptosis-associated proteins,
such as Noxa, Puma and Bim, thereby inducing cell apoptosis
(8,9). In vivo studies have
demonstrated that (-)-gossypol presents good antitumor activity in
lymphoma, head and neck tumors (4,10,11).
However, (-)-gossypol has not be used as an antitumor agent due to
a number of limitations, including poor water solubility,
single-route drug administration and low bioavailability. In
addition, at high concentrations, (-)-gossypol may be highly toxic
to the liver and intestinal tract (12).
In order to improve the application of gossypol as
an antitumor agent, the polymer carrier, methoxy polyethylene
glycol-maleimide (mPEG-Mal), was loaded on (-)-gossypol
nanoparticles using an emulsification-volatilization method. The
aim of the present study was to further investigate the toxicity of
the mPEG-Mal polymer carrier and the antitumor effect of
(-)-gossypol nanoparticles.
Materials and methods
Cell lines and reagents
Human prostate RWPE-1 and prostate cancer PC-3 cell
lines were obtained from the Animal Experiment Center of the Fourth
Military Medical University (Xi’an, China), and (-)-gossypol was
obtained from the College of Life Science of Xi’an Jiaotong
University (Xi’an, China). Written informed consent was obtained
from the patient prior to this. The polymer carrier, mPEG-Mal
(5,000 D; Beijing Kaizheng Biotech Development Co., Ltd., Beijing,
China), MTT dye (Shanghai Sangon Biotech Co., Ltd., Shanghai,
China) and acridine orange (AO) dye (One Lambda, Beijing China)
were purchased for the purpose of the experiments. Reverse
transcription polymerase chain reaction (RT-PCR) primers were
synthesized by Shanghai Sangon Biotech Co., Ltd.
Main instruments
The following instruments were used in the
experiments: NuAire AutoFlow CO2 cell incubator (NuAire,
Plymouth, MN, USA); PCR EDC-810 amplifier (Dongsheng Biotech Co.,
Ltd., Beijing, China); multifunctional gel imaging system (GL2200;
Kodak, Rochester, NY, USA); JEM-2000EX transmission electron
microscope (Electronic optical Company, Osaka, Japan); and BX60
inverted fluorescence microscope (Olympus Corporation, Tokyo,
Japan).
Preparation of the mPEG-Mal nanoparticles
and their main features
An emulsification-volatilization method was used to
prepare the loaded (-)-gossypol nanoparticles. Blank nanoparticles
were also prepared using the same method, after which they were
frozen. The average diameter of the nanoparticles was 65.1 nm, the
(-)-gossypol-loading efficiency was 97.5±1.57% and the loading
capacity was 37.5±0.27%. In vitro release experiments
demonstrated that the (-)-gossypol nanoparticles had
controlled-release characteristics.
In vitro detection of the toxicity of
(-)-gossypol nanoparticles using an MTT assay
PC-3 cells were adjusted to a concentration of
5×106 cells/ml and inoculated in 96-well culture plates,
with each well holding up to a volume of 100 μl. Free (-)-gossypol
or (-)-gossypol nanoparticles at different concentrations were
added to a plate (one plate for each concentration of gossypol
nanoparticles), and the final concentrations in the wells were 2.5,
5, 10 or 20 μg/ml. Each well was followed by three duplicate wells.
After 48 h, 20 μl MTT (5 mg/ml) was added and the plates were
cultured for 4 h. The culture solution was centrifugally removed.
Next, 150 μl DMSO was added to each well and the plate was vortexed
for 10 min until the crystals were fully dissolved. ELISA was used
to detect the absorbance (optical density) at a wavelength of 490
nm, and the median inhibitory concentration (IC50) was
calculated. The same method was used to measure the growth
inhibition of the blank nanoparticles (control sample) on the PC-3
and RWPE-1 cells, in order to assess the toxicity of the polymer
carrier.
AO staining
PC-3 cells were inoculated in 96-well plates, with
each well containing 100 μl cell suspension. In each well, 100-μl
samples of the different (-)-gossypol nanoparticle concentrations
were added. For the control group, 100 μl RPMI-1640 culture medium
(ScienCell, Carlsbad, CA, USA), supplemented with 10% fetal bovine
serum (Gibco®, Invitrogen Life Technologies, Grand
Island, NY, USA), was added. After 48 h, 10 μl AO dye (Bio-Teck,
Beijing, China) was added to each well and cultured for 15 min. The
morphological changes were observed under an inverted fluorescence
microscope (Olympus Corporation). Each sample was found to contain
≥100 cells, and the percentage of apoptotic cells was
calculated.
Cellular ultrastructure observations
Cells were inoculated in culture bottles at a
concentration of 2×105 cells/ml, with each bottle
containing 4 ml cell suspension. After 24 h, 4 ml (-)-gossypol
nanoparticles, at a concentration of 10 μg/ml, was added to each
bottle. For the control, 4 ml DMSO (Beyotime, Hangzhou, China) at
the same concentration was added to the RPMI-1640 culture medium.
After 48 h, trypsin was used to digest and wash any unreacted
RPMI-1640 culture medium, and the solution was centrifugally
subsided. Next, 4% glutaraldehyde (Huakang, Suzhou, China) was
added and incubated for 2 h, which was followed by two washes with
phosphate-buffered saline. Osmic acid (1%) that had been precooled
at 4°C was added, and after 1 h, the samples were dehydrated,
embedded in paraffin and cut into 70-nm segments. Uranyl acetate
and citrate staining were used to dye the samples, and their
cellular morphology was observed under a transmission electron
microscope.
Semi-quantitative RT-PCR detection of
Bcl-2 and Bak mRNA expression
The concentration of the cells was adjusted to
5×105 cells/ml. A total of 2 ml cells was added to a
cell culture bottle. Samples from the control group and the
experimental group (containing 10.0 μg/ml (-)-gossypol
nanoparticles) were placed into the cell culture bottle; 8 ml cell
culture fluid was added and cultured for 48 h. The primer design
and the experimental methods followed in these experiments were
based on the methods of a previous study (13).
Statistical analysis
SPSS 10.0 software (SPSS, Inc., Chicago, IL, USA)
was used to conduct statistical analysis. χ2 analysis
and the t-test were used to evaluate the results, where P<0.05
was considered to a indicate statistically significant
difference.
Results
Effect of (-)-gossypol nanoparticles on
the proliferation of PC-3 cells
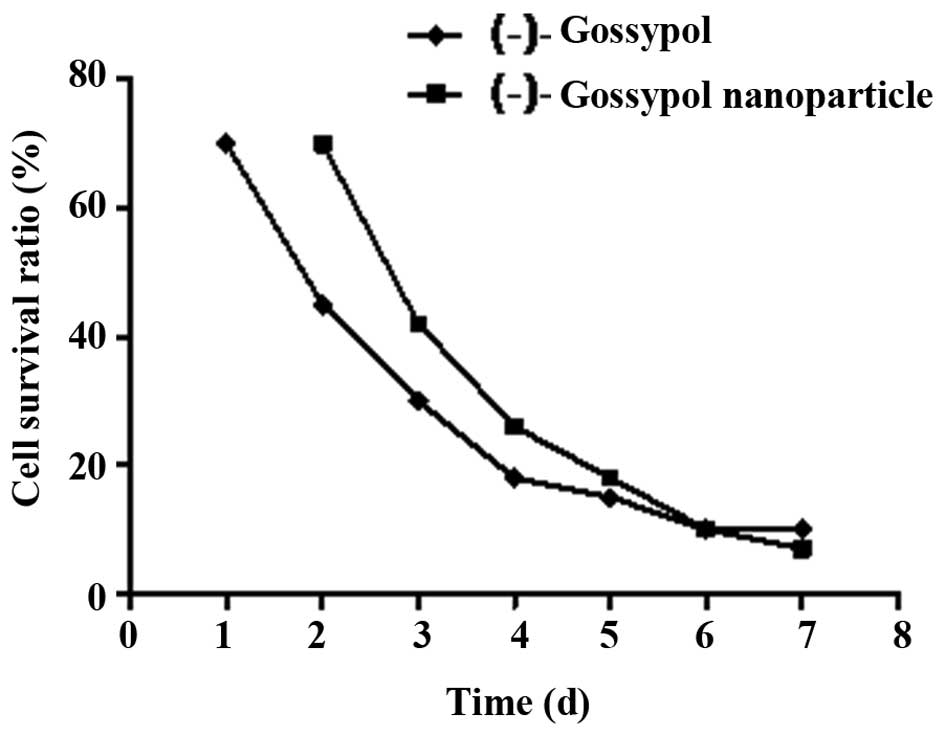
When the (-)-gossypol nanoparticles and free
(-)-gossypol reached a concentration of 10.0 μg/ml, they
demonstrated evident antitumor activity against prostate cancer
PC-3 cells in vitro (Fig.
1). As shown in Fig. 1, the
inhibition effects of (-)-gossypol nanoparticles and free
(-)-gossypol on the proliferation of PC-3 cells increased with
time. In addition, following culture for 72 h, the inhibition
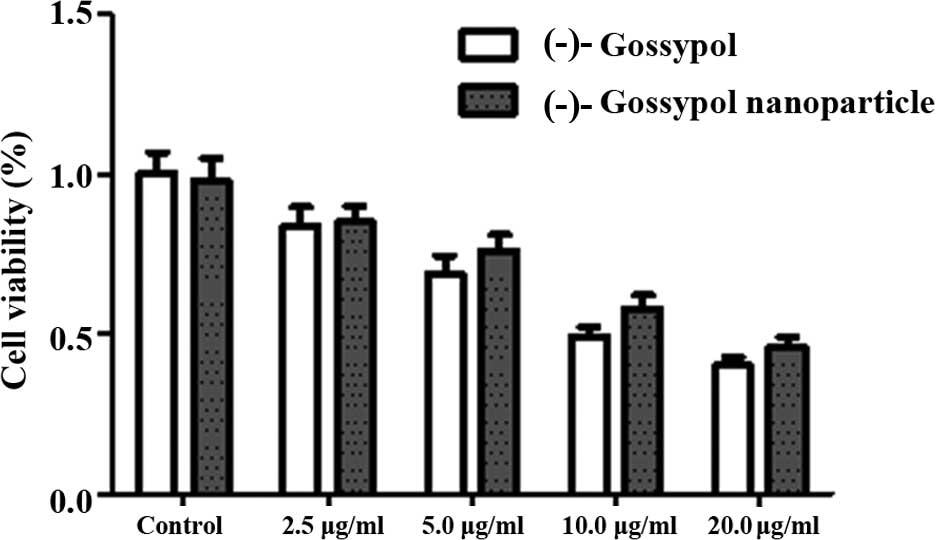
effects of (-)-gossypol nanoparticles and free (-)-gossypol on the
proliferation of PC-3 cells increased with increasing concentration
(Fig. 2). At the various time
points, the IC50 of the (-)-gossypol nanoparticles was
slightly higher compared with the free (-)-gossypol; however, no
statistically significant difference was observed (P>0.05).
Toxicity assessment of the blank
carrier
Following the addition of the blank carrier in the
PC-3 and RWPE-1 cells for 48 h, no evident change was observed with
regard to the survival rate of the cells. When the concentration of
the blank carrier reached 200 μg/ml, the survival rate of the PC-3
and RWPE-1 cells decreased; however, the rate remained >95%, and
no evident change in the cell shape was observed (data not
shown).
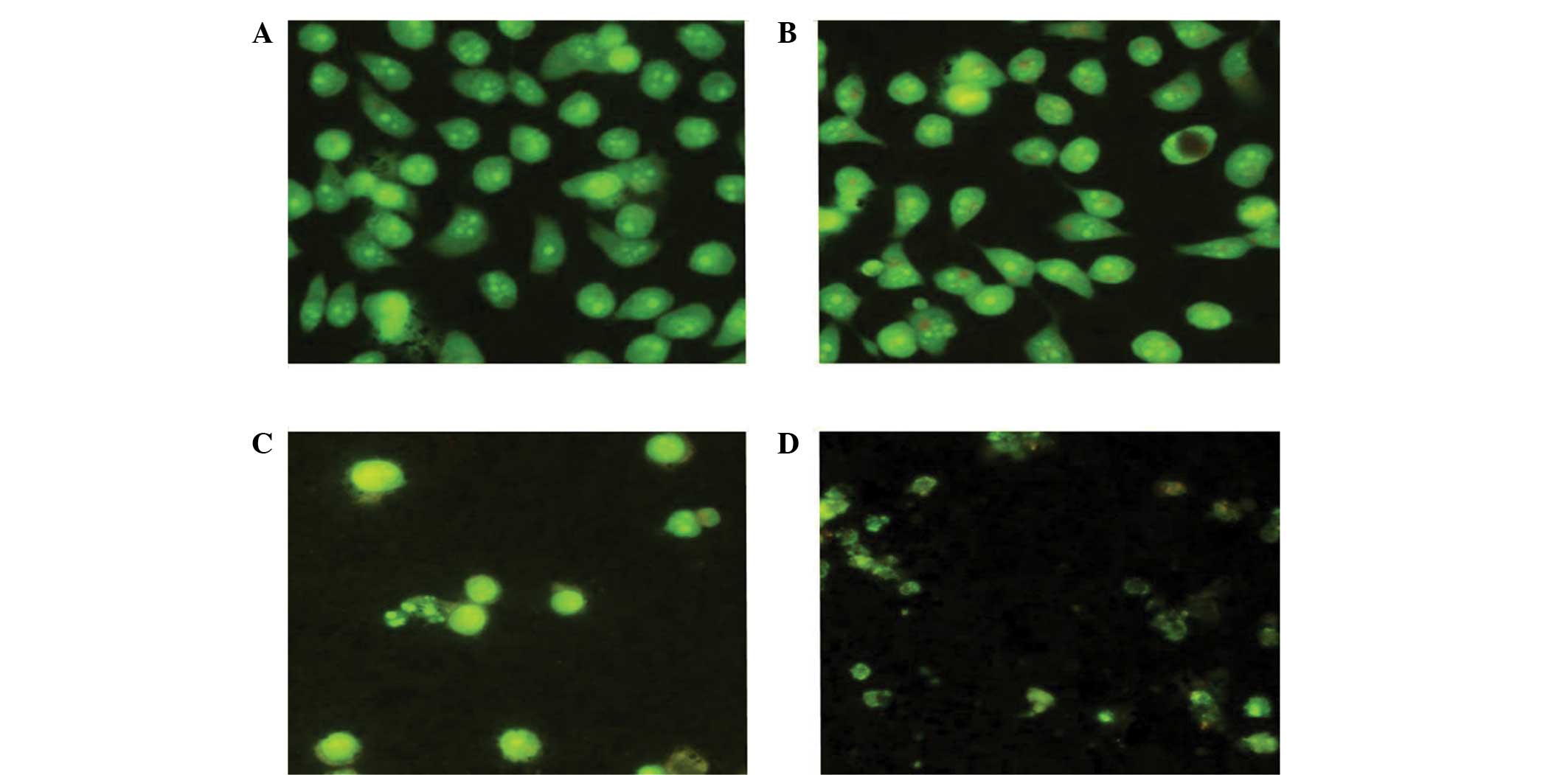
AO staining results
After culturing the control group cells for 48 h,
the cells were concentrated together and the cell chromatin was
evenly distributed (Fig. 3). Upon
culturing with 5.0 μg/ml (-)-gossypol nanoparticles for 48 h, only
part of the cell nucleus was pyknotic and cell apoptosis was
observed. Following culture with 10.0 μg/ml (-)-gossypol
nanoparticles for 48 h, the number of apoptotic cells was markedly
increased, the cell chromatin was not evenly distributed and a
number of cells had burst. After culturing with 20.0 μg/ml
(-)-gossypol nanoparticles for 48 h, the number of cells decreased,
cell apoptosis was evident, the chromatin was arranged along the
nuclear membrane in a crescent-shape and cell fragmentation was
observed.
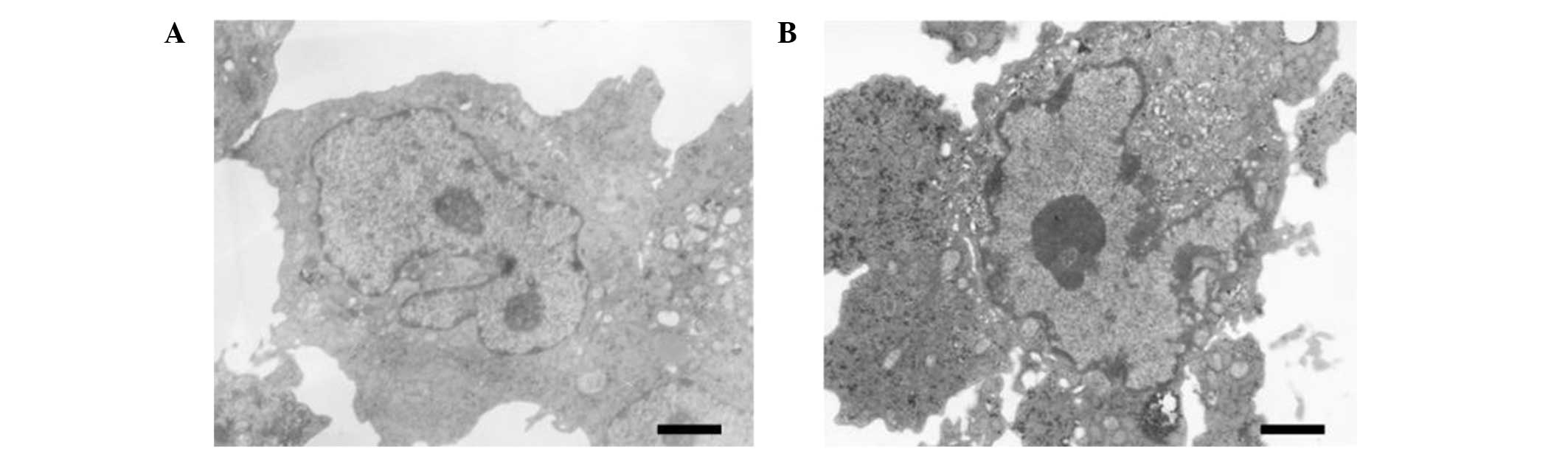
Changes in the cellular
ultrastructure
In the normal PC-3 cells, microvilli were detected
on the surface, small fat droplets and lipofuscin particles were
observed, and the nucleus chromatin was shown to mainly consist of
euchromatin (Fig. 4A). Following
culture with 10.0 μg/ml (-)-gossypol for 48 h, the PC-3 cells
presented typical features of apoptotic cells, including the
disappearance of microvilli from the cell surface, smooth edges and
agglutinated nuclear chromatin that was arranged close to the edge
of the nuclear membrane (Fig.
4B).
Semi-quantitative RT-PCR detection of
Bcl-2 and Bak mRNA expression levels
Through semi-quantitative RT-PCR detection, the size
of the Bcl-2, Bak and GAPDH genes were determined as 387, 360 and
142 bp, respectively, consistent with the expected values.
Following culture with 10.0 μg/ml (-)-gossypol nanoparticles for 48
h, the mRNA expression levels of Bcl-2 were downregulated in the
PC-3 cells, and the Bcl-2/GAPDH ratio decreased from 0.17 to 0.08.
In addition, the mRNA expression levels of Bak were upregulated,
and the Bak/GAPDH ratio increased from 0.62 to 0.89.
Discussion
As traditional in vivo medicine drug
carriers, nanomaterials have become increasingly important in
modern medicine and possess a good application potential (14,15).
PEG is a synthetic polymer material, which can dissolve in water
and is soluble in certain organic solvents. PEG is the only polymer
material approved by the US Food and Drug Administration for use in
food and pharmaceuticals (16).
Coating lipophilic drugs with PEG may improve their solubility and
stability, while reducing or eliminating the body’s rejection of
the drug effects, and lowering the rate of drug metabolism. In
addition, coating drugs with PEG can extend the cycling time of the
drug, improve distribution in the body and diminish any adverse
reactions (17). mPEG-Mal is a
modified PEG polymer material with good biodegradability and
biocompatibility. In addition, the maleimide functional group
allows the polymer to chemically connect with proteins containing a
sulfhydryl group or with antibodies at room temperature. The mild
reaction conditions do not destroy the proteins and the activity of
antibodies, providing the necessary structural basis for future
research on nanoactive targeting drugs.
In the present study, (-)-gossypol nanoparticles
were shown to effectively inhibit the growth of prostate cancer
PC-3 cells in vitro, with their toxicity similar to that of
free (-)-gossypol. In a previous study (18), the slow-release ability of
(-)-gossypol was revealed, with a potential release of ~40% in 48
h. At the same dose, (-)-gossypol nanoparticles release less
compared with free (-)-gossypol, indicating that the antitumor
effect of (-)-gossypol nanoparticles is stronger than that of free
(-)-gossypol. This may result from the ability of nanoparticles to
penetrate into cells through cell endocytosis, which is not
possible for small-molecule drugs (19,20).
With regard to the mechanisms, (-)-gossypol
nanoparticles can function as micromolecule inhibitors to inhibit
the expression of the anti-apoptotic protein Bcl-2 (21). In the present study, apoptosis was
induced in prostate cancer cells and the mechanism was similar to
that observed in previous studies (8,9),
indicating that the preparation of nanoparticles has no effect on
the biological activity and molecular structure of (-)-gossypol.
The preparation process was simple and the reaction conditions were
mild. In addition, the blank carrier was found to be safe and
non-toxic; thus, demonstrated good application potential.
Tumor-bearing animal models should be used in future studies to
further investigate the antitumor effects and pharmacokinetic
properties of nanoparticles, after which active targeting studies
may be performed.
References
|
1
|
Keshmiri-Neghab H and Goliaei B:
Therapeutic potential of gossypol: an overview. Pharm Biol.
52:124–128. 2014. View Article : Google Scholar
|
|
2
|
Wang X, Howell CP, Chen F, Yin J and Jiang
Y: Gossypol - a polyphenolic compound from cotton plant. Adv Food
Nutr Res. 58:215–263. 2009. View Article : Google Scholar
|
|
3
|
Lopez LM, Grimes DA and Schulz KF:
Nonhormonal drugs for contraception in men: a systematic review.
Obstet Gynecol Surv. 60:746–752. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oliver CL, Bauer JA, Wolter KG, et al: In
vitro effects of the BH3 mimetic, (-)-gossypol, on head and neck
squamous cell carcinoma cells. Clin Cancer Res. 10:7757–7763. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stein RC, Joseph AE, et al: A preliminary
clinical study of gossypol in advanced human cancer. Cancer
Chemother Pharmacol. 30:480–482. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Le Blanc M, Russo J, Kudelka AP and Smith
JA: An in vitro study of inhibitory activity of gossypol, a
cottonseed extract, in human carcinoma cell lines. Pharmacol Res.
46:551–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kapoor S: Attenuating effect of gossypol
on tumor growth in systemic malignancies. Cell Biochem Biophys.
67:1551–1552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oliver CL, Miranda MB, Shangary S, et al:
(-)-Gossypol acts directly on the mitochondria to overcome Bcl-2-
and Bcl-X(L)-mediated apoptosis resistance. Mol Cancer Ther.
4:23–31. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meng Y, Tang W, Dai Y, et al: Natural BH3
mimetic (-)-gossypol chemosensitizes human prostate cancer via
Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol
Cancer Ther. 7:2192–2202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mohammad RM, Wang S, Aboukameel A, et al:
Preclinical studies of a nonpeptidic small-molecule inhibitor of
Bcl-2 and Bcl-X(L) [(-)-gossypol] against diffuse large cell
lymphoma. Mol Cancer Ther. 4:13–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wolter KG, Wang SJ, Henson BS, et al:
(-)-Gossypol inhibits growth and promotes apoptosis of human head
and neck squamous cell carcinoma in vivo. Neoplasia. 8:163–172.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang XQ, Huang XF, Mu SJ, et al:
Inhibition of proliferation of prostate cancer cell line, PC-3, in
vitro and in vivo using (-)-gossypol. Asian J Androl. 12:390–399.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang WT, Huang XF, Mu SJ, et al: Study on
the effects of (-)-gossypol in inducing apoptosis of human prostate
cancer PC-3 cells in vitro. Xian Dai Zhong Liu Yi Xue. 12:251–254.
2011.(In Chinese).
|
|
14
|
Allen TM and Cullis PR: Drug delivery
systems: entering the mainstream. Science. 303:1818–1822. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang SX and Li QF: Application on
nano-scale drug vehicles in cancer therapy. Zhong Hua Zhong Liu
Fang Zhi Za Zhi. 17:1031–1034. 2010.(In Chinese).
|
|
16
|
Agarwal A, Saraf S, Asthana A, et al:
Ligand based dendritic systems for tumor targeting. Int J Pharm.
350:3–13. 2008. View Article : Google Scholar
|
|
17
|
Gabizon AA: Pegylated liposomal
doxorubicin: metamorphosis of an old drug into a new form of
chemotherapy. Cancer Invest. 19:424–436. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaza N, Kohli L, Graham CD, et al: BNIP3
regulates AT101 [(-)-gossypol] induced death in malignant
peripheral nerve sheath tumor cells. PLoS One. 9:e967332014.
View Article : Google Scholar
|
|
19
|
Chawla JS and Amiji MM: Biodegradable
poly(epsilon-caprolactone) nanoparticles for tumor-targeted
delivery of tamoxifen. Int J Pharm. 249:127–138. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oyewumi MO, Yokel RA, Jay M, et al:
Comparison of cell uptake, biodistribution and tumor retention of
folate-coated and PEG-coated gadolinium nanoparticles in
tumor-bearing mice. J Control Release. 95:613–626. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu L, Wang G, Tang N, et al: 27th Annual
The Charles A. Coltman, Jr. San Antonio Breast Cancer Symposium:
Discovery and therapeutic potential of novel Bcl-2/Bcl-xL
small-molecule inhibitors in human breast cancer. Breast Cancer Res
Treat. 88(1 Suppl): S662004.
|