Introduction
Pleural effusion may be caused by a variety of
diseases, including malignant tumors, pneumonia, tuberculosis,
pancreatitis and heart failure. However, pleural effusion caused by
multiple myeloma (MM) is extremely rare, with a frequency of only
6%, and is usually associated with benign conditions such as
sepsis, pulmonary embolism, heart failure secondary to amyloidosis
or chronic renal failure. Myelomatous pleural effusion (MPE) is
rare, occurring in <1% of cases, and may result in a delayed
diagnosis of MM (1). Although
diagnostic procedures for MPE have not been well defined, the
preferred methods defined in previously published studies are
pleural fluid cytology or pleural biopsy via a thoracoscope, as
well as bone marrow biopsy (2,3).
Furthermore, diffuse osteoporosis or bone loss may be regarded as
indirect diagnostic evidence for MPE. Despite considerable progress
in the treatment of MM in the past decade, including a number of
drugs with highly active agents, such as thalidomide, bortezomib
and lenalidomide (4,5), the prognosis of MM patients with MPE
remains poor and the median survival time is <4 months (6). The present study reports the case of
a 78-year-old patient who initially presented with bilateral
pleural effusion and elevated adenosine deaminase (ADA) activity,
but was ultimately diagnosed with MPE.
Case report
The study was conducted in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of Taizhou People’s Hospital (Taizhou, China). Written informed
consent was obtained from the patient. The 78-year-old patient was
admitted to the Department of Respiratory Medicine at Taizhou
People’s Hospital in March 2014, complaining of persistent dyspnea
over the preceding 20 days. The patient had no history of smoking
or lung disease. Following admission, a physical examination of the
patient revealed a body weight of 62 kg, a height of 175 cm and a
body temperature of 36.7°C. In addition, the patient had a pulse of
90 bpm, a respiratory rate of 22 bpm and a blood pressure of 120/80
mmHg. The patient appeared fatigued, however, no cyanosis of the
lips was observed. A respiratory examination revealed decreased
breath sounds in the bilateral lower hemithorax, with dullness of
percussion.
Laboratory results of the initial examination were
as follows: Red blood cells, 4.59×1012/l (normal range,
4–5.5×1012/l); hemoglobin, 138 g/l (normal range,
120–160 g/l); white blood cells (WBC), 10.95×109/l
(normal range, 4–10×109/l); platelets,
121×109/l (normal range, 100–300×109/l); and
erythrocyte sedimentation rate, 12 mm/h (normal range, 0–15 mm/h).
In addition, biochemical examinations revealed the following
results: Total serum protein, 69.6 g/l (normal range, 66–87 g/l);
albumin, 28.6 g/l (normal range, 35–54 g/l); globulin, 41 g/l
(normal range, 20–40 g/l); IgD, 127 mg/l (normal range, 1–4 mg/l);
IgG, 11.5 g/l (normal range, 8–17 g/l); IgA, 0.31 g/l (normal
range, 0.7–4.0 g/l); IgM, 0.57 g/l (normal range, 0.4–2.3 g/l);
C-reactive protein, 22.1 mg/l (normal range, 0–5.0 mg/l); serum
carcinoembryonic antigen, 4.67 ng/ml (normal range, 0–6.5 ng/ml);
neuron-specific enolase, 10.01 ng/ml (normal range, 0–20.0 ng/ml);
and CYFRA 21-1, 2.19 ng/ml (normal range, 0.1–3.3 ng/ml). The
levels of lactate dehydrogenase (LDH), electrolytes, glucose and
fat, and the patient’s renal function were all normal and no
Bence-Jones protein was detected in the patient’s urine. Computed
tomography examination of the chest revealed bilateral pleural
effusion and a small amount of pericardial effusion, but no evident
mass lesion (Fig. 1A and B).
Thoracentesis was performed and the pleural effusion was found to
be exudative and slightly bloody. The pleural fluid was shown to
contain 13.1×1012/l WBC (neutrophils, 41%; lymphocytes,
25%; mesothelial cells, 34%), 35.8 g/l total protein, 4,187 U/l LDH
and an elevated total ADA level of 80 U/l (0–35 U/l). In addition,
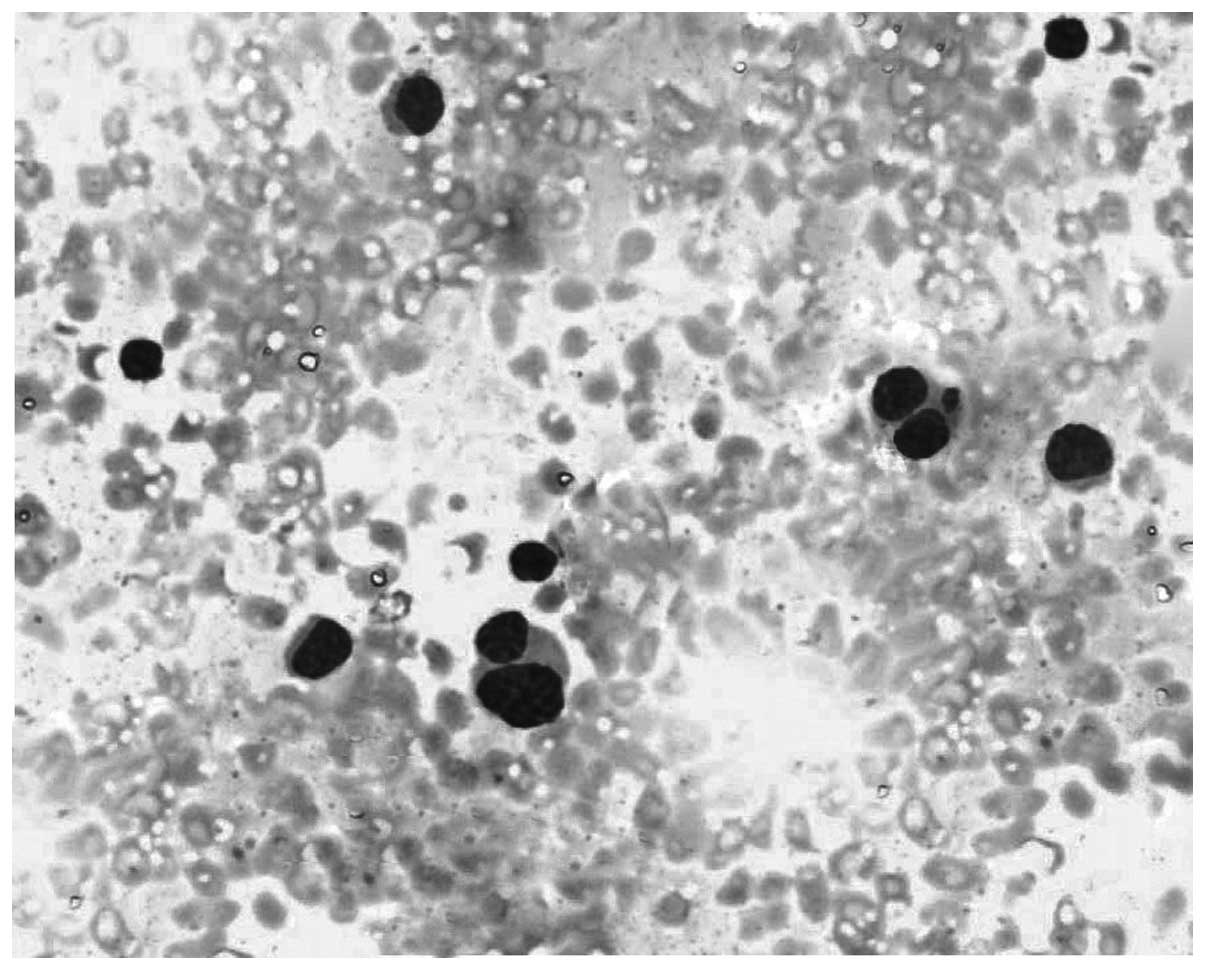
pleural fluid cytology revealed atypical plasma cells with
binucleated forms (Fig. 2). The
results of the mycobacterial and bacterial cultures were negative.
The patient was ultimately diagnosed with MM (IgD type).
Following confirmation of the diagnosis, a bone
marrow biopsy was performed, revealing active hyperplasia of the
lymphoid plasma cells (Fig. 3). A
plain radiograph showed degeneration in the thoracic and lumbar
vertebra, and diffuse osteoporosis in the ilium, pubis and sciatic
nerve. The patient was subsequently transferred to the Department
of Hematology at Taizhou People’s Hospital to receive chemotherapy
with CHOP (cyclophosphamide: 750 mg/m2, i.v., day 1;
doxorubicin: 50 mg/m2, i.v., day 1; vincristine: 2 mg,
i.v., day 1; and prednisolone: 100 mg, p.o., days 1–5) which was
repeated every 21 days. Following two rounds of chemotherapy, the
pleural effusion was reduced and partial remission of MM was
achieved.
Discussion
MM, also known as plasma cell myeloma, is a
malignant plasma cell disorder that accounts for 1% of all
malignant neoplasms and 10% of hematological malignant neoplasms
(7). The main clinical
pathological characteristics of MM are the clonal proliferation of
plasma cells in the bone marrow and the overproduction of
structurally homogeneous immunoglobulins. These changes eventually
lead to a series of clinical symptoms, which include anemia,
hemorrhage, recurrent infection, bone loss, hypercalcemia, renal
failure, highly viscous hematic disease, nervous system damage and
amyloidosis (8).
Fewer than 5% of MM patients present clinically with
apparent extraosseous symptoms (9). Clinicopathological studies have
revealed that the spleen, lymph nodes and liver are the most
frequent sites of MM occurrence. However, the development of MM in
the kidneys, central nervous system, skin, pleura, testes,
pancreas, thyroid, adrenal glands and omentum have also been
reported (10). Cases of MM in
which the respiratory system is involved are extremely uncommon,
particularly where bilateral pleural effusion with an elevated ADA
activity is the only initial symptom. This rarity may result in a
delayed diagnosis of MM. Pleural effusion occurs in ~6% of patients
with MM during the course of the disease, while myelomatous pleural
effusion (MPE) is even rarer, presenting in <1% of patients
(11). Cho et al (2) retrospectively reviewed the records of
734 patients with MM and found that only 19 cases had been
diagnosed with MPE. Kintzer et al (11) reported a study of 958 patients with
MM in which the rate of accompanied pleural effusion was 6.1%
(58/958), while only eight of these cases (0.8%) were diagnosed
with MPE. A number of mechanisms have been proposed for the
pathogenesis of pleural effusion in MM (12), which include the infiltration of
the pleural fluid by malignant plasma cells or directly from
adjacent tissues, nephrotic syndrome secondary to renal tubular
infiltration with paraprotein, the development of glomerular damage
and congestive heart failure secondary to amyloidosis, and
lymphatic drainage obstruction by tumor infiltration.
Diagnosis of MM in the present study was based on
the criteria of the International Myeloma Working Group (13). Increasing levels of awareness and
improved diagnosis of MM have significantly increased the rate at
which MPE has been reported (2,3,11,14).
Although diagnostic procedures for MPE have not been well defined,
the preferred methods in all published studies are pleural fluid
cytology or pleural biopsy via a thoracoscope, as well as bone
marrow biopsy (2,3). In addition, diffuse osteoporosis or
bone loss may be regarded as indirect diagnostic evidence of MPE.
To the best of our knowledge, the positive rate of pleural fluid
cytology is low and closely associated with the competence of
pathologists. Therefore, the diagnostic value of a pleural biopsy
using a thoracoscope for MPE has been associated with a higher
positive rate of diagnosis in previous studies (3,15).
In the present study, significantly elevated ADA
activity was observed in the exudative pleural effusion of the
patient. To the best of our knowledge, the majority of
pulmonologists would interpret the present symptoms as tuberculous
pleural effusion, even though no evidence of active pulmonary
tuberculosis was found in the patient. However, elevated ADA
activity in the pleural fluid has been associated with a number of
malignant neoplasms, including non-Hodgkin’s lymphoma and breast
cancer (14,17). Previous studies have also reported
high levels of ADA in patients with MPE (2,3). Cho
et al (2) hypothesized that
a small fraction of myeloma cells express ADA on their surface,
which may be associated with the high levels of ADA observed in MPE
patients.
There have been major advances in the treatment of
MM in the past decade, with a number of drugs emerging as highly
active agents, including thalidomide, bortezomib and lenalidomide
(4,5,18).
However, the prognosis of MM patients with MPE remains poor and the
median survival time is less than four months (6). Hematopoietic stem cell
transplantation may improve response rates and prolong the median
overall survival rate in MM patients; however, this treatment is
not suitable for patients with MPE who are frequently characterized
by an aggressive clinical course, as well as a bad performance
status.
In conclusion, the occurrence of bilateral pleural
effusion with an elevated ADA activity as the initial symptom in MM
is extremely rare, and may result in a delayed diagnosis. In such
cases, improved awareness of MM as a potential diagnosis and
knowledge concerning the clinical presentation of MPE are key
factors in ensuring timely diagnosis and effective
intervention.
References
|
1
|
Elloumi M, Frikha M, Masmoudi H, et al:
Plasmocytic pleural effusion disclosing multiple myeloma. Rev Mal
Respir. 17:495–497. 2000.(In French). PubMed/NCBI
|
|
2
|
Cho YU, Chi HS, Park CJ, Jang S, Seo EJ
and Suh C: Myelomatous pleural effusion: a case series in a single
institution and literature review. Korean J Lab Med. 31:225–230.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu XL, Shen YH, Shen Q and Zhou JY: A case
of bilateral pleural effusion as the first sign of multiple
myeloma. Eur J Med Res. 18:72013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singhal S, Mehta J, Desikan R, et al:
Antitumor activity of thalidomide in refractory multiple myeloma. N
Engl J Med. 341:1565–1571. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Richardson PG, Blood E, Mitsiades CS, et
al: A randomized phase 2 study of lenalidomide therapy for patients
with relapsed or relapsed and refractory multiple myeloma. Blood.
108:3458–3464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamble R, Wilson CS, Fassas A, et al:
Malignant pleural effusion of multiple myeloma: prognostic factors
and outcome. Leuk Lymphoma. 46:1137–1142. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kyle RA and Rajkumar SV: Multiple myeloma.
Blood. 111:2962–2972. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kyle RA and Rajkumar SV: Multiple myeloma.
N Engl J Med. 351:1860–1873. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin M, Zhu J, Shen H and Huang J:
Gastrointestinal bleeding as an initial symptom in asymptomatic
multiple myeloma: A case report and review of the literature. Oncol
Lett. 5:218–220. 2013.
|
|
10
|
Kapadia SB: Multiple myeloma: a
clinicopathologic study of 62 consecutively autopsied cases.
Medicine (Baltimore). 59:380–392. 1980. View Article : Google Scholar
|
|
11
|
Kintzer JS Jr, Rosenow EC III and Kyle RA:
Thoracic and pulmonary abnormalities in multiple myeloma: A review
of 958 cases. Arch Intern Med. 138:727–730. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alexandrakis MG, Passam FH, Kyriakou DS
and Bouros D: Pleural effusions in hematologic malignancies. Chest.
125:1546–1555. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
International Myeloma Working Group.
Criteria for the classification of monoclonal gammopathies,
multiple myeloma and related disorders: a report of the
International Myeloma Working Group. Br J Haematol. 121:749–757.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oudart JB, Maquart FX, Semouma O, Lauer M,
Arthuis-Demoulin P and Ramont L: Pleural effusion in a patient with
multiple myeloma. Clin Chem. 58:672–674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wan YY, Lin DJ, Guo HS, Li XL, Yao ZH and
Qiu YT: Multiple myeloma with pleural effusion as initial symptom:
a report of one case and literature review. Guo Ji Hu Xi Za Zhi.
30:1097–1099. 2010.(In Chinese).
|
|
16
|
Buyukberber M, Sevinc A, Cagliyan CE,
Gulsen MT, Sari I and Camci C: Non-Hodgkin lymphoma with high
adenosine deaminase levels mimicking peritoneal tuberculosis: an
unusual presentation. Leuk Lymphoma. 47:565–568. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aghaei M, Karami-Tehrani F, Salami S and
Atri M: Adenosine deaminase activity in the serum and malignant
tumors of breast cancer: the assessment of isoenzyme ADA1 and ADA2
activities. Clin Biochem. 38:887–891. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Richardson PG, Sonneveld P, Schuster MW,
et al; Assessment of Proteosome Inhibition for Extending Remissions
(APEX) Investigators. Bortezomib or high-dose dexamethasone for
relapsed multiple myeloma. N Engl J Med. 352:2487–2498. 2005.
View Article : Google Scholar : PubMed/NCBI
|