Introduction
Hand, foot and mouth disease (HFMD) is a highly
contagious viral disease caused by the foot and mouth disease
virus. The most common strains causing HFMD are Coxsackie A virus
A16 and enterovirus 71 (EV-71) (1); however, it may be caused by various
strains of the Coxsackie virus or enterovirus (2). The disease is characterized by severe
vesicular disease in cloven-hoofed animals, including domestic
animals and wild species (3). The
clinical symptoms usually start with fever, followed by oral
vesicles and blisters on the feet. Although these symptoms tend to
be self-limiting in adult animals, HFMD causes immense economic
loss as the epidemic tends to result in the slaughter of an
extremely large number of animals.
In 2008, a large wave of HFMD epidemics occurred in
mainland China, Taiwan, Malaysia, Singapore, Hong Kong and other
places. In mainland China, the epidemics began in Fuyang City in
Anhui province, resulting in 353 severe cases and 22 mortalities,
and then rapidly developed into a national-scale epidemic, covering
28 provinces within 3 months with 345,159 reported cases
(accounting for 70.59% of the total reported cases in the year)
(4,5). The Chinese Ministry of Health has
listed HFMD as a notifiable class-C communicable disease since May
2008 (6,7). According to the national network’s
surveillance data, a total of 5,031,044 cases were officially
reported in China between May 2008 and December 2011.
The magnitude of HFMD epidemics varies in response
to the scale of the original infection challenge, the geographical
distribution of the animal population at risk and the effectiveness
of control efforts during eradication. In dynamic outbreak
situations (where, as a result of pre-emptive culling, the animal
population at risk is constantly changing, and control measures
vary in their effectiveness and intensity of application), the
spatial and temporal components of disease risk change markedly
throughout an epidemic’s course.
In recent years, the HFMD epidemic has rapidly
spread in Liaocheng City, China. The incident cases have increased
year by year and caused serious social harm. Therefore, in this
study we analyzed the prevalence of hand, foot and mouth disease
characteristics and trends in Liaocheng City from 2007 to 2011 and
study the epidemic strain. We analyzed risk factors in several
publications of HFMD, explore prevention and control measures,
preliminarily evaluate the effect of prevention and control, and
provide a scientific basis for prevention and control work in the
future.
Materials and methods
Study area and period
Liaocheng is a prefecture-level city in western
Shandong province, China. Its geographical location is 36.27N and
115.59E, and it has a population of 5.79 million (data from 2011
census). The Round-Robin Database occupies a land area of 8,715
km2. It borders the provincial capital of Jinan to the
southeast, Dezhou to the northeast, Tai’an to the south, and the
provinces of Hebei and Henan to the west.
The city of Liaocheng administers eight county-level
divisions, including one district, one county-level city and six
counties: Dongchangfu district, Linqing City, Yanggu County, Dong’e
County, Chiping County, Gaotang County, Guan County and Shen
County. These are further divided into 134 township-level
divisions.
The period of interest was from January 1, 2007 to
December 31, 2011. Data relating to HFMD cases were obtained from
the National Center for Public Health Surveillance and Information
Services, China Center for Disease Control and Prevention. The date
recorded was the date of symptom onset, and every district was
required to report HFMD cases via the web-based surveillance system
in a unified format, with information including the name, gender,
age and address of patients.
Geographical analyses
For the geographical analysis, techniques available
through the Geographical Information System (GIS) were employed. To
conduct a GIS-based analysis on the spatial distribution of HFMD, a
town-level polygon map at a scale of 1:100,000 was obtained, on
which the town-level point layer containing information with regard
to latitudes and longitudes of central points of each county was
created. Geographical data was used from digital maps from the
National Fundamental Geographic Information System, China
(http://nfgis.nsdi.gov.cn). All HFMD cases were
geo-coded and matched to the town-level polygon and point layers by
the administrative code using the software ArcGIS9.3 (ESRI Inc.,
Redlands, CA, USA).
To assess the risk of HFMD in each town, an excess
hazard map was produced. The excess hazard represents the ratio of
the observed incidence in each town over the average incidence of
all areas; the latter is calculated by the number of cases over the
total number of people at risk instead of the annualized incidence
of a town (8). Mapping raw
estimates of disease occurrence can lead to spurious spatial
features. To overcome this problem spatial empirical Bayes
smoothing was implemented using SpaceStat software (http://www.biomedware.com/?module=Page&sID=spacestat).
Spatial autocorrelation analysis
Spatial autocorrelation analyses were performed
using SpaceStat. Global Moran’s I statistics were used to discern
spatial autocorrelation and detect the spatial distribution pattern
of HFMD in Liaocheng City. Local Moran’s I statistics were used to
examine the local level of spatial autocorrelation and determine
locations of clusters or hotspots. A calculated value of local
Moran’s I Z-score (LMiZScore) ≥1.96 indicated that the town and its
neighboring towns had a HFMD incidence rate statistically
significantly higher than other towns. The number of permutations
was set to 999 and P<0.05 was considered to indicate a
statistically significant difference.
Space-time scan statistic
The spatial scan statistic developed by Kulldorff
(9) implemented in a software
program, SaTScan™ version 9.1 (http://www.satscan.org/), was used to test the
presence of statistically significant spatial as well as space-time
clusters of HFMD and to identify their approximate locations. The
method is defined by a cylindrical window with a circular
geographic base and with height corresponding to time (10). The null hypothesis assumed that the
relative risk (RR) of HFMD was the same within the window compared
with outside.
For this analysis, a Poisson-based model was used,
where the number of events in an area is Poisson distributed
according to a known underlying population at risk (11). The geographical size of the window
was limited to half the expected number of cases and the length of
time was limited to half the total time period (10). The test of significance of the
identified clusters was based on comparing the likelihood ratio
test statistics against a null distribution obtained from Monte
Carlo simulation (12). The number
of permutations was set to 999 and P<0.05 was considered to
indicate a statistically significant difference.
Results
Descriptive analysis of HFMD
Between January 1, 2007 and December 31, 2011, there
were a total of 35,163 HFMD cases reported in Liaocheng City. Of
these, 34,176 (98.73%) had complete information, including mapping
of their place of residence. Annualized average incidence at the
town-level ranged from 2.53% in 2007 to 5.69% in 2009. During the
five-year study period, a summer peak was observed in April and
June with a second smaller peak in July and August, with the
exception of the year 2009, when the peak appearing in April
happened to coincide with the influenza (H1N1) pandemic period
(Fig. 1).
The excess hazard map reveals the distribution of
the excess risk, which was defined as a ratio of the observed
number over the expected number of cases. Towns with an excess
hazard distribution <0.25 had lower incidences than expected, as
indicated by excess risk values <1. In contrast, towns with an
excess hazard distribution >0.4 had higher incidences than
expected or excess risk values >1 (Fig. 2).
A spatial empirical Bayes smoothed map for
annualized average incidence was created by correcting the variance
in the variability of incidence (data not shown). The resulting
smoothed regional estimates demonstrate a variance stabilizing side
effect by using from local or global neighborhood information.
Spatial autocorrelation analysis of
HFMD
The global spatial autocorrelation analyses for
annualized incidence of HFMD in Liaocheng City from 2007 to 2011
demonstrated that the Moran’s I value was significant for every
year (Table I), implying that the
distribution of HFMD was spatially autocorrelated in Liaocheng
City, China.
 | Table IGlobal spatial autocorrelation
analyses for annualized incidence of hand, foot and mouth disease
in Liaocheng City from 2007 to 2011. |
Table I
Global spatial autocorrelation
analyses for annualized incidence of hand, foot and mouth disease
in Liaocheng City from 2007 to 2011.
| Year | Moran’s I | E (I) | SD | P-value | Pattern |
|---|
| 2007 | 0.1440 | −0.0075 | 0.0013 | 0.000026 | Clustered |
| 2008 | 0.3316 | −0.0075 | 0.0029 | 0.000000 | Clustered |
| 2009 | 0.5878 | −0.0075 | 0.0038 | 0.000000 | Clustered |
| 2010 | 0.5263 | −0.0075 | 0.0035 | 0.000000 | Clustered |
| 2011 | 0.5903 | −0.0075 | 0.0036 | 0.000000 | Clustered |
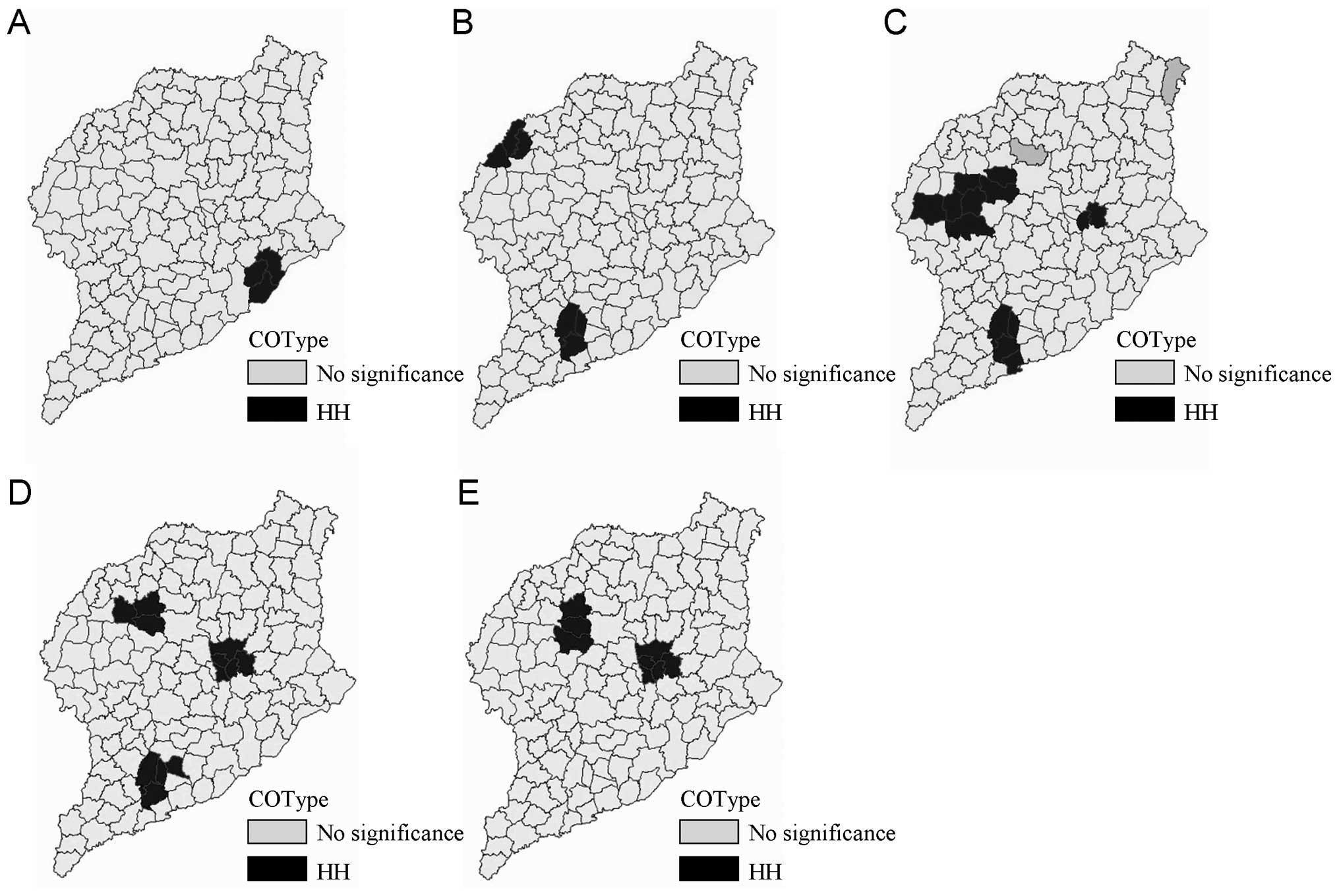
Three significant spatial clusters of HFMD were
identified using the LMiZScore for spatial autocorrelation
(Fig. 3). The hotspots persisted
in Dongchangfu district, Guan county and Yanggu county from 2007 to
2011.
Space-time analysis of HFMD
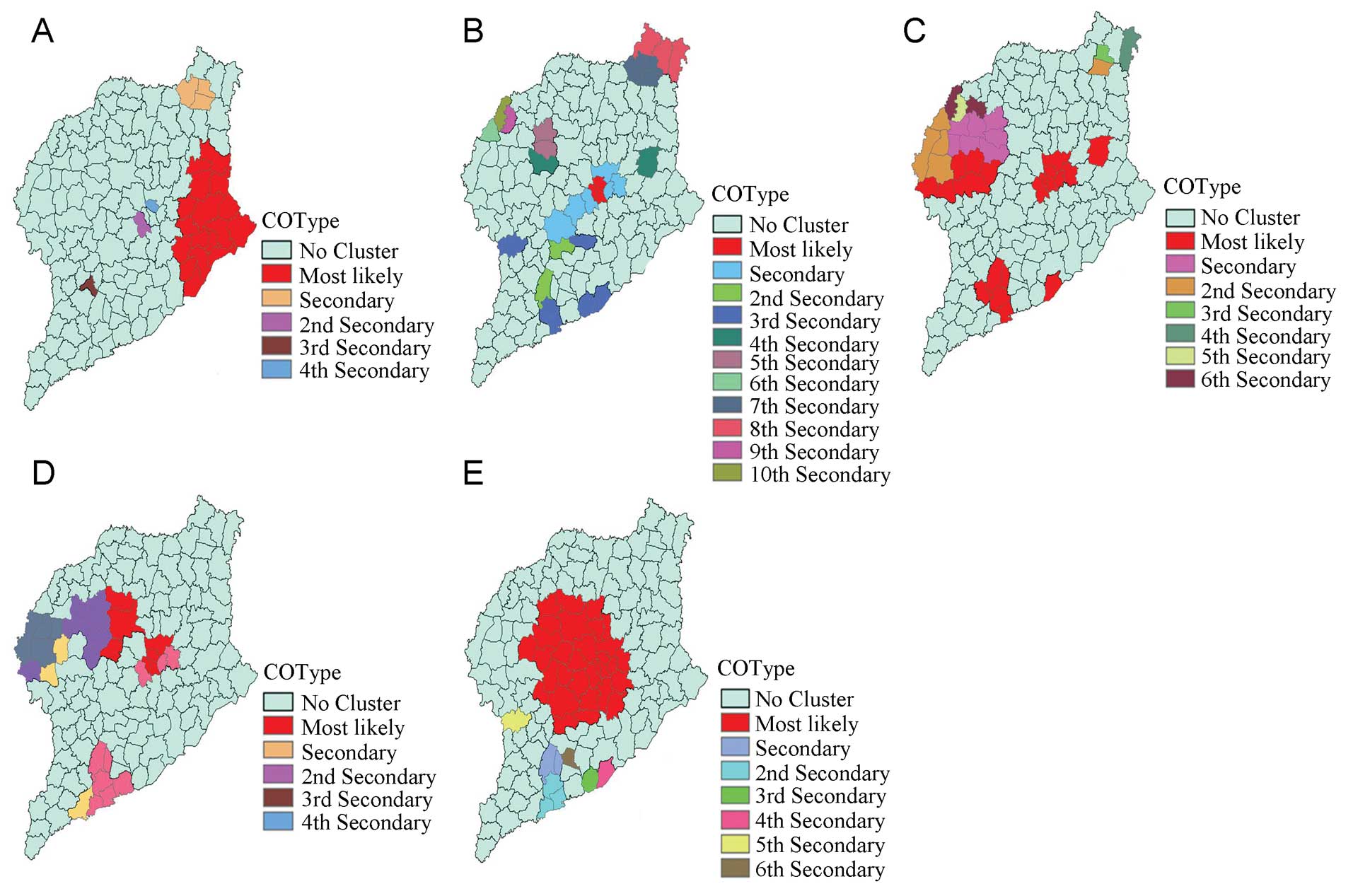
The space-time cluster analysis of HFMD from 2007 to
2011 revealed that HFMD was not distributed randomly in space-time.
The most likely statistically significant cluster for high
incidence of HFMD was found to exist in Dongchangfu district for
the year 2008 (RR=12.83, P<0.01), with 520 observed cases and
235.93 expected cases.
Twenty-five statistically significant secondary
clusters were also detected for high incidence of HFMD. These
results are listed in Table II,
and are also depicted on the map in Fig. 4.
 | Table IISaTScan statistics for space-time
clusters with significant higher incidence in Liaocheng City, China
from 2007 to 2011. |
Table II
SaTScan statistics for space-time
clusters with significant higher incidence in Liaocheng City, China
from 2007 to 2011.
| Time | Cluster type | Cluster areas
(n) | Observed cases | Expected cases | Relative risk | P-value |
|---|
| 2007 | Most likely | 17 | 206 | 52.18 | 6.49 | <0.01 |
| 2007 | Secondary | 3 | 32 | 8.38 | 4.04 | <0.01 |
| 2008 | Most likely | 3 | 85 | 6.91 | 12.83 | <0.01 |
| 2008 | Secondary | 6 | 193 | 61.26 | 3.40 | <0.01 |
| 2008 | 2nd secondary | 3 | 58 | 9.80 | 6.08 | <0.01 |
| 2008 | 3rd secondary | 7 | 223 | 108.40 | 2.20 | <0.01 |
| 2008 | 4th secondary | 2 | 33 | 6.72 | 4.98 | <0.01 |
| 2008 | 5th secondary | 2 | 32 | 8.36 | 3.88 | <0.01 |
| 2008 | 6th secondary | 1 | 27 | 6.31 | 4.33 | <0.01 |
| 2008 | 7th secondary | 3 | 77 | 40.11 | 1.96 | <0.01 |
| 2008 | 8th secondary | 3 | 34 | 12.78 | 2.69 | <0.01 |
| 2009 | Most likely | 20 | 2668 | 912.45 | 3.33 | <0.01 |
| 2009 | Secondary | 7 | 2003 | 866.30 | 2.51 | <0.01 |
| 2009 | 2nd secondary | 5 | 750 | 193.30 | 4.03 | <0.01 |
| 2009 | 3rd secondary | 1 | 137 | 33.44 | 4.13 | <0.01 |
| 2009 | 4th secondary | 1 | 356 | 182.97 | 1.97 | <0.01 |
| 2009 | 5th secondary | 1 | 55 | 8.35 | 6.61 | <0.01 |
| 2009 | 6th secondary | 2 | 242 | 167.53 | 1.45 | <0.01 |
| 2010 | Most likely | 7 | 2117 | 604.05 | 4.10 | <0.01 |
| 2010 | Secondary | 9 | 1262 | 407.26 | 3.37 | <0.01 |
| 2010 | 2nd secondary | 3 | 344 | 57.39 | 6.16 | <0.01 |
| 2010 | 3rd secondary | 7 | 477 | 241.84 | 2.02 | <0.01 |
| 2011 | Most likely | 31 | 2964 | 1230.19 | 4.08 | <0.01 |
| 2011 | Secondary | 2 | 69 | 15.60 | 4.47 | <0.01 |
| 2011 | 2nd secondary | 3 | 52 | 21.29 | 2.46 | <0.01 |
Discussion
Cluster analyses are essential in epidemiology in
order to detect aggregation of disease cases, and to test the
occurrence of any statistically significant clusters. Cluster
analysis identifies whether geographically grouped cases of disease
can be explained by chance or are statistically significant. It
detects true clusters of disease from cases grouped around
population centers. The use of GIS with spatial statistics
including spatial filtering and cluster analysis has been applied
to a number of diseases to analyze and more clearly demonstrate the
spatial patterns of these diseases (13–18).
Spatial scan statistics (19)
implemented in SaTScan software are being widely used to detect
clusters of various diseases worldwide (10,19–29).
The results of our space-time analyses clearly demonstrate that the
HFMD outbreaks were clustered in space and time in Liaocheng City
during the four years studied. The study revealed that the spatial
distribution of HFMD in Liaocheng City was non-random and clustered
with a significant Moran’s I value every year. LMiZScore detected
25 significant spatial clusters for high incidence of HFMD when
only space distribution was considered.
The results of the present study provide useful
information on the prevailing epidemiological situation of HFMD in
Liaocheng City. This new knowledge of the presence of clusters of
HFMD in Liaocheng City may help the Liaocheng Institute to
intensify their remedial measures in the identified areas of high
HFMD prevalence and determine future strategies for more effective
HFMD control. The district health authorities should put more focus
into controlling the spread of HFMD in the district. In particular,
vigorous efforts are required to intensify case-finding activities
in these three HFMD-infested areas of the district. Compulsory HFMD
immunization of children, increased coordination between the
government and private sector, further promotion of general health
and hygiene, and improvement in the nutritional status of the towns
analyzed in the study may lead to better control of HFMD in the
district.
The present study only analyzes the statistically
significant clusters of HFMD in Liaocheng City. Future research
could focus on the effect of various socio-economic and
environmental factors on the high occurrence of HFMD. It is an
established fact that the incidence of HFMD increases with age.
There are several other risk factors responsible for the disease,
including malnutrition. This adversely affects the immune system,
and may therefore enhance HFMD incidence. The factors mainly
responsible for the high occurrence of HFMD may be attributable to
the poor socio-economic conditions of the inhabitants and poor
nutrition. Now that the statistically significant clusters of HFMD
have been identified in the region, a survey-based study is planned
to identify the role of these factors in the spread of HFMD.
The present study has revealed the presence of three
hotspots of HFMD in Liaocheng City, China. Spatial statistics and
GIS may provide public health officials with necessary feedback on
the prevalence of statistically significant clusters of HFMD in the
region, and thus enable them to develop more effective strategies
to control HFMD. Since the efficacy of HFMD control measures in
specific areas could be assessed by a longitudinal change in HFMD
prevalence, the space-time scan statistics also may contribute to a
health program evaluation. More detailed individual-level
investigations are needed in the identified clusters to evaluate
the most significant determinants of disease distribution.
References
|
1
|
Shang L, Xu M and Yin Z: Antiviral drug
discovery for the treatment of enterovirus 71 infections. Antiviral
Res. 97:183–194. 2013. View Article : Google Scholar
|
|
2
|
Deng X, Jia C, Chen F, Liu J and Zhou Z:
Effects of heat stress on the expression of the coxsackievirus and
adenovirus receptor in mouse skin keratinocytes. Exp Ther Med.
6:1029–1033. 2013.PubMed/NCBI
|
|
3
|
Alexandersen S, Zhang Z and Donaldson AI:
Aspects of the persistence of foot-and-mouth disease virus in
animals - the carrier problem. Microbes Infect. 4:1099–1110. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Y, Zhu Z, Yang W, Ren J, Tan X, Wang
Y, Mao N, Xu S, Zhu S and Cui A: An emerging recombinant human
enterovirus 71 responsible for the 2008 outbreak of hand foot and
mouth disease in Fuyang city of China. Virol J. 7:942010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
The Ministry of Health of The People’s
Republic of China. Ministry of Health of The People’s Republic of
China reported national notifiable infectious diseases from
January, 2008 to January, 2009. Gazette Minist Health People’s
Repub China. 65–68. 2009.(in Chinese).
|
|
6
|
The Ministry of Health of The People’s
Republic of China. Guide for the preparedness and control measures
of hand, foot, and mouth disease in China. (2008 version). Cap J
Public Health. 146–148. 2008.(in Chinese).
|
|
7
|
Chinese Center for Disease Control and
Prevention (China CDC). National incidence and death cases of
notifiable class A or class B infectious disease
(2008,2009,2010,2011). http://www.chinacdc.cnuri.
Accessed March 14, 2013
|
|
8
|
Anselin L, Syabri I and Kho Y: GeoDa: An
introduction to spatial data analysis. Geogr Anal. 38:5–22. 2006.
View Article : Google Scholar
|
|
9
|
Kulldorff M: A spatial scan statistic.
Communi Statist Theory Meth. 26:1481–1496. 1997. View Article : Google Scholar
|
|
10
|
Kulldorff M, Athas WF, Feurer EJ, Miller
BA and Key CR: Evaluating cluster alarms: a space-time scan
statistic and brain cancer in Los Alamos, New Mexico. Am J Public
Health. 88:1377–1380. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kulldorff M, Heffernan R, Hartman J,
Assuncao R and Mostashari F: A space-time permutation scan
statistic for disease outbreak detection. PLoS Med. 2:e592005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kulldorff M, Feuer EJ, Miller BA and
Freedman LS: Breast cancer clusters in the northeast United States:
a geographic analysis. Am J Epidemiol. 146:161–170. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Curtis A: Using a spatial filter and a
geographic information system to improve rabies surveillance data.
Emerg Infect Dis. 5:603–606. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Frank C, Fix AD, Pena CA and Strickland
GT: Mapping Lyme disease incidence for diagnostic and preventive
decisions, Maryland. Emerg Infect Dis. 8:427–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Glass GE, Schwartz BS, Morgan JM, Johnson
DT, Noy PM and Israel E: Environmental risk factors for Lyme
disease identified with geographic information systems. Am J Public
Health. 85:944–948. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morrison AC, Getis A, Santiago M,
Rigau-Perez JG and Reiter P: Exploratory space-time analysis of
reported dengue cases during an outbreak in Florida, Puerto Rico,
1991–1992. Am J Trop Med Hyg. 58:287–298. 1998.PubMed/NCBI
|
|
17
|
Mott KE, Nuttall I, Desjeux P and Cattand
P: New geographical approaches to control of some parasitic
zoonoses. Bull World Health Organ. 73:247–257. 1995.PubMed/NCBI
|
|
18
|
Zeman P: Objective assessment of risk maps
of tick-borne encephalitis and Lyme borreliosis based on spatial
patterns of located cases. Int J Epidemiol. 26:1121–1129. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang L, Kulldorff M and Gregorio D: A
spatial scan statistic for survival data. Biometrics. 63:109–118.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Michelozzi P, Capon A, Kirchmayer U,
Forastiere F, Biggeri A, Barca A and Perucci CA: Adult and
childhood leukemia near a high-power radio station in Rome, Italy.
Am J Epidemiol. 155:1096–1103. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Viel JF, Arveux P, Baverel J and Cahn JY:
Soft-tissue sarcoma and non-Hodgkin’s lymphoma clusters around a
municipal solid waste incinerator with high dioxin emission levels.
Am J Epidemiol. 152:13–19. 2002. View Article : Google Scholar
|
|
22
|
Turnbull BW, Iwano EJ, Burnett WS, Howe HL
and Clark LC: Monitoring for clusters of disease: application to
leukemia incidence in upstate New York. Am J Epidemiol.
132:S136–S143. 1990.PubMed/NCBI
|
|
23
|
Hjalmars U, Kulldorff M, Gustafsson G and
Nagarwalla N: Childhood leukaemia in Sweden: using GIS and a
spatial scan statistic for cluster detection. Stat Med. 15:707–715.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sheehan TJ and DeChello LM: A space-time
analysis of the proportion of late stage breast cancer in
Massachusetts, 1988 to 1997. Int J Health Geogr. 4:152005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cousens S, Smith PG, Ward H, Everington D,
Knight RS, Zeidler M, Stewart G, Smith-Bathgate EA, Macleod MA and
Mackenzie J: Geographical distribution of variant Creutzfeldt-Jakob
disease in Great Britain, 1994–2000. Lancet. 357:1002–1007. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Y, Liu H, Xing J, Yang H, Ma X, Xu J,
Shi J and Yan G: Molecular typing of mycobacterium tuberculosis
isolates circulating in Henan, central China. Exp Ther Med.
4:949–953. PubMed/NCBI
|
|
27
|
Sabel CE, Boyle PJ, Loytonen M, Gatrell
AC, Jokelainen M, Flowerdew R and Maasilta P: Spatial clustering of
amyotrophic lateral sclerosis in Finland at place of birth and
place of death. Am J Epidemiol. 157:898–905. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Green C, Hoppa RD, Young TK and Blanchard
JF: Geographic analysis of diabetes prevalence in an urban area.
Soc Sci Med. 57:551–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu Y, Ding S, Liang Y, Zheng Y, Li W, Yang
L, Zheng X and Jiang J: Expression of ERCC1, TYMS, TUBB3, RRM1 and
TOP2A in patients with esophageal squamous cell carcinoma: A
hierarchical clustering analysis. Exp Ther -Med. 7:1578–1582.
2014.PubMed/NCBI
|