Introduction
Ganglioneuromas (GNs) of the gastrointestinal (GI)
tract are rare tumors characterized by hyperplasia of ganglion
cells, nerve fibers and supporting cells. Shekitka et al
(1) divided GNs into three groups:
Polypoid GN, ganglioneuromatous polyposis (GP) and diffuse
ganglioneuromatosis (DG). Polypoid GN is the most common type, a
benign solitary polyp involving the mucosa and submucosa that
resembles an adenoma or a juvenile polyp. GP is usually
distinguished by numerous discrete sessile or pedunculated mucosal
and/or submucosal lesions mimicking familial adenomatous polyposis,
which may be associated with multiple cutaneous lipomas and a
family history of multiple intestinal polyps (2). DG is characterized by a transmural
proliferation of the neural plexus in the bowel wall and is closely
associated with neurofibromatosis type 1 (NF 1) (3) and multiple endocrine neoplasia type
2B (MEN 2B) (4). Schwannomas of
the GI tract have been reported relatively rarely and have occurred
predominantly in the stomach, accounting for 3.3–12.8% of all GI
mesenchymal tumors (5,6). DG with multiple schwannomas is a rare
condition. It is yet to be elucidated whether the occurrence of DG
with multiple schwannomas is incidental or whether the two lesions
are connected through a causal association. The present case report
describes a male with DG of the GI and schwannomas. In combination
with the relevant literature, the diagnosis and treatment of the
patient are discussed in the present study.
Case report
Patient history
In October 2012, a 54 year-old Chinese male was
admitted to West China Hospital (Chengdu, China) with a one-month
history of intermittent bloody stools and abdominal pain, without
diarrhea or vomiting. The colonoscopy revealed >50 sessile,
bead-like polyps ranging grossly in size from 0.1 to 8 cm
throughout the entire colon. The patient also underwent an
esophagogastroduodenoscopy to exclude other similar lesions in the
GI. Endoscopic examination and biopsy specimens from the gastric
cardia revealed no specific histopathology. No pigmented skin
lesions were identified on physical examination. Tumor marker
studies revealed that calcitonin, α-fetoprotein, carcinoembryonic
antigen and cancer antigen 19-9 levels were normal.
The patient gave a medical history of two previous
laparotomies in a local hospital. The first time was 43 years
previously when the patient was 11 years old. At this time, the
patient was admitted to a local hospital with colicky abdominal
pain, vomiting and the passage of bloody stools. From the
laparotomy, a small intestinal intussusception was identified and
reduced. Resection of a segment of the small intestine and a
primary anastomosis were carried out. A polyp was found in the
small intestine but the patient could not remember any details of
the pathological diagnosis.
The second laparotomy was eight years previously at
the age of 46. The patient was admitted to a local hospital with
abdominal pain. The colonoscopy showed multiple polyps in the small
intestinal, which were removed by surgery. Since the pathological
change was uncommon, the slices of specimen were transferred to
West China Hospital for consultation.
There was no history of polyposis or colonic disease
among the parents, siblings or children of the proband. The patient
and his family had no known history of familial adenomatous
polyposis, NF 1, MEN 2B or Cowden syndrome (CS).
Diagnosis
The specimen that was sent to the hospital consisted
of the ascending, transverse and descending colons of the splenic
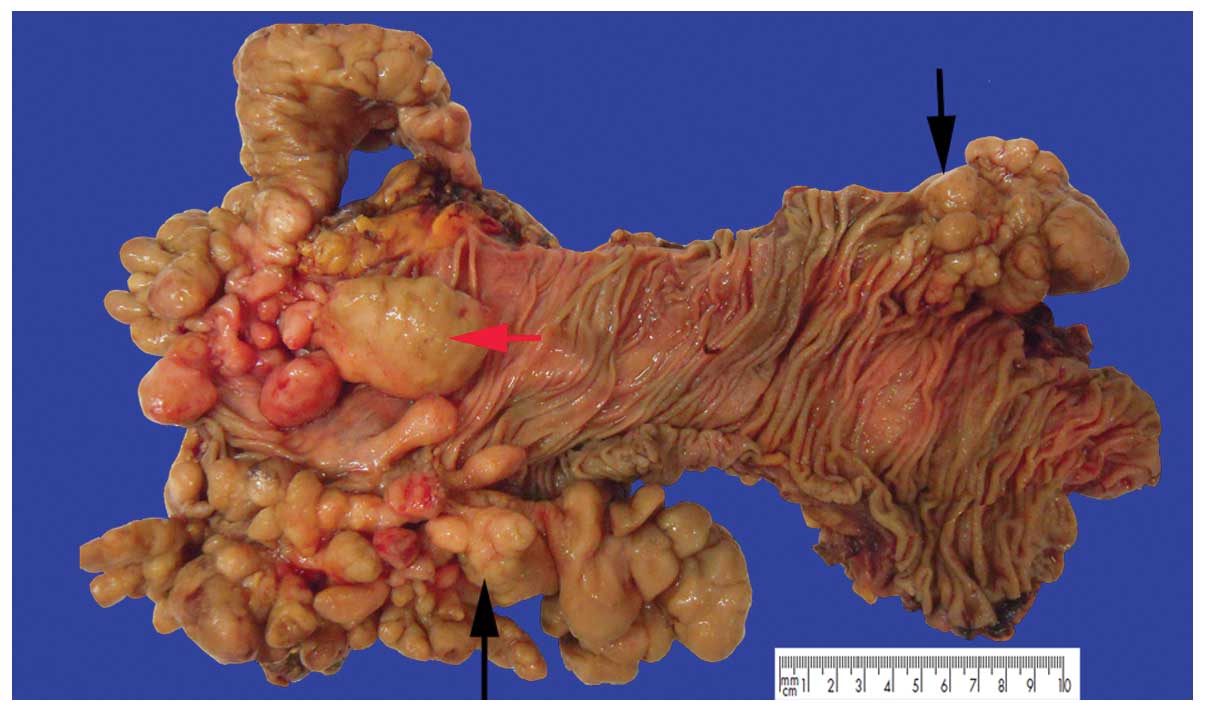
flexure. The specimen measured 30 cm in length. There was a diffuse
thickening of the intestinal wall and no evidence of perforation.
Numerous (50 to 80) sessile or pedunculated polyps ranging in size
from 0.1 to 8 cm in the colon were observed. The sessile polyps
were small, linked together and hard to count, and produced
stricture-like thickenings of segments of the bowel. By contrast,
the pedunculated polyps were large, with diameters ranging from 1
to 5.2 cm, and formed large, irregular, nodular lesions. The
overlying mucosa between the lesions was intact (Fig. 1). Two histological growth patterns
were identified: i) The proliferation of ganglion and nerve sheath
cells was mainly found in the lamina propria and submucosa
(Fig. 2A), and constituted the
predominant lesions of the colon; ii) a plexiform or band-like
enlargement of the nerve fibers and ganglion cells was observed in
the myenteric plexuses (Fig. 2B).
The ganglion cells were large, with rich eosinophilic cytoplasm,
enlarged nuclei and prominent nucleoli; however, no significant
nuclear pleomorphism or mitotic activity was noted. In addition,
large numbers of eosinophils and sparse histiocytes, lymphocytes
and neutrophils were observed. The lesion was transmural, without
the involvement of the subserosa or the serosal fat.
Immunohistochemical (IHC) stains revealed that the ganglion cells
were positive for neuron-specific nuclear protein (NeuN) (Fig. 2C), neurofilament (NF) and
neuron-specific enolase, while the nerve fibers were negative for
NeuN and cluster of differentiation (CD) 117, but positive for NF
(Fig. 2D) and S100. The positive
rate of Ki67 in the ganglion cells and nerve fibers was <2%. A
review of the hematoxylin and eosin and IHC slices from eight years
previously, which showed similar findings, also confirmed the
pathological diagnosis of DG.
 | Figure 2Histology of diffuse
ganglioneuromatosis. (A) Ganglion and nerve sheath cell
proliferation was predominantly found in the lamina propria and
submucosa (HE staining; magnification, ×400). The ganglion cells
were large, with rich eosinophilic cytoplasm, enlarged nuclei and
prominent nucleoli. (B) Plexiform or band-like enlargement of the
nerve fibers and ganglion cells was observed in the myenteric
plexuses (HE staining; magnification, ×100). (C) Ganglion cells
were positive for NeuN, while the nerve fibers were negative for
NeuN (IHC staining; magnification, ×400). (D) Nerve fibers were
positive for S100, while the ganglion cells were negative for S100
(IHC staining; magnification, ×400). HE, hematoxylin and eosin;
IHC, immunohistochemical; NeuN, neuron-specific nuclear
protein. |
In addition to the DG, >30 nodules of schwannomas
without capsules, ranging in size from 0.1 to 1.5 cm, were found in
the subserosa, some even involving the surrounding adipose tissue.
These showed typical features of schwannoma, with Antoni A (cells
forming a typical palisade arrangement in a well-organized pattern)
and Antoni B (loose cells without palisade architecture) regions in
variable proportions (Fig. 3A) and
cells with strong positivity for S100 protein (Fig. 3B) but negative results for CD117,
discovered on gastrointestinal stromal tumors protein 1 (DOG1),
desmin, smooth muscle actin (SMA), CD34, CgA and pancytokeratin, as
determined using IHC assay.
Treatment and follow-up
The patient underwent surgery and made an uneventful
recovery. The patient also received Traditional Chinese Medicine to
coordinate the intestines and stomach, but was not administered any
other treatments. A follow-up observation with repeat endoscopic
evaluation was performed once every six months; two years later the
patient showed no evidence of recurrence and was in good
health.
Discussion
GP is characterized by aggregates of ganglion cells
and nerve fibers within the colonic mucosa, while DG can be mucosal
or transmural with a diffuse, band-like enlargement of nerve fibers
and ganglion cells of the submucosal and myenteric plexuses and
more pronounced changes in the latter (1,7).
Notably, the present case appeared to combine the two patterns of
histological change together with involvement from the lamina
propria to the muscularis propria (Fig. 4). The condition was classified as
DG due to its transmural growth pattern and giant polyps. The
intramural growth pattern and florid hyperplasia of the submucosal
or myenteric plexus are distinct to DG and often occur with NF 1
and the first manifestations of MEN 2B.
NF 1, also named Von Recklinghausen’s disease, is an
autosomal dominant disease characterized by mucocutaneous
neurofibromas and café-au-lait spots and involves numerous organs,
including the GI (8). NF 1 is
caused by the mutation of a gene on chromosome 17 that is
responsible for the control of cell division. The patient in the
present case showed no skin pigment deposition or skin
neurofibromas, and no osseous lesion or optic glioma by computed
tomography. MEN 2B (medullary thyroid carcinoma, pheochromocytoma,
oral mucosal neuromas and skeletal deformities) typically manifests
before a child reaches 10 years of age. Variations in the RET
proto-oncogene cause MEN 2B, and DNA analysis for M918T mutation is
the preferred method of establishing the diagnosis (9,10).
The patient in the present case had none of the manifestations
associated with NF 1 or MEN 2B.
The patient had a long disease history of 43 years.
Although there was insufficient pathological evidence to support a
diagnosis of DG when the patient was 11 years old, the possibility
of DG could not be excluded. The patient underwent three surgical
procedures due to polyps; the main symptoms were abdominal pain and
bloody stools. The recurrence of the DG may have been associated
with the extensive involvement of the lesions and incomplete
excision. The lesions were so extensive that it was difficult to
achieve a complete excision, even in the third surgery. The growth
index, Ki67, of the ganglion cells and nerve fibers was <2%, and
no mitotic figures or nuclear pleomorphism were observed, which
suggested that DG exhibits an indolent or benign biological
behavior. Malignant transformation of DG itself has not been
reported, to the best of our knowledge; however, there are a number
of case reports of DG coincident with adenocarcinoma (11–14),
carcinoid tumor (15) or malignant
peripheral nerve sheath tumor (1,16).
Kanter et al (17)
suggested that DG should be considered as a premalignant condition,
but this association is controversial (13,18).
Genetic testing has revealed PTEN mutations in a handful of DGs
with CS. Heald et al (19)
reported that nine (13%) out of a total of 65 patients undergoing
colonoscopy who were PTEN mutation carriers were diagnosed with
colorectal cancer, all before the age of 50 years; however, the
pathogenesis of these ganglioneuromatous lesions is yet to be
elucidated. It is not known whether or not such an association with
malignant tumors is a simple incidental coexistence or whether the
two lesions are connected by a causal association.
Schwannomas in the GI tract are rare, while
occurrence in the colon is extremely rare (20). Schwannomas are derived from the
Schwann cells that form the neuronal sheath (21), while DG is believed to represent an
unusual hyperplasia of the nerve plexuses, including a mixed
hyperplasia of three types of cells (neuronal cells, nerve fibers
and supporting cells). The presence of ganglion cells in DG makes
the condition notably different from schwannoma. In general,
schwannomas behave in a benign manner; however, they have the
tendency to recur locally and become malignant if left untreated
(5,22). Occasionally, benign schwannomas may
invade several layers of the bowel wall and even involve the
surrounding adipose tissue (22).
The immunophenotype of schwannomas differs from that of other
intestinal mesenchymal neoplasms, such as smooth muscle tumors, GI
stromal tumors and neurofibromas. This difference in
immunophenotype is represented by the spindle cells of schwannomas
showing strong diffuse positivity for S100 and an absence of
staining for CD117, DOG1, CD34, desmin, SMA and actin. With regard
to neurofibromas in the GI, tumors consist of a mixture of spindle
cells with wavy nuclei and strands of collagen, as well as Schwann
cells and perineural fibroblasts. Tumor cells are also weakly
positive for S100. Wavy nuclei and strands of collagen can
facilitate differentiation (23).
Treatment of GNs depends on the number, size,
anatomical location and clinical history (24). Polypectomy with hot biopsy forceps
is curative for polypoid GN; therefore, radical surgery is the
accepted standard treatment of GP and DG. Radiotherapy or
chemotherapy is not recommended. Due to the association that exists
between DG and other systemic diseases, it has been suggested that
patients with DG should undergo careful screening for malignancies
in the colon and elsewhere, as well for the two associated
syndromes (25); however, certain
authors have stated that this screening is unnecessary due to the
benign nature, slow-growing and indolent behavior of DGs (26). Furthermore, screening could lead to
over-treatment, increased financial costs and health risks due to
increased endoscopic surveillance.
In conclusion, the present study describes the case
of a male with recurrent DG of the GI who developed multiple
schwannomas in the subserosa of the colon. To the best of our
knowledge, this case report of the synchronous occurrence of DG and
schwannomas in the colon is extremely rare. The nature and
significance of this association are unclear. The two diseases are
slow growing and well-differentiated neurogenic neoplasias. In this
particular case, it was not known whether there was an association
between the DG and schwannomas or whether their coexistence was
coincidental. Further studies are required to clarify the molecular
alterations in such cases and reveal the etiology of this
association.
References
|
1
|
Shekitka KM and Sobin LH: Ganglioneuromas
of the gastrointestinal tract. Relation to Von Recklinghausen
disease and other multiple tumor syndromes. Am J Surg Pathol.
18:250–257. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weidner N, Flanders DJ and Mitros FA:
Mucosal ganglioneuromatosis associated with multiple colonic
polyps. Am J Surg Pathol. 8:779–786. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fuller CE and Williams GT:
Gastrointestinal manifestations of type 1 neurofibromatosis (von
Recklinghausen’s disease). Histopathology. 19:1–11. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brauckhoff M, Gimm O, Weiss CL, et al:
Multiple endocrine neoplasia 2B syndrome due to codon 918 mutation:
clinical manifestation and course in early and late onset disease.
World J Surg. 28:1305–1311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hou YY, Tan YS, Xu JF, et al: Schwannoma
of the gastrointestinal tract: a clinicopathological,
immunohistochemical and ultrastructural study of 33 cases.
Histopathology. 48:536–545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Daimaru Y, Kido H, Hashimoto H and Enjoji
M: Benign schwannoma of the gastrointestinal tract: a
clinicopathologic and immunohistochemical study. Hum Pathol.
19:257–264. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soccorso G, Puls F, Richards C, et al: A
ganglioneuroma of the sigmoid colon presenting as leading point of
intussusception in a child: a case report. J Pediatr Surg.
44:e17–e20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Agaimy A, Märkl B, Kitz J, et al:
Peripheral nerve sheath tumors of the gastrointestinal tract: a
multicenter study of 58 patients including NF1-associated gastric
schwannoma and unusual morphologic variants. Virchows Arch.
456:411–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krampitz GW and Norton JA: RET gene
mutations (genotype and phenotype) of multiple endocrine neoplasia
type 2 and familial medullary thyroid carcinoma. Cancer.
120:1920–1931. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wells SJ Jr, Pacini F, Ribinson BG and
Santoro M: Multiple endocrine neoplasia type 2 and familial
medullary thyroid carcinoma: an update. J Clin Endocrinol Metab.
98:3149–3164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trufant JW, Greene L, Cook DL, et al:
Colonic ganglioneuromatous polyposis and metastatic adenocarcinoma
in the setting of Cowden syndrome: a case report and literature
review. Hum Pathol. 43:601–604. 2012. View Article : Google Scholar
|
|
12
|
Qiao S, Iwashita T, Ichihara M, et al:
Increased expression of glial cell line-derived neurotrophic factor
and neurturin in a case of colon adenocarcinoma associated with
diffuse ganglioneuromatosis. Clin Neuropathol. 28:105–112. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Snover DC, Weigent CE and Sumner HW:
Diffuse mucosal ganglioneuromatosis of the colon associated with
adenocarcinoma. Am J Clin Pathol. 75:225–229. 1981.PubMed/NCBI
|
|
14
|
Prakash K, Varma D, Ramesh GN, et al:
Ileal ganglioneuromatosis with adenocarcinoma in a patient with
multiple neurofibromatosis. Indian J Surg. 73:375–376. 2011.
View Article : Google Scholar :
|
|
15
|
Haraguchi M, Kinoshita H, Koori M, et al:
Multiple rectal carcinoids with diffuse ganglioneuromatosis. World
J Surg Oncol. 5:192007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ricci A Jr, Parham DM, Woodruff JM, et al:
Malignant peripheral nerve sheath tumors arising from
ganglioneuromas. Am J Surg Pathol. 8:19–29. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanter AS, Hyman NH and Li SC:
Ganglioneuromatous polyposis: a premalignant condition. Report of a
case and review of the literature. Dis Colon Rectum. 44:591–593.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shousha S and Smith PA: Colonic
ganglioneuroma. Report of a case in a patient with
neurofibromatosis, multiple colonic adenomas and adenocarcinoma.
Virchows Arch A Pathol Anat Histol. 392:105–109. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heald B, Mester J, Rybicki L, et al:
Frequent gastrointestinal polyps and colorectal adenocarcinomas in
a prospective series of PTEN mutation carriers. Gastroenterology.
139:1927–1933. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng L, Wu X, Kreis ME, et al:
Clinicopathological and immunohistochemical characterisation of
gastric schwannomas in 29 cases. Gastroenterol Res Pract.
2014:2029602014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Agaimy A and Michal M: Hybrid
schwannoma-perineurioma of the gastrointestinal tract: a
clinicopathologic study of 2 cases and reappraisal of perineurial
cells in gastrointestinal schwannomas. Appl Immunohistochem Mol
Morphol. 19:454–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jacobson BC, Hirsch MS, Lee JH, et al:
Multiple asymptomatic plexiform schwannomas of the sigmoid colon: a
case report and review. Gastrointest Endosc. 53:801–804. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Donk W, Poyck P, Westenend P, et al:
Recurrent abdominal complaints caused by a cecal neurofibroma: a
case report. World J Gastroenterol. 17:3953–3956. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fiori E, Pozzessere C, Lamazza A, et al:
Endoscopic treatment of ganglioneuroma of the colon associated with
a lipoma: a case report. J Med Case Rep. 6:3042012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ledwidge SF, Moorghen M, Longman RJ and
Thomas MG: Adult transmural intestinal ganglioneuromatosis is not
always associated with multiple endocrine neoplasia or
neurofibromatosis: a case report. J Clin Pathol. 60:222–223. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dahl EV, Waugh JM and Dahlin DC:
Gastrointestinal ganglioneuromas; brief review with report of a
duodenal ganglioneuroma. Am J Pathol. 33:953–965. 1957.PubMed/NCBI
|