Introduction
Skin wound healing is one of the most complex
biological processes and is affected by multiple factors. The
process involves three steps of inflammation, cell proliferation
and tissue remodeling, in order to maintain the body homeostasis
(1). This complex process is
regulated by a signaling network system with the same complexity,
which includes numerous growth factors, cytokines and chemokines
(2,3). Following activation, transforming
growth factor (TGF)-β, a key regulator in wound healing, can
stimulate extracellular matrix deposition and angiogenesis by
regulating the proliferation of fibroblasts (2,4). The
action of TGF-β is accomplished through the Smad signaling pathways
(5).
Autophosphorylation of TGF-β occurs after binding to
the heterodimer receptor complex, which activates the downstream
transcription factor signaling molecules that belong to the Smad
protein family (2,3). Following activation, TGF-β receptors
induce the phosphorylation of Smad2 and Smad3, forming
hetero-oligomeric complexes with Smad4 (6–8). The
complexes are transported into the nucleus where they regulate
ligand-induced gene transcription. The signaling pathway following
the activation of the TGF-β receptor is further regulated by Smad7,
which functions as an intracellular antagonist (9). Smad7 can firmly bind to the activated
TGF-β1 receptor, inhibit the phosphorylation of R-Smad and hence
inhibit the signaling pathway (9).
Mast cell chymase is a type of serine proteinase
that is included in the secretory granules of mast cells. Following
activation, mast cell chymase can transform inactivated TGF-β1 into
an activated form (10,11). Taipale et al (12) demonstrated that in the
extracellular matrix of human epithelial and endothelial cells,
chymase facilitates the release of TGF-β1 from the bound protein.
Mast cell chymase can increase the concentration of TGF-β1 in
cultured fibroblasts; however, the increase in TGF-β1 can be
attenuated using a chymase inhibitor (13). In addition, a neutralizing antibody
of TGF-β1 has been shown to completely inhibit chymase-induced
fibroblast proliferation, indicating that chymase promotes cell
proliferation through TGF-β1 (13). Furthermore, chymase regulates the
formation of angiotensin II (Ang II) (14), degrades procollagen in tissue
remodeling (15,16) and participates in inflammatory
responses (17,18).
Mast cells originate from bone marrow
CD34+ hematopoietic stem cells, and are distributed to
various tissues via the blood circulation. Mature mast cells are
only found in tissues, and those found in the blood are precursors.
Previous studies have revealed that chymase promotes the
proliferation of skin fibroblasts in a dose- and time-dependent
manner (19), and chymase may be
involved in the wound healing process (20,21).
Chymase is known to activate TGF-β1 (10,11),
which plays a central role in wound healing and fibrosis (22). In addition, chymase has been
reported to induce myocardial fibrosis via the activation of the
TGF-β1/Smad signaling pathway (23). However, the effect of chymase on
the TGF-β1/Smad signaling pathway in skin fibroblasts remains
unknown. In the present study, the effects of different
concentrations of mast cell chymase were investigated on the
TGF-β1/Smad signaling pathway in skin fibroblasts.
Materials and methods
Cell culture
Skin tissues were obtained from patients treated at
the Department of Burns and Plastic Surgery in the First Affiliated
Hospital of Xinjiang Medical University (Ürümqi, China). The
collection and use of tissue samples were approved by the Ethics
Committee of the First Affiliated Hospital of Xinjiang Medical
University. Written informed consent was obtained from all the
participants.
Skin tissues were cut in sterile conditions and
placed into phosphate-buffered saline containing 100,000 U/l
penicillin and 100 mg/l streptomycin. After soaking for 30 min, the
tissues were transferred to Petri dishes where the subcutaneous
tissues were eliminated, and the samples were cut into small
strips. The tissues were digested with 0.25% Dispase II
(Sigma-Aldrich, St. Louis, MO, USA) at 4°C overnight. After
removing the epidermis, the isolated dermis was cut into sections
of 1–2 mm3. The tissue sections were digested at 37°C
with shaking for 3 h. The filtrate was collected via a 150 μm mesh
(Tiantai Global Screen Mesh Co., Ltd., Taizhou, China), and the
remainder was centrifuged at 1,000 × g for 10 min to collect the
cells. The cells were seeded onto Petri dishes at a density of
2×104 cells/cm2 and cultured at 37°C in the
presence of 5% CO2. The medium was changed after 4 h
incubation, following which the medium was changed every three
days. Cell growth and shapes were observed under an inverted
microscope (BX50; Olympus, Tokyo, Japan). The third to sixth
generations of the cells were used for further study.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cell proliferation was analyzed using an MTT assay.
Cultured fibroblasts were trypsinized, made into a single cell
suspension (1×106 cells/ml) and seeded onto 96-well
plates for incubation for 24 h. The cells were divided into five
groups for the addition of different concentrations (0, 15, 30, 60
and 120 ng/ml) of chymase (C8118; Sigma-Aldrich). The five groups
of cells were cultured for 24, 48, 72 and 96 h, followed by the
addition of 20 μl MTT (0.5%) per well prior to continued culture
for an additional 4 h. The supernatants were discarded, and 100 μl
dimethyl sulfoxide was added to each well prior to shaking for 10
min. Optical density (490 nm) values were measured using a
microplate reader (Thermo Plate TP-Reader; Thermo Fisher
Scientific, Waltham, MA, USA). All the experiments were performed
in triplicate.
Quantitative polymerase chain reaction
(qPCR)
Skin fibroblasts were cultured in the presence of
different concentrations (0, 15, 30, 60 and 120 ng/ml) of chymase
for 6, 12 and 24 h. Following cell culture, the total RNA was
isolated using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). qPCR was conducted using SYBR Premix Ex Taq™
(Takara Bio, Inc., Otsu, Japan) on an IQ5 qRT-PCR system (Bio-Rad
Laboratories, Hercules, CA, USA). PCR amplification conditions were
as follows: Initial denaturation at 95°C for 30 sec, followed by 40
cycles of amplification at 95°C for 5 sec, 55°C for 30 sec and 72°C
for 60 sec. The temperature range for the dissolution curve was
65–95°C. The 2−ΔΔCt method was used to calculate the
gene expression levels of TGF-β1 relative to
glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The sequences of
the specific primers were as follows: TGF-β1 (158 bp),
5′-ACACCAACTATTGCTTCAG-3′ (sense) and 5′-TGTCCAGGCTCCAAATG-3′
(antisense); GAPDH (137 bp), 5′-GCACCGTCAAGGCTGAGAAC-3′ (sense) and
5′-TGGTGAAGACGCCAGTGGA-3′ (antisense). Each PCR trial was performed
with three samples and repeated a minimum of three times.
Bicinchoninic acid (BCA) assay
Cells were seeded into six-well plates at a density
of 5×104 cells/well. The cells were incubated with
various concentrations of chymase for 6, 12 and 24 h. Following
incubation, the cells were washed with ice-cold phosphate-buffered
saline, and lysed in 500 μl ice-cold radioimmunoprecipitation assay
buffer (BioTeke Corporation, Beijing, China), containing 1 μg/ml
phosphatase inhibitors and 1 mM phenylmethanesulfonyl fluoride, for
30 min. The mixture was centrifuged at 12,000 × g (4°C) for 10 min,
after which the supernatants were stored at −80°C. The
concentration of TGF-β1, phosphorylated Smad2/3 (P-Smad2/3),
Smad2/3, Smad7 and GAPDH proteins were measured using a BCA protein
assay kit (BioTeke Corporation).
Western blot analysis
A prestained marker with a low molecular weight and
30 μg protein from the tissue samples were subject to sodium
dodecyl sulfate polyacrylamide gel electrophoresis. The separated
proteins were transferred onto polyvinylidene fluoride membranes
(EMD Millipore, Billerica, MA, USA) and blocked in Tris-buffered
saline and Tween-20 [10 mM Tris (pH 7.6), 150 mM NaCl and 0.1%
Tween-20] containing 5% skimmed milk for 1 h at room temperature.
Subsequently, the membranes were incubated with primary antibodies
against TGF-β1 (sc-146; 1:300; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), P-Smad2/3 (8828s; 1:1,000; Cell Signaling
Technology, Inc., Beverly, MA, USA), Smad2/3 (sc-8332; 1:300; Santa
Cruz Biotechnology, Inc.), Smad7 (sc-11392; 1:300; Santa Cruz
Biotechnology, Inc.) and GAPDH (sc-25778; 1:300; Santa Cruz
Biotechnology, Inc.) at 4°C overnight. The blots were rinsed in
Tris-buffered saline and Tween-20, and incubated with an alkaline
phosphatase-conjugated secondary antibody (77054s; 1:1,000; Cell
Signaling Technology, Inc.) for 2 h at room temperature. Bands on
the western blots were visualized using a
5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium kit
(Invitrogen Life Technologies), according to the manufacturer’s
instructions. Optical densities of the bands were scanned and
quantified using Quantity One image analysis software (Bio-Rad
Laboratories).
Statistical analysis
All statistical analyses were performed using SPSS
17.0 software for Windows (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. Statistical differences
between groups were assessed by one-way analysis of variance,
followed by the least significant difference post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Mast cell chymase increases skin
fibroblast proliferation in a dose- and time-dependent manner
To analyze the effect of different concentrations of
mast cell chymase on the proliferation of skin fibroblasts
following incubation for 24, 48, 72 and 96 h, an MTT assay was
performed. The results revealed that chymase (15, 30, 60 and 120
ng/ml) enhanced the proliferation of skin fibroblasts following
incubation for 48, 72 and 96 h (P<0.01). As the incubation time
increased, the proliferation was enhanced (Fig. 1). These results indicated that mast
cell chymase increased skin fibroblast proliferation in a dose- and
time-dependent manner.
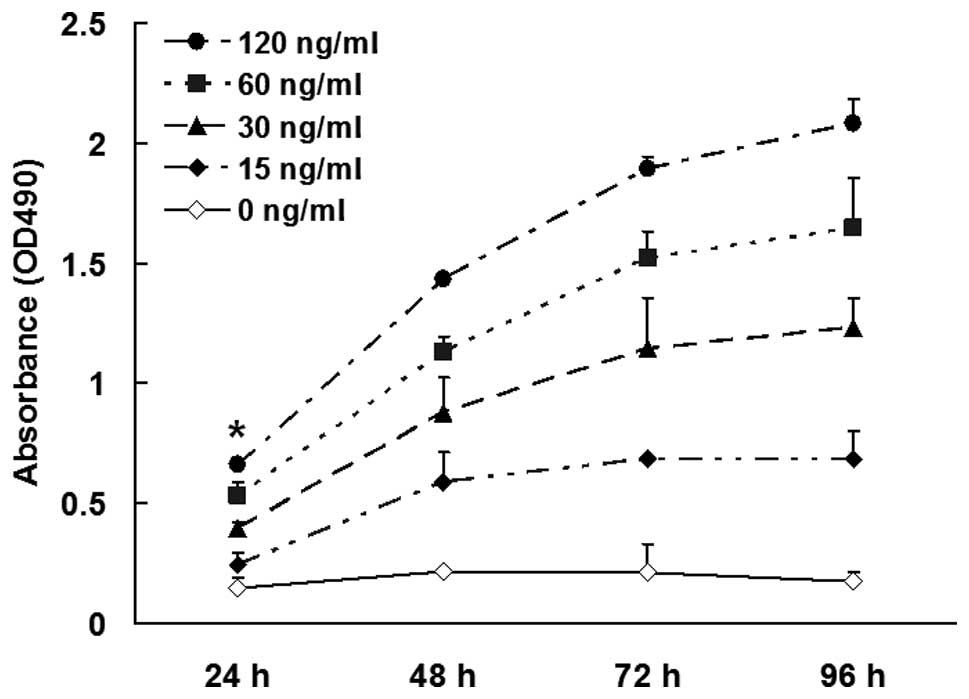 | Figure 1Proliferation of the skin fibroblasts
was analyzed using an
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Cultured fibroblasts were trypsinized, made into a single
cell suspension (1×106 cells/ml) and seeded onto 96-well
plates for incubation for 24 h. The cells were divided into five
groups for the addition of different concentrations (0, 15, 30, 60
and 120 ng/ml) of chymase and cultured for 24, 48, 72 and 96 h,
followed by the addition of 20 μl MTT (0.5%) per well and continued
culture for an additional 4 h. OD (490 nm) values were measured and
the experiments were performed in triplicate. Data are expressed as
the mean ± standard deviation. *P<0.01, vs. other
groups. OD, optical density. |
High concentrations of mast cell chymase
enhance the mRNA and protein expression levels of TGF-β1 after
long-term incubation
To analyze the effect of various concentrations of
mast cell chymase on the mRNA and protein expression levels of
TGF-β1 after incubation for 6, 12 and 24 h, qPCR and western blot
analysis were employed, respectively. The qPCR results revealed
that the mRNA expression levels of TGF-β1 following incubation with
chymase at concentrations of 60 and 120 ng/ml for 6, 12 and 24 h
were significantly higher compared with those incubated with
chymase at concentrations of 0, 15 and 30 ng/ml (P<0.01;
Fig. 2A). In addition, western
blot analysis demonstrated that TGF-β1 protein expression following
incubation with chymase at 60 and 120 ng/ml for 6, 12 and 24 h was
significantly higher compared with that in the control group (0
ng/ml; P<0.01). However, TGF-β1 protein expression levels
following incubation with 15 and 30 ng/ml chymase were slightly
lower compared with that in the control group (0 ng/ml; Fig. 2B). These results indicated that
high concentrations of mast cell chymase (60 and 120 ng/ml)
enhanced the mRNA and protein expression levels of TGF-β1 following
incubation for ≥6 h.
High concentrations of mast cell chymase
increase P-Smad2/3 and Smad2/3 protein expression, whereas low
concentrations of mast cell chymase increase Smad7 protein
expression
To measure the protein expression levels of
P-Smad2/3, Smad2/3 and Smad7, western blot analysis was performed.
The assay revealed that the protein expression levels of P-Smad2/3
and Smad2/3 following incubation with chymase (60 and 120 ng/ml)
for 6, 12 and 24 h were significantly higher compared with those in
the control group (0 ng/ml; P<0.05). However, P-Smad2/3 and
Smad2/3 protein expression levels following incubation with chymase
at a concentration of 15 and 30 ng/ml were slightly lower compared
with those in the control group (0 ng/ml; Figs. 3A, B and 4). By contrast, protein expression levels
of Smad7 following incubation with 60 and 120 ng/ml chymase for 6 h
were slightly lower compared with those in the control group (0
ng/ml), whereas Smad7 protein expression levels following
incubation with 15 and 30 ng/ml chymase for 6 h were significantly
higher compared with those in the control group (0 ng/ml)
(P<0.05; Figs. 3C and 4). In addition, the protein expression
levels of Smad7 following incubation with chymase (15, 30, 60 and
120 ng/ml) for 12 and 24 h were all higher compared with those in
the control group (0 ng/ml). A statistically significant difference
was observed in the Smad7 protein expression levels between those
incubated with 15 and 30 ng/ml chymase and those incubated with
chymase at 60 and 120 ng/ml (P<0.05; Figs. 3C and 4). These results indicated that high
concentrations of mast cell chymase (60 and 120 ng/ml) increased
P-Smad2/3 and Smad2/3 protein expression, whereas low
concentrations of mast cell chymase (15 and 30 ng/ml) increased
Smad7 protein expression.
Discussion
One of the most important functions of mast cell
chymase is the regulation of Ang II formation (14). Ang II directly acts on vascular
smooth muscle to regulate the blood pressure and is associated with
tissue fibrosis. In addition, chymase promotes the proliferation of
fibroblasts in heart muscle and skin tissues (23–25),
and promotes the release of TGF-β1 bound to the extracellular
matrix (26). Furthermore, chymase
is known to degrade procollagen for tissue remodeling (15,16)
and participate in the inflammatory response (17,18).
A previous study demonstrated that chymase can
activate the potential of TGF-β1. In cultured fibroblasts, chymase
promotes the release of TGF-β1 from the bound protein (27). In human skin fibroblasts, chymase
significantly increases the proliferation of fibroblasts; however,
this process can be completely inhibited by chymase inhibitors,
rather than Ang II receptor blockers (26). Chymase can facilitate the protein
expression of TGF-β1 in fibroblasts; however, increased TGF-β1
levels can be inhibited by the chymase inhibitor, Suc-Val-Pro-Phep
(OPh)2. Furthermore, an anti-TGF-β1 neutralizing
antibody has been shown to completely inhibit chymase-induced cell
proliferation, which may be mediated by the activation of TGF-β1
(28).
TGF-β is a secretory polypeptide signaling molecule
that participates in a variety of pathophysiological processes in
mammals. TGF-β affects cell proliferation and differentiation, and
plays an important role in embryo development, extracellular matrix
formation and immunoregulation. The subtype, TGF-β1, is closely
associated with extracellular matrix deposition and fibrotic
diseases, and is one of the main factors that affects wound healing
and scar formation (29). In the
present study, different concentrations of chymase were shown to
affect the proliferation of skin fibroblasts in a dose- and
time-dependent manner, which was consistent with the results of a
previous study (19).
TGF-β1 mRNA expression levels in the skin
fibroblasts following treatment with mast cell chymase (15 and 30
ng/ml) were higher compared with those in the control group;
however, the protein expression levels following treatment with
chymase at a concentration of 15 and 30 ng/ml were lower compared
with those in the control group. In addition, TGF-β1 mRNA and
protein expression levels following treatment with mast cell
chymase at concentrations of 60 and 120 ng/ml were significantly
higher compared with those following treatment with mast cell
chymase at a concentration of 15 and 30 ng/ml.
Smads are signaling intermediates and antagonists of
the TGF-β superfamily that are responsible for the intracellular
signaling and regulation of TGF-β1 (7). The initial process of Smad pathway
activation is R-Smad phosphorylation. Smad7 prevents the activation
of R-Smad and downregulates TGF-β1 signaling. The TGF-β1/Smad
signaling pathway plays an important role in skin development and
wound healing (30–32). Chymase has been reported to induce
myocardial fibrosis via the TGF-β1/Smad signaling pathway in
cultured mouse cardiac fibroblasts (23). However, the effect of chymase on
the TGF-β1/Smad signaling pathway in cultured human skin
fibroblasts has not been reported previously.
In the present study, Smad2/3 and P-Smad2/3 protein
expression levels in the fibroblasts treated with mast cell chymase
(60 and 120 ng/ml) for 6, 12 and 24 h were higher compared with
those in the control group. However, in the fibroblasts treated
with 15 and 30 ng/ml chymase, the protein expression levels were
lower compared with the control group. By contrast, Smad7 protein
expression levels in the skin fibroblasts treated with 15 and 30
ng/ml chymase were higher compared with the control group and the
60 and 120 ng/ml chymase groups. The higher the TGF-β1 protein
expression levels, the lower the Smad7 protein expression levels,
and vice versa.
Phosphorylation of Smad2/3 is a key step in the
activation of the Smad signaling pathway (33). The amount of P-Smad2/3 protein
represents the degree of activation of the Smad signaling pathway.
The aforementioned results demonstrated that chymase can activate
the Smad signaling pathway. However, Smad proteins are not the only
proteins activated by TGF-β (34,35),
and may be associated with other signaling pathways. Thus, whether
chymase is able to activate other signaling pathways requires
further investigation.
Smad7 is the primary inhibitory protein in the
TGF-β1 signaling pathway. The protein competitively binds to
activated TGF-β receptor 1 to prevent the phosphorylation of
R-Smad; thus, causing the downregulation of the Smad signaling
pathway (36). In the present
study, chymase at concentrations of 15 and 30 ng/ml significantly
enhanced Smad7 protein expression, which was consistent with a
previous study that reported TGF-β1 induced Smad7 protein
expression in skin fibroblasts (37). Chymase can activate Smad2/3, and
can increase Smad7 expression, forming an autocrine negative
feedback loop. However, the expression of endogenous Smad7 induced
by 60 and 120 ng/ml chymase was much lower compared with the
protein expression levels of TGF-β1, Smad2/3 and P-Smad2/3, which
promoted the Smad signaling pathway. The evidence that chymase (60
and 120 ng/ml) upregulated the mRNA and protein expression levels
of TGF-β1 and promoted the phosphorylation of Smad2/3 protein
indicated that chymase activated TGF-β1 and the intracellular
signaling transduction Smad protein molecules, which promoted the
transduction of the TGF-β1/Smad signaling pathway.
In conclusion, the present study investigated the
effects of various concentrations of mast cell chymase on the
TGF-β1/Smad signaling pathway in cultured skin fibroblasts. The
results demonstrated that high concentrations of chymase facilitate
the transduction of the TGF-β1/Smad signaling pathway.
Acknowledgements
The study was supported by a grant from the Youth
Science Foundation of the First Affiliated Hospital of Xinjiang
Medical University (no. 2012QN02).
References
|
1
|
Li J, Chen J and Kirsner R:
Pathophysiology of acute wound healing. Clin Dermatol. 25:9–18.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Werner S and Grose R: Regulation of wound
healing by growth factors and cytokines. Physiol Rev. 83:835–870.
2003.PubMed/NCBI
|
|
3
|
Barrientos S, Stojadinovic O, Golinko MS,
Brem H and Tomic-Canic M: Growth factors and cytokines in wound
healing. Wound Repair Regen. 16:585–601. 2008. View Article : Google Scholar
|
|
4
|
Li J, Zhang YP and Kirsner RS:
Angiogenesis in wound repair: angiogenic growth factors and the
extracellular matrix. Microsc Res Tech. 60:107–114. 2003.
View Article : Google Scholar
|
|
5
|
Itoh S, Itoh F, Goumans MJ and Ten Dijke
P: Signaling of transforming growth factor-beta family members
through Smad proteins. Eur J Biochem. 267:6954–6967. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ashcroft GS and Roberts AB: Loss of Smad3
modulates wound healing. Cytokine Growth Factor Rev. 11:125–131.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Derynck R, Zhang Y and Feng XH: Smads:
transcriptional activators of TGF-beta responses. Cell. 95:737–740.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Massagué J: TGF-beta signal transduction.
Annu Rev Biochem. 67:753–791. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayashi H, Abdollah S, Qiu Y, et al: The
MAD-related protein Smad7 associates with the TGFbeta receptor and
functions as an antagonist of TGFbeta signaling. Cell.
89:1165–1173. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lyons RM, Gentry LE, Purchio AF and Moses
HL: Mechanism of activation of latent recombinant transforming
growth factor beta 1 by plasmin. J Cell Biol. 110:1361–1367. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miyazono K and Heldin CH: Role for
carbohydrate structures in TGF-beta 1 latency. Nature. 338:158–160.
1989. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taipale J, Lohi J, Saarinen J, Kovanen PT
and Keski-Oja J: Human mast cell chymase and leukocyte elastase
release latent transforming growth factor-beta 1 from the
extracellular matrix of cultured human epithelial and endothelial
cells. J Biol Chem. 270:4689–4696. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okamoto Y, Takai S and Miyazaki M: Effect
of chymase-dependent transforming growth factor beta on peritoneal
adhesion formation in a rat model. Surg Today. 34:865–867. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Urata H, Kinoshita A, Misono KS, Bumpus FM
and Husain A: Identification of a highly specific chymase as the
major angiotensin II-forming enzyme in the human heart. J Biol
Chem. 265:22348–22357. 1990.PubMed/NCBI
|
|
15
|
Saarinen J, Kalkkinen N, Welgus HG and
Kovanen PT: Activation of human interstitial procollagenase through
direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J
Biol Chem. 269:18134–18140. 1994.PubMed/NCBI
|
|
16
|
Vartio T, Seppä H and Vaheri A:
Susceptibility of soluble and matrix fibronectins to degradation by
tissue proteinases, mast cell chymase and cathepsin G. J Biol Chem.
256:471–477. 1981.PubMed/NCBI
|
|
17
|
Takai S and Miyazaki M: A novel
therapeutic strategy against vascular disorders with chymase
inhibitor. Curr Vasc Pharmacol. 1:217–224. 2003. View Article : Google Scholar
|
|
18
|
Doggrell SA and Wanstall JC: Vascular
chymase: pathophysiological role and therapeutic potential of
inhibition. Cardiovasc Res. 61:653–662. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong X, Chen J, Zhang Y and Cen Y: Mast
cell chymase promotes cell proliferation and expression of certain
cytokines in a dose-dependent manner. Mol Med Rep. 5:1487–1490.
2012.PubMed/NCBI
|
|
20
|
Dong X, Geng Z, Zhao Y, Chen J and Cen Y:
Involvement of mast cell chymase in burn wound healing in hamsters.
Exp Ther Med. 5:643–647. 2013.PubMed/NCBI
|
|
21
|
Nishikori Y, Kakizoe E, Kobayashi Y, et
al: Skin mast cell promotion of matrix remodeling in burn wound
healing in mice: relevance of chymase. Arch Dermatol Res.
290:553–560. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Massagué J: How cells read TGF-beta
signals. Nat Rev Mol Cell Biol. 1:169–178. 2000. View Article : Google Scholar
|
|
23
|
Zhao XY, Zhao LY, Zheng QS, et al: Chymase
induces profibrotic response via transforming growth
factor-beta1/Smad activation in rat cardiac fibroblasts. Mol Cell
Biochem. 310:159–166. 2008. View Article : Google Scholar
|
|
24
|
Maruichi M, Takai S, Sugiyama T, et al:
Role of chymase on growth of cultured canine Tenon’s capsule
fibroblasts and scarring in a canine conjunctival flap model. Exp
Eye Res. 79:111–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Algermissen B, Hermes B,
Feldmann-Boeddeker I, Bauer F and Henz BM: Mast cell chymase and
tryptase during tissue turnover: analysis on in vitro mitogenesis
of fibroblasts and keratinocytes and alterations in cutaneous
scars. Exp Dermatol. 8:193–198. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takai S, Jin D, Sakaguchi M, et al: A
novel chymase inhibitor,
4-[1-([bis-(4-methyl-phenyl)-methyl]-carbamoyl)3-
(2-ethoxy-benzyl)-4-oxo-azetidine-2-yloxy]-benzoic acid (BCEAB),
suppressed cardiac fibrosis in cardiomyopathic hamsters. J
Pharmacol Exp Ther. 305:17–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanzaki T, Olofsson A, Morén A, et al:
TGF-beta 1 binding protein: a component of the large latent complex
of TGF-beta 1 with multiple repeat sequences. Cell. 61:1051–1061.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Simard E, Jin D, Takai S, et al:
Chymase-dependent conversion of Big endothelin-1 in the mouse in
vivo. J Pharmacol Exp Ther. 328:540–548. 2009. View Article : Google Scholar
|
|
29
|
Rhett JM, Ghatnekar GS, Palatinus JA,
O’Quinn M, Yost MJ and Gourdie RG: Novel therapies for scar
reduction and regenerative healing of skin wounds. Trends
Biotechnol. 26:173–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Owens P, Han G, Li AG and Wang XJ: The
role of Smads in skin development. J Invest Dermatol. 128:783–790.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ponugoti B, Xu F, Zhang C, Tian C, Pacios
S and Graves DT: FOXO1 promotes wound healing through the
up-regulation of TGF-β1 and prevention of oxidative stress. J Cell
Biol. 203:327–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kajdaniuk D, Marek B, Borgiel-Marek H and
Kos-Kudła B: Transforming growth factor β1 (TGFβ1) in physiology
and pathology. Endokrynol Pol. 64:384–396. 2013. View Article : Google Scholar
|
|
33
|
Attisano L and Wrana JL: Signal
transduction by the TGF-beta superfamily. Science. 296:1646–1647.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Euler-Taimor G and Heger J: The complex
pattern of SMAD signaling in the cardiovascular system. Cardiovasc
Res. 69:15–25. 2006. View Article : Google Scholar
|
|
35
|
Rodríguez-Vita J, Sánchez-López E, Esteban
V, Rupérez M, Egido J and Ruiz-Ortega M: Angiotensin II activates
the Smad pathway in vascular smooth muscle cells by a transforming
growth factor-beta-independent mechanism. Circulation.
111:2509–2517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakao A, Afrakhte M, Morén A, et al:
Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta
signalling. Nature. 389:631–635. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mori Y, Chen SJ and Varga J: Modulation of
endogenous Smad expression in normal skin fibroblasts by
transforming growth factor-beta. Exp Cell Res. 258:374–383. 2000.
View Article : Google Scholar : PubMed/NCBI
|


















