Introduction
Cobalt (Co) is a ferromagnetic transition metal,
essential to human health, that plays a critical role in the
synthesis of vitamin B12. The toxic effect of Co was
first described in 1966, when beer drinkers developed a
cardiomyopathy characterized by pericardial effusion, elevated
hemoglobin concentrations and congestive heart failure, due to the
addition of Co sulfate to the beer as a stabilizer (1).
The mechanism by which Co acts on cells is
controversial. Previous studies have demonstrated that Co modifies
mitochondrial permeability by opening the transition pores, which
leads to mitochondrial swelling and electrical membrane potential
collapse (2), and inhibits crucial
enzymes that participate in the mitochondrial respiratory chain due
to its high affinity for sulfhydryl groups (3). In addition, Co has been demonstrated
to decrease neurotransmitter-induced postsynaptic responses by
inhibiting synaptic transmission through the presynaptic blockage
of calcium (Ca2+) channels (4). Co has also been hypothesized to
function as a Ca2+ channel antagonist, competing for
intracellular Ca2+-binding proteins and thus exerting
inhibitory effects on Ca2+ signaling (5). Furthermore, Co administration in rats
has been shown to induce a depletion of neurotransmitters,
including dopamine, norepinephrine and serotonin, which suggests
that Co can cause memory impairment (6).
A previous study indicated that Co chloride
(CoCl2) activates hypoxia-inducible factor (HIF)-1α,
acting as a hypoxia-mimetic and inducing reactive oxygen species
(ROS)-mediated toxicity (7).
Furthermore, CoCl2 is able to stabilize HIF-1α, a key
determinant of the cellular response to hypoxia, which has made the
compound one of the most commonly used hypoxia-mimetic agents
(8). Previous animal studies have
demonstrated that CoCl2 preconditioning increases
mitochondrial biogenesis, glucose uptake and metabolism in the
skeletal muscles (9), and
attenuates vascular leakage and ROS-induced hypoxia generation in
the brain (10). Research
indicates that CoCl2 preconditioning has
cardioprotective (11),
renoprotective (12) and
neuroprotective effects (13).
There is increasing evidence that superoxide, a
member of the ROS family produced during hypoxia, and mitochondrial
activation, are involved in the development of chronic pain, in the
transition from acute to chronic pain, in opiate-induced
hyperalgesia and in antinociceptive tolerance (14).
The aim of the present study was to investigate the
effects of modulating mitochondrial function and cellular
antioxidant capacity by CoCl2 preconditioning on
nociception and locomotor activity in mice.
Materials and methods
Animals
Experiments were conducted on 80 male BALB/c mice
(weight, 28–34 g), housed at 21±2°C under a 12-h light/dark cycle,
with access to food and water ad libitum. The study was
conducted in accordance with the European Communities Council
Directive 2010/63/EU (15), the
‘Guidelines for the use of animals in research (1991)’ (16) and the Grigore T. Popa University of
Medicine and Pharmacy (Iaşi, Romania) ethical guidelines for the
experimental investigation of pain in conscious animals.
Drugs
CoCl2, glacial acetic acid and
formaldehyde solution (37 wt% in H2O) were obtained from
Sigma-Aldrich (St. Louis, MO, USA). The compound was freshly
diluted in a saline solution (0.9% NaCl; B. Braun Melsungen AG,
Melsungen, Germany) and was administered via an intraperitoneal
(i.p.) route, with doses expressed as mg per kg of body weight
(mg/kg b.w.).
Tests and models of nociception and
pain
Hot-plate test (HPT)
The HPT was performed on 12 mice in the
CoCl2 group and 10 mice in the saline group, as
previously described (17). The
animals were placed in an open Plexiglas tube on a hot-plate device
(model-DS 37; Ugo Basile Srl, Varese, Italy) at a temperature of
55±0.1°C. The time between the placing of the animal and the
occurrence of licking, shaking of hind paws or jumping off the
surface was recorded as a response latency. The experiment cut-off
time was set to 15 sec to prevent tissue damage.
Tail-flick test (TFT)
A TFT was performed on 12 mice in the
CoCl2 group and 10 mice in the saline group (18). The distal portion of each mouse
tail was placed on the heat source of the apparatus (Tail Flick
Unit-37360; Ugo Basile Srl) and the time until the animal removed
its tail was defined as the tail-flick latency, with a 12-sec
cut-off time.
Plantar test
A Plantar test using the Hargreaves method was
applied for 12 mice in the CoCl2 group and nine mice in
the saline group (19). The mice
were placed into clear acrylic boxes on a Plexiglas floor, and the
radiant heat source from the Hargreaves unit (Plantar Test-37370;
Ugo Basile Srl) was placed under the hind paw. The thermal
withdrawal threshold was recorded as the time between the start of
the radiant heat stimulus and the withdrawal or licking of the hind
paw, with a 20-sec cut-off time. The mean paw withdrawal latency
was obtained from the average of three separate trials.
Mechanical sensitivity test
The mechanical withdrawal threshold was measured
using an automatic Von Frey method with a Dynamic Plantar
Aesthesiometer 37450 (Ugo Basile Srl). A total of 12 mice in the
CoCl2 group and nine mice in the saline group were
placed into acrylic chambers with wire mesh floors. A
servo-controlled mechanical stimulus was applied repeatedly and
alternately to the plantar surface of each hind paw. The time
elapsed until the pressure exerted by the filament evoked a clear
voluntary hind paw withdrawal response was automatically recorded.
To yield the mean value, mechanical withdrawal thresholds were
measured in triplicate for each animal.
For the aforementioned tests, hyperalgesia was
quantified as the percentage decrease in the withdrawal threshold:
(Baseline value - CoCl2 value) × 100/baseline value
(20).
Writhing test (WT)
A WT was performed to measure visceral pain, as
described by Koster et al (21). After becoming accustomed to the
acrylic chambers, the mice (eight in the CoCl2 group and
eight in the saline group) received i.p. injections of 1.0% (v/v)
acetic acid (0.1 ml/10 mg b.w.). The number of abdominal writhes
was counted over a period of 30 min.
Paw formalin test (PFT) and orofacial
formalin test (OFT)
These tests consisted of injecting 20 μl formalin
(5%) subcutaneously into the plantar surface of the right hind paw
(PFT) or the right whisker pad (OFT). The total time (sec) that the
mice (n=10 in the CoCl2 and saline groups for PFT and
OFT) spent licking and/or biting the injected paw/whisker during
the first/neurogenic phase (PFT, 0–9 min; OFT, 0–6 min) and the
second/inflammatory phase (PFT, 10–40 min; OFT, 7–40 min) were
recorded (22,23).
For formalin- and acetic acid-induced pain, the
antinociceptive activity was expressed as the percentage inhibition
of nociceptive behavior (INB), using the following formula: %INB =
(mean saline group - mean CoCl2 group) × 100/(mean
saline group).
Study design
The 80 mice were divided into two groups. The
CoCl2 group mice (n=30) received 12.5 mg/kg b.w.
CoCl2 (i.p.) daily for 21 days, while the saline group
mice (n=50) received an equivalent volume of saline. The dose of
12.5 mg/kg b.w. was selected in accordance with published data
concerning CoCl2 preconditioning (10,24).
A total of 12 out of the 30 mice from the
CoCl2 group underwent testing prior to CoCl2
administration (baseline) and thereafter each day for the TFT and
HPT, and weekly for the mechanical and thermal hyperalgesia tests.
All the tests were performed prior to the daily CoCl2
injection. The other 18 mice from the 30 CoCl2 mice
received CoCl2 daily for 21 days. During
CoCl2 preconditioning two mice died. The remaining 28
mice were divided as follows: 8 underwent the WT, 10 mice underwent
PDT, and 10 mice underwent OFT. The control group received an
equivalent volume of saline daily. A total of 10 mice out of the 50
mice from the control group underwent baseline testing for the TF
and HPT, and another 10 mice were tested for mechanical and thermal
hyperalgesia. Throughout the experiment, three mice from the
control group died, therefore the number of mice tested for thermal
hyperalgesia was nine, and the number of mice included in the WT
was eight. Data from the tests performed at baseline and days 7, 14
and 21 are presented for both the CoCl2 and saline
groups. In addition, the WT, OFT and PFT were performed 24 h after
the last dose of CoCl2 and compared with the saline
group.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. The following values are presented in the results:
F ratio and the number of degrees of freedom, outcome and
sigificance value. Statistical evaluations were performed using
SPSS v20.0 software (IBM, Armonk, NY, USA). An unpaired Student’s
t-test and repeated analysis of variance (ANOVA), followed by the
Bonferroni post hoc test, were used when appropriate. P<0.05 was
considered to indicate a statistically significant difference.
Results
TFT
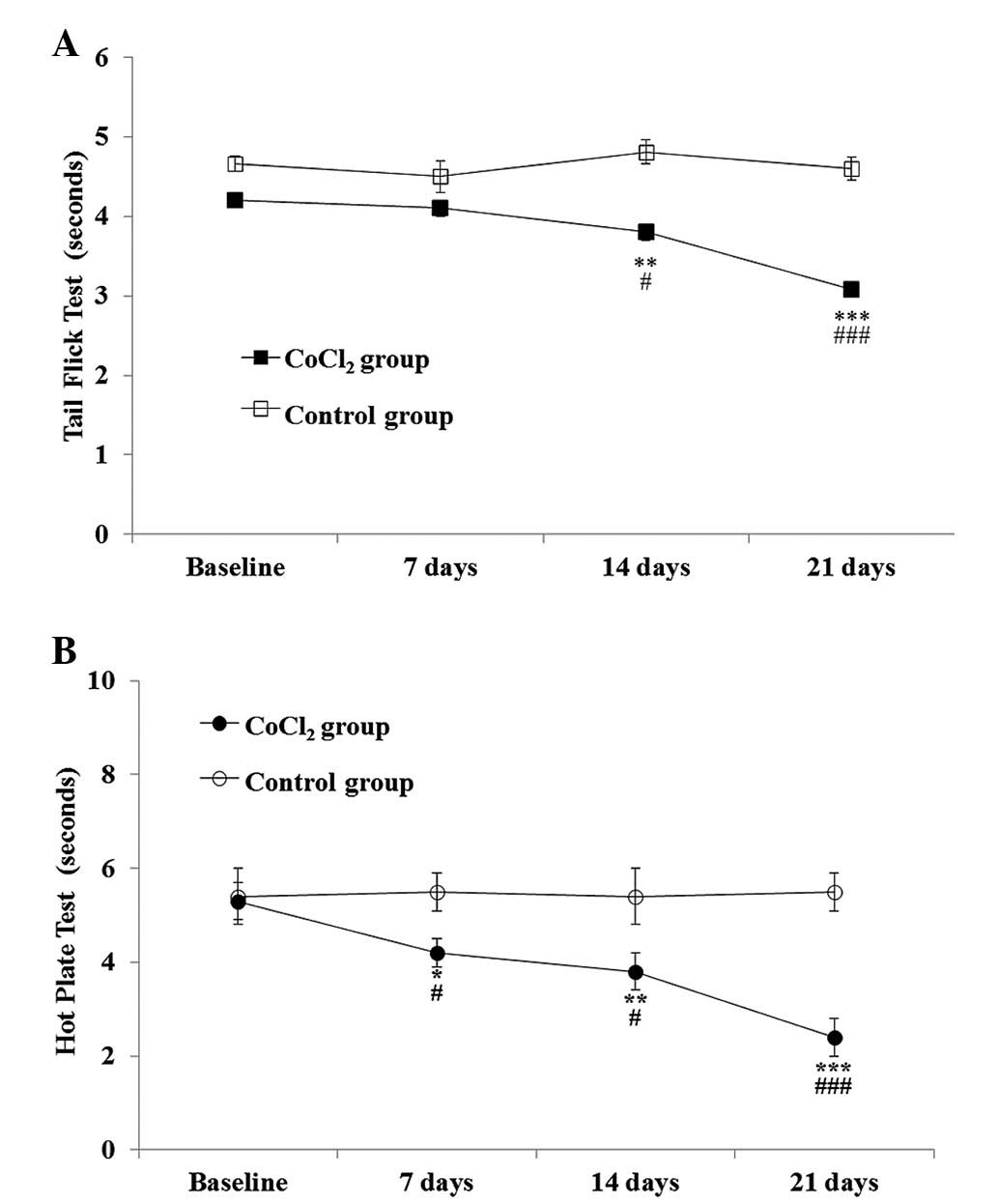
CoCl2 preconditioning was shown to have a
statistically significant effect on the TFT when compared with
saline administration [F(1,20)=52.7; P<0.001], and this effect
became more pronounced over time [F(3,33)=10.9; P<0.001], as
assessed by repeated measures ANOVA. The Student’s t-test for
independent samples showed a statistically significant lower
tolerance for thermal stimuli in the CoCl2 group, which
was observed in the second week (P=0.01) and persisted until the
end of the experiment (day 21; P<0.001). The decrease in the
withdrawal threshold ranged between 8.52±3.8% at week one and
25.56±3.8% at week three (Fig.
1).
HPT
With regard to the HPT, repeated measures ANOVA
indicated that CoCl2 preconditioning had a statistically
significant effect on the response latency when compared with the
saline group [F(1,20)=17.08; P=0.001]. The effect became more
pronounced over time [F(3,33)=12.7; P<0.001].
Statistically significant differences were observed
between the saline and CoCl2 groups one week after
CoCl2 administration (P=0.03). By the end of the second
week, HPT latencies significantly decreased (P=0.03, vs. saline
group and P=0.01, vs. baseline) and remained lower than the
baseline values until the end of the experiment (day 21;
P<0.001, vs. saline group and baseline). By the final day of the
experiment, the decrease in the withdrawal threshold was
49.35±5.64% (Fig. 1).
WT
A similar number of writhes were recorded over a
30-min period in the CoCl2 group when compared with the
saline group (P=0.1).
PFT
No statistically significant differences were
observed between the CoCl2 and saline group mice for the
first phase (P=0.1); however, the CoCl2 group mice spent
23.2% less time grooming the affected paw when compared with the
saline group. In the second phase of the PFT, the CoCl2
group mice spent significantly less time grooming/licking the
affected paw when compared with the saline group, with an INB of
35.7% (P=0.01; Fig. 2).
OFT
Pain behavior did not statistically differ between
the two groups in the two phases of the test (P=0.8, first phase;
P=0.06, second phase). However, the INB for the CoCl2
group mice in the second phase was 27.08% (Fig. 2).
Plantar test
In the preconditioned mice, the CoCl2 had
a statistically significant effect [F(1,40)=7.3; P=0.01], while
time had a marginal effect [F(2.08,48.01)=2.5; P=0.08]. At the end
of the first week, a significant thermal withdrawal threshold
difference was observed in the CoCl2 group when compared
with the saline group (P=0.01). However, these changes returned to
baseline values in week three (8.19±0.5 sec at baseline vs.
8.57±0.6 sec at week three; P=0.6). Throughout the experiment, no
statistically significant differences were observed in the
CoCl2 group when compared with the baseline values
(Fig. 3).
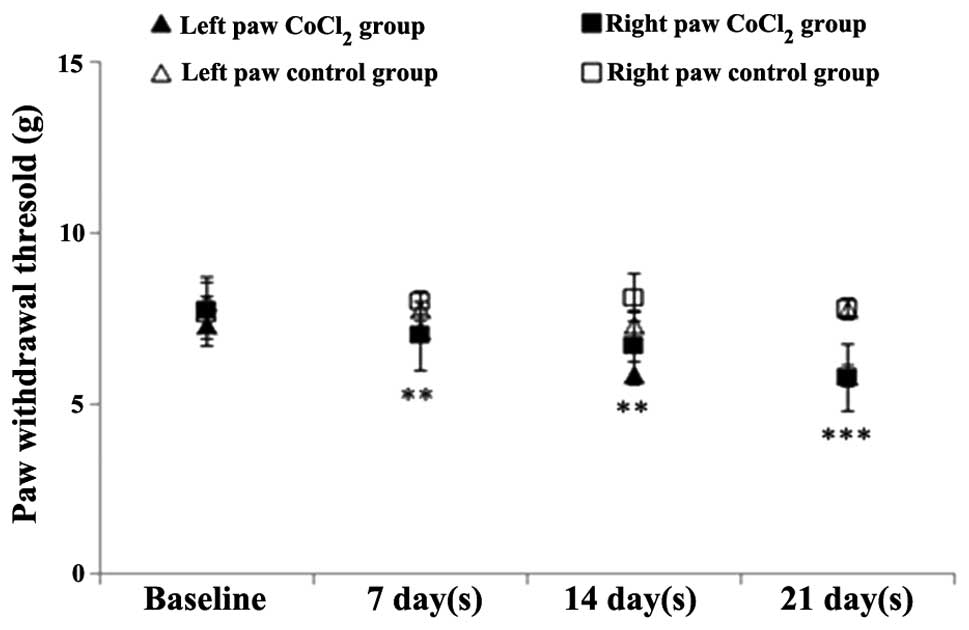
Mechanical sensitivity test
Repeated measures ANOVA indicated that
CoCl2 preconditioning had a statistically significant
effect on the mechanical sensitivity test when compared with saline
administration [F(1,40)=27.9; P<0.001]. In addition, this effect
became more pronounced over time [F(3,69)=9.76; P<0.001].
Throughout the experiment, the mechanical withdrawal
thresholds progressively decreased in the CoCl2 group.
In the first week, a statistically significant difference was
observed in the CoCl2 group when compared with the
saline group (P=0.01), and in the second week when compared with
the CoCl2 baseline levels (P=0.001). At the end of the
experiment, the mechanical withdrawal threshold was 20% lower than
the baseline value of the CoCl2 group, and the
difference with the saline group was statistically significant
(P<0.0001; Fig. 4).
Discussion
The present study examined the effects of hypoxic
preconditioning on nociception and pain. The results indicated that
CoCl2 hypoxic preconditioning resulted in a
pronociceptive effect on the HPT and TFT after one and two weeks of
daily administration, respectively. These pronociceptive effects
persisted until day 21 of treatment. Mechanical allodynia was
similar to the HPT. After the first week, thermal hyperalgesia
(Plantar test) was observed; however, this change was temporary and
by the end of the experiment the mean thermal withdrawal thresholds
had returned to baseline values. In addition, CoCl2
preconditioning was shown to modify pain perception in the second
phase of formalin-induced pain; however, no effects were observed
on visceral and acute formalin pain.
Previous studies have indicated that the TFT is
affected by stimuli that act primarily at a spinal level (25), whereas the HPT reflects supraspinal
sensory integration (26). By
comparing the time frame for these two tests, the results of the
present study indicate that supraspinal pain structures are
affected one week following CoCl2 administration,
whereas spinal structures are affected after two weeks. Once
established, these changes persisted throughout the duration of the
experiment. These data are consistent with the literature, showing
that spinal somatosensory-evoked potentials are more resistant to
severe hypoxia than cortical somatosensory-evoked potentials
(27). In addition, the data
indicate that the spinal nociceptive reflexes (TFT) are more
resistant to CoCl2 hypoxic preconditioning than
supraspinal responses to nociception (HPT).
Mechanical allodynia appeared in the first week of
the study and persisted until the last day of the experiment, while
thermal hyperalgesia returned to baseline levels in the second
week. Previous studies have also reported behavioral differences
between mechanical and thermal hyperalgesia (28–30).
Thermal hyperalgesia appears to be dependent on opioid-sensitive
small-diameter primary afferent fibers, while mechanical allodynia
is considered to rely on a largely independent small-fiber input.
In addition to the differences in neuroanatomical pathways, changes
in 5-hydroxytryptamine (29),
opioid (30), nitric
oxide/cGMP/ATP-sensitive K+ channels (31) or in ATP distribution along the
peripheral and central nervous system have been considered as
alternative explanations for the behavioral differences. The
results of the present study, with regard to thermal and mechanical
hyperalgesia, suggest a different response of A and C fibers to
hypoxia.
Apostoli et al hypothesized that Co may have
a specific neurotropism; therefore, CoCl2 may have a
neurotoxic effect (32). In
addition, chronic occupational exposure to Co has been shown to
induce sensory-motor polyneuropathy in humans (33), while in animal models, Co has been
shown to have neurotoxic effects in vitro and in vivo
(34).
Contrary to the hyperalgesic effect on thermal pain,
CoCl2 preconditioning decreased the time spent grooming
the affected area in the inflammatory phase of formalin-induced
pain in the paw and orofacial models. However, no effect on the
nociceptive phase of the formalin tests was observed.
Hypoxic preconditioning has been documented to
protect the brain (and other tissues) from ischemic insults and
increase resistance to other types of injury. However, hypoxia
causes a rapid reduction in intracellular ATP and a delayed
increase in extracellular purine levels (35). Thus, the adenosine released
secondary to hypoxia may act on the P2Y (a protein G-coupled
receptor) and P2X (a ligand-gated cation channel receptor)
adenosine receptors, which have different effects according to the
concentration of the receptor along the nervous system and the ATP
levels in the synapses or nervous cells (36).
Previous studies have suggested that the sensory
experience of pain depends on descending pain modulator circuits
arising from the rostral ventromedial medulla (RVM) (37). Thus, nociception is the consequence
of enhancement (ON cells-pain facilitatory cells) or inhibition
(OFF cells-pain inhibitory cells) of the spinal dorsal horn neurons
by the RVM projection (38).
In formalin-induced pain, adenosine injected into
the periaqueductal gray region has been shown to produce
antinociceptive effects as a consequence of its ability to act on
the ON and OFF cells from the RVM (39). By contrast, in the TFT and HPT, the
ON and OFF cells were shown to be activated only during motor
reactions, indicating that the RVM does not play an important role
in the latencies observed in these tests. Therefore, it was
hypothesized that the RVM may be one of the nervous system
structures involved in the analgesic effect of CoCl2
hypoxic preconditioning.
The results obtained in the formalin-induced pain
models are unlikely to be a consequence of motor disturbance, since
locomotor activity and coordination did not exhibit statistically
significant differences between the CoCl2 and saline
groups (data not shown).
The results of the present study indicate that
CoCl2 hypoxic preconditioning may have a neurotoxic
effect, with observations of pronociception on thermal nociception,
increased mechanical hyperalgesia and diminished pain sensibility
on formalin-induced persistent pain. These effects may result from
the capacity of CoCl2 to inhibit mitochondrial function
and modulate ROS. Furthermore, an increased tolerance to pain was
observed in the second phase of the formalin-induced pain models.
Thus, in addition to a possible Co-induced neuropathy, a
centrally-mediated analgesic effect of hypoxic preconditioning
cannot be excluded.
In conclusion, the present study demonstrated that
CoCl2 hypoxic preconditioning is a multifaceted
phenomenon, and a balance between beneficial and detrimental
effects must be taken into account in future experimental
studies.
Acknowledgements
This study was supported by a grant from the
Romanian National Authority for Scientific Research (CNCS-UEFISCDI;
no. PN-II-ID-PCE-2011-3-0875).
References
|
1
|
Sullivan J, Parker M and Carson SB: Tissue
cobalt content in ‘beer drinkers’ myocardiopathy’. J Lab Clin Med.
71:893–911. 1968.PubMed/NCBI
|
|
2
|
Battaglia V, Compagnone A, Bandino A, et
al: Cobalt induces oxidative stress in isolated liver mitochondria
responsible for permeability transition and intrinsic apoptosis in
hepatocyte primary cultures. Int J Biochem Cell Biol. 41:586–594.
2009. View Article : Google Scholar
|
|
3
|
Barceloux DG: Cobalt. J Toxicol Clin
Toxicol. 37:201–206. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gerber U and Gähwiler BH: Cobalt blocks
postsynaptic responses induced by neurotransmitters in the
hippocampus in vitro. Neurosci Lett. 134:53–56. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akbar M, Brewer JM and Grant MH: Effect of
chromium and cobalt ions on primary human lymphocytes in vitro. J
Immunotoxicol. 8:140–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Czarnota M, Whitman D and Berman R:
Activity and passive-avoidance learning in cobalt-injected rats.
Int J Neurosci. 93:29–33. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen SL, Yang CT, Yang ZL, et al: Hydrogen
sulphide protects H9c2 cells against chemical hypoxia-induced
injury. Clin Exp Pharmacol Physiol. 37:316–321. 2010. View Article : Google Scholar
|
|
8
|
Lee M, Lapham A, Brimmell M, et al:
Inhibition of proteasomal degradation of Mcl-1 by cobalt chloride
suppresses cobalt chloride-induced apoptosis in HCT116 colorectal
cancer cells. Apoptosis. 13:972–982. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saxena S, Shukla D and Bansal A:
Augmentation of aerobic respiration and mitochondrial biogenesis in
skeletal muscle by hypoxia preconditioning with cobalt chloride.
Toxicol Appl Pharmacol. 264:324–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kalpana S, Dhananjay S, Anju B, et al:
Cobalt chloride attenuates hypobaric hypoxia induced vascular
leakage in rat brain: molecular mechanisms of action of cobalt
chloride. Toxicol Appl Pharmacol. 231:354–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh M, Shukla D, Thomas P, et al:
Hypoxic preconditioning facilitates acclimatization to hypobaric
hypoxia in rat heart. J Pharm Pharmacol. 62:1729–1739. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumoto M, Makino Y, Tanaka T, et al:
Induction of renoprotective gene expression by cobalt ameliorates
ischemic injury of the kidney in rats. J Am Soc Nephrol.
14:1825–1832. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shrivastava K, Shukla D, Bansal A, et al:
Neuroprotective effect of cobalt chloride on hypobaric
hypoxia-induced oxidative stress. Neurochem Int. 52:368–375. 2008.
View Article : Google Scholar
|
|
14
|
Salvemini D, Little JW, Doyle T and
Neumann WL: Roles of reactive oxygen and nitrogen species in pain.
Free Radic Biol Med. 51:951–966. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Directive 2010/63/EU of the European
Parliament and of the Council of 22 September 2010 on the
protection of animals used for scientific purposes. OJ L.
276:33–79. 2010.
|
|
16
|
1991 Guidelines for the Use of Animals in
Research. Animal Behav. 41:183–186. 1991. View Article : Google Scholar
|
|
17
|
Woolfe G and MacDonald AD: The evaluation
of the analgesic action of pethidine hydrochloride (Demerol). J
Pharmacol Exp Ther. 80:300–307. 1944.
|
|
18
|
D’Amour FE and Smith DL: A method for
determining loss of pain sensation. J Pharmacol Exp Ther. 72:74–79.
1941.
|
|
19
|
Hargreaves K, Dubner R, Brown F, et al: A
new and sensitive method for measuring thermal nociception in
cutaneous hyperalgesia. Pain. 32:77–88. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tegeder I, Del Turco D, Schmidtko A, et
al: Reduced inflammatory hyperalgesia with preservation of acute
thermal nociception in mice lacking cGMP-dependent protein kinase
I. Proc Natl Acad Sci USA. 101:3253–3257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koster R, Anderson M and Beer EJ: Acetic
acid for analgesic screening. Federation Proceeds. 18:412–416.
1959.
|
|
22
|
Hunskaar S, Fasmer OB and Hole K: Formalin
test in mice, a useful technique for evaluating mild analgesics. J
Neurosci Methods. 14:69–76. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luccarini P, Childeric A, Gaydier AM, et
al: The orofacial formalin test in the mouse: a behavioral model
for studying physiology and modulation of trigeminal nociception. J
Pain. 7:908–914. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shukla D, Saxena S, Purushothaman J, et
al: Hypoxic preconditioning with cobalt ameliorates hypobaric
hypoxia induced pulmonary edema in rat. Eur J Pharmacol.
656:101–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gebhart GF and Ossipov MH:
Characterization of inhibition of the spinal nociceptive tail-flick
reflex in the rat from the medullary lateral reticular nucleus. J
Neurosci. 6:701–713. 1986.PubMed/NCBI
|
|
26
|
Kubo K, Nishikawa K, Ishizeki J, et al:
Thermal hyperalgesia via supraspinal mechanisms in mice lacking
glutamate decarboxylase 65. J Pharmacol Exp Ther. 331:162–169.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haghighi SS, Oro JJ, Gibbs SR and McFadden
M: Effect of graded hypoxia on cortical and spinal somatosensory
evoked potentials. Surg Neurol. 37:350–355. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bian D, Ossipov MH, Zhong C, et al:
Tactile allodynia, but not thermal hyperalgesia, of the hindlimbs
is blocked by spinal transection in rats with nerve injury.
Neurosci Lett. 241:79–82. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vogel C, Mössner R, Gerlach M, et al:
Absence of thermal hyperalgesia in serotonin transporter-deficient
mice. J Neurosci. 23:708–715. 2003.PubMed/NCBI
|
|
30
|
Huang C, Hu ZP, Long H, et al: Attenuation
of mechanical but not thermal hyperalgesia by electroacupuncture
with the involvement of opioids in rat model of chronic
inflammatory pain. Brain Res Bull. 63:99–103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Curto-Reyes V, Juárez L, García-Pérez E,
et al: Local loperamide inhibits thermal hyperalgesia but not
mechanical allodynia induced by intratibial inoculation of melanoma
cells in mice. Cell Mol Neurobiol. 28:981–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Apostoli P, Catalani S, Zaghini A, et al:
High doses of cobalt induce optic and auditory neuropathy. Exp
Toxicol Pathol. 65:719–727. 2013. View Article : Google Scholar
|
|
33
|
Catalani S, Rizzetti MC, Padovani A and
Apostoli P: Neurotoxicity of cobalt. Hum Exp Toxicol. 31:421–437.
2012. View Article : Google Scholar
|
|
34
|
Wang P, Zhang H, Chu F, et al: Synthesis
and protective effect of new ligustrazine-benzoic acid derivatives
against CoCl2-induced neurotoxicity in differentiated
PC12 cells. Molecules. 18:13027–13042. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Björklund O, Shang M, Tonazzini I, et al:
Adenosine A1 and A3 receptors protect astrocytes from hypoxic
damage. Eur J Pharmacol. 596:6–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Burnstock G: Purinergic signalling:
pathophysiology and therapeutic potential. Keio J Med. 62:63–73.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
De Felice M, Sanoja R, Wang R, et al:
Engagement of descending inhibition from the rostral ventromedial
medulla protects against chronic neuropathic pain. Pain.
152:2701–2709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fields HL, Malick A and Burstein R: Dorsal
horn projection targets of ON and OFF cells in the rostral
ventromedial medulla. J Neurophysiol. 74:1742–1759. 1995.PubMed/NCBI
|
|
39
|
Maione S, Piscitelli F, Gatta L, et al:
Non-psychoactive cannabinoids modulate the descending pathway of
antinociception in anaesthetized rats through several mechanisms of
action. Br J Pharmacol. 162:584–596. 2011. View Article : Google Scholar :
|