Introduction
Liver cancer remains the fifth most common
malignancy in males and the seventh in females worldwide. An
estimate indicated that 748,300 new liver cancer cases and 695,900
cancer mortalities occurred worldwide in 2008 (1). HCC is the major histological subtype
among primary liver cancer, accounting for 70–85% of all primary
liver cancer cases (2). Although
there have been advances in the diagnosis and treatment of HCC in
recent years, the prognosis for patients with HCC remains poor
(3). Diagnosis during the later
stages of HCC, with the development of clinical symptoms and a high
rate of recurrence or metastases following curative resection, are
considered the main reasons for poor prognosis (4,5).
Thus, further investigation into the mechanisms underlying HCC may
provide a reliable scientific basis for improving treatment methods
and diagnosis.
Semaphorins comprise a large family of secreted or
membrane-bound proteins that function as crucial regulators of
morphogenesis and homeostasis in a wide range of organ systems,
subsequently influencing a variety of biological processes from
cell migration to cytokine release (6–8). In
addition, semaphorins regulate tumor angiogenesis, tumor growth,
cancer cell invasiveness and metastatic spreading (9). The cellular functions of semaphorins
are the result of the receptors, plexins and neuropilins (10). Plexins can be divided into four
homology groups, named the plexin-A (plexin-A1, plexin-A2,
plexin-A3 and plexin-A4), -B (plexin-B1, plexin-B2 and plexin-B3),
-C (plexin-C1) and -D (plexin-D1) subfamilies (10).
Plexin-B3 plays an important role as a regulator in
a multitude of biological processes and the occurrence of tumors
(11,12). The functions of plexin-B3 are
associated with semaphorin 5A (Sema5A), as a high-affinity receptor
(13,14). Upon semaphorin-independent
signaling mechanisms, plexin-B3 influences neuronal morphogenesis
or function and interacts with Rin (15). Li et al reported that
plexin-B3, upon stimulation by its ligand Sema5A, can inhibit the
migration and invasion of glioma cells (16). Plexin-B3 mutations have been
identified in prostate cancer (17), breast cancer (18) and melanoma (19). In addition, the overexpression of
Sema5A and plexin-B3 in gastric carcinoma have been shown to
correlate with the invasion and metastasis of tumors (20). However, the expression and
localization of plexin-B3 in HCC remain unknown.
In the present study, the mRNA and protein
expression levels of plexin-B3 were analyzed in HCC samples and
corresponding adjacent non-cancerous tissue, in order to
preliminarily analyze the association with the occurrence of
HCC.
Materials and methods
Samples and clinicopathological data
Paired HCC samples and the corresponding adjacent
non-cancerous tissues were obtained from 14 patients who had
undergone a liver resection at the Xiangya Hospital of Central
South University (Changsha, China). The tissue samples were
immediately snap-frozen in liquid nitrogen, and stored long-term at
−80°C in a freezer. Of the 14 patients, 12 were male and two were
female, with a gender ratio of 6:1 (male/female). The mean age of
the patients was 52 years old.
In total, 84 HCC archived specimens were obtained
from the tissue bank of the Department of Pathology in the Xiangya
Hospital of Central South University. All patients had been treated
surgically between 2011 and 2012, and the HCC specimens had been
routinely processed with 10% formalin fixation and paraffin
embedding prior to archiving. The clinical and pathological
features of the 84 HCC cases were described briefly. Of the 84
patients, 68 were male and 16 were female, with a gender ratio
(male/female) of 4.25:1. The mean age of the patients was 50 years.
According to the microscopic pathological characteristics, the
histological grade of tumor differentiation was assigned. In total,
14% of tumors were well-differentiated, 64% were
moderately-differentiated and 21% of tumors were classified with
poor differentiation. The majority of patients (70 cases) had a
single tumor, while 11 cases had multiple tumors. A microscopic
capsule and/or vascular invasion was observed in ~69% of the
patients. Tumor staging was conducted and 36 cases were assigned as
stage I, 40 cases were stage II, seven cases were stage III and one
case was classified as stage IV. Hepatic cirrhosis was recorded in
58% of the patients. All pathological diagnoses were based on the
World Health Organization’s criteria (21), while histological classification
and tumor differentiation were conducted according to the Edmondson
and Steiner grading system (22).
Tumor staging was defined according to to the Sixth Edition of TNM
Classification guidelines, which was jointly promulgated by the
American Joint Committee on Cancer and the International Union
Against Cancer (23).
Written informed consent was provided by all the
patients, and all the experimental protocols of the study were
approved by the Ethics Committee of Xiangya Hospital of Central
South University.
Quantitative polymerase chain reaction
(PCR)
Total RNA was extracted from the frozen tumor
specimens using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). A 2-μg sample of total RNA was used for the
synthesis of Oligo (dT)-primed single stranded cDNA using a First
Strand cDNA Synthesis kit (Fermentas, Burlington, ON, Canada). The
cDNA products were amplified using a SYBR PrimeScript RT-PCR kit
(Takara Bio, Inc., Otsu, Japan), according to the manufacturer’s
instructions. GAPDH served as an internal control for the total
cDNA content. The sequence of oligonucleotides used as PCR primers
were as follows: Plexin-B3 forward, 5′-GGCTGGTCACCTGACCCTAT-3′ and
reverse, 5′-CCCACTGTTGCTCCATCTG-3′; GAPDH forward,
5′-AGGCTAGCTGGCCCGATTTC-3′ and reverse,
5′-TGGCAACAATATCCACTTTACCAGA-3′. The relative mRNA expression
levels of plexin-B3 were measured using Ct values, corrected for
GAPDH expression, according to the following equation:
2−ΔCt [ΔCt = Ct (target gene) − Ct (internal control)].
All experiments were performed in triplicate.
Western blot analysis
Frozen tumor specimens were treated in
radioimmunoprecipitation assay lysis buffer (50 mM Tris-HCl, 150 mM
NaCl, 1% Triton X-100, 1% sodium deoxycholate and 0.1% SDS)
containing a protease inhibitor cocktail (Roche Diagnostics, Basel,
Switzerland) for 30 min on ice. Equal amounts of protein were
separated by 10% SDS-PAGE, and transferred onto polyvinylidene
difluoride membranes (Sigma-Aldrich Shanghai Trading Co., Ltd.,
Shanghai, China). After blocking with 5% skim milk solution for 2
h, the membranes were incubated with a rabbit polyclonal plexin-B3
antibody (1:200; cat. no. sc-67144, Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) or a mouse monoclonal β-actin antibody
(1:1,000; cat. no. 60008-1-Ig, Proteintech, Chicago, IL, USA) at
4°C overnight. The membranes were then incubated with appropriate
secondary antibodies: Goat anti-rabbit (cat. no. sc-2004) or goat
anti-mouse (cat. no. sc-2005) horseradish peroxidase
(HRP)-conjugated immunoglobulin G (1:2,000; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature, and the signal of
the protein was revealed using an enhanced chemiluminescence method
(Auragene Bioscience, Inc., Changsha, China). Band intensities were
quantified using image analysis software (Bio-Rad Laboratories,
Hercules, CA, USA). Each experiment was repeated a minimum of three
times.
Immunohistochemistry (IHC)
IHC analysis of the paraffin-embedded sections was
performed according to a two-step protocol (Polink-2 Plus Polymer
HRP Detection System; Golden Bridge International, Inc., Bothell,
WA, USA). Briefly, the sections were deparaffinized in xylene and
hydrated in a graded series of ethanol (100–50%) and tap water. A
high pressure method was selected to perform antigen retrieval in
citrate buffer (0.01 M, pH 6.0). Subsequently, the sections were
incubated in 3% H2O2 at room temperature for
10 min to block the endogenous peroxidase activity. After washing
in phosphate-buffed saline, the sections were treated with an
anti-plexin-B3 antibody (Santa Cruz Biotechnology, Inc.) at 4°C
overnight. The sections were incubated with a polymer helper
(Golden Bridge International, Inc.) at 37°C for 20 min, followed by
incubation with a poly-horseradish peroxidase-conjugated
anti-mouse/rabbit IgG at the same temperature and duration.
Diaminobenzidine solution was used to stain the sections. The
staining reactions were observed carefully under a microscope
(BX51; Olympus Corporation, Tokyo, Japan) and stopped with tap
water. Finally, the sections were counterstained with hematoxylin.
Negative controls were obtained by omission of the primary
antibodies in all the IHC procedures.
The sections were observed by two independent
pathologists. IHC staining was classified according to the
percentage of cells with a positive score for staining. Firstly,
the staining intensity was divided into four levels and scored as
follows: 0, negative; 1, weak; 2, moderate; and 3, high. Secondly,
the percentage of positive cells was divided into four levels and
scored as follows: 0, 0% positive cells; 1, <30% positive cells;
2, 30–70% positive cells; and 3, >70% positive cells.
Subsequently, the sum of the two scores for staining intensity and
the percentage of positive cells was used as a final score for each
sample. Samples were classified as negative (−) if the final score
was 0, weak positive (+) if the final scores were 1–2, moderate
positive (++) if the final scores were 3–4 and strong positive
(+++) if the final scores were 5–6. Plexin-B3 demonstrated membrane
and cytoplasm staining; however, no signal was observed in the
negative controls.
Statistical analysis
Comparisons between groups were statistically
analyzed using the two-tailed Student’s t-test and the
χ2 test. Statistical analyses were performed using SPSS
19.0 (IBM, Armonk, NY, USA) software for Windows, where P<0.05
was considered to indicate a statistically significant
difference.
Results
mRNA expression levels of plexin-B3 in
HCC
To examine the biological significance of plexin-B3
in HCC, 14 pairs of HCC samples and corresponding adjacent
non-cancerous tissues were analyzed by quantitative PCR. The mRNA
expression levels of plexin-B3 were found to be significantly
decreased in 11 of the 14 (78.6%) HCC samples when compared with
the corresponding adjacent non-cancerous tissue (P<0.05,
two-tailed Student’s t-test; Fig.
1A). These results indicated that plexin-B3 may play a tumor
suppressor role in hepatocarcinogenesis.
Protein expression levels of plexin-B3 in
HCC
Protein expression levels of plexin-B3 were analyzed
in HCC samples and the corresponding adjacent non-cancerous tissue
by western blot analysis. A representative result of western blot
analysis for the expression of plexin-B3 is shown in Fig. 1. The protein expression levels of
plexin-B3 were found to be downregulated in the HCC samples when
compared with the corresponding adjacent non-cancerous tissue
(P<0.05, two-tailed Student’s t-test; Fig. 1B and C), which was consistent with
the results from the quantitative PCR analysis.
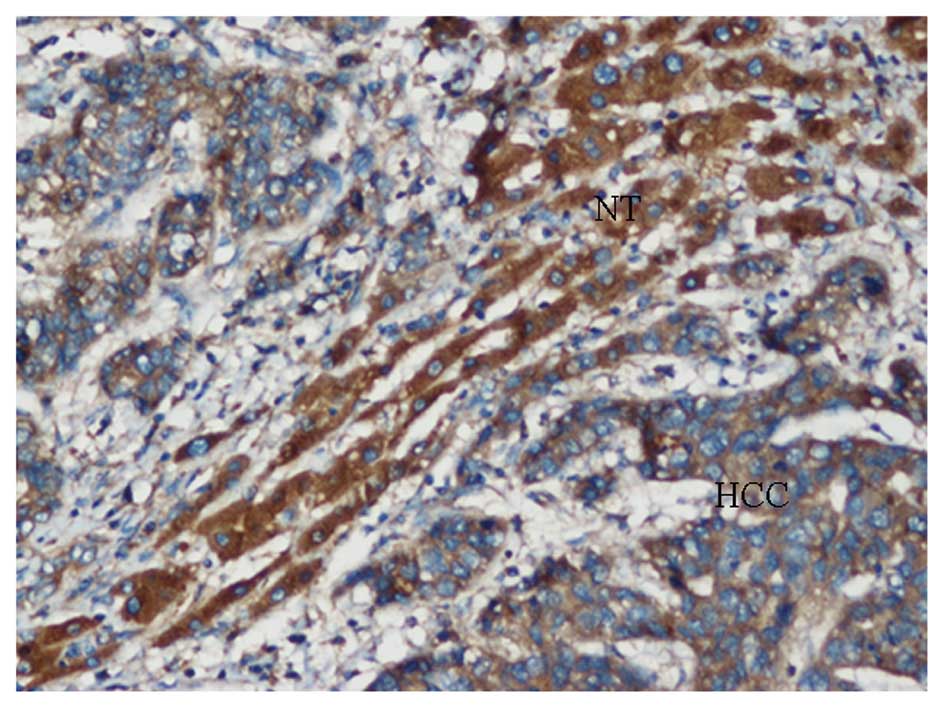
Immunohistochemical characteristics
Observations of the hematoxylin and eosin-stained
sections revealed the HCC cells to be relatively homogenous when
the necrotic, hemorrhagic and fibrotic components were excluded.
Plexin-B3 expression was negative or low in the majority of HCC
samples, with positive staining observed in the membrane and
cytoplasm of the tumor cells in positive cases. Only 40 samples of
the 84 cases exhibited positive expression, with a positive
expression rate of 47.6%. In the corresponding adjacent
non-cancerous tissue, membrane and cytoplasm staining of plexin-B3
were observed in hepatocyte cells. The majority of cases (70.2%;
59/84) revealed staining patterns with an intermediate or strong
staining intensity, with a positive score for plexin-B3 expression
in 84 HCC patients (Figs. 2 and
3). Statistical analysis indicated
that plexin-B3 expression was significantly downregulated in the
HCC samples when compared with the adjacent non-cancerous tissue
(P<0.05, χ2 test; Table
I).
 | Table IExpression of plexin-B3 in 84 cases of
HCC samples and adjacent non-cancerous tissue by IHC analysis. |
Table I
Expression of plexin-B3 in 84 cases of
HCC samples and adjacent non-cancerous tissue by IHC analysis.
| | Plexin-B3
expression | |
|---|
| |
| |
|---|
| Tissue type | Cases (n) | (+++) | (++) | (+) | (−) | P-value |
|---|
| HCC samples | 84 | 10 | 17 | 13 | 44 | 0.003 |
| Adjacent
non-cancerous tissue | 84 | 12 | 20 | 27 | 25 |
In order to improve the understanding of the
potential roles of plexin-B3 in HCC development and progression,
associations between plexin-B3 expression and clinicopathological
characteristics of HCC patients were analyzed. The results
indicated that the loss of plexin-B3 expression was associated with
the patient gender (P=0.01, χ2 test; Table II) and tumor size (P=0.001,
χ2; Table II);
however, there were no correlations with age, histology, tumor
stage, tumor number, capsule invasion, microvascular invasion and
liver cirrhosis (Table II).
 | Table IIClinicopathological features of the
HCC patients with positive and negative expression of
plexin-B3. |
Table II
Clinicopathological features of the
HCC patients with positive and negative expression of
plexin-B3.
| | Plexin-B3 expression
(n) | |
|---|
| |
| |
|---|
| Pathological
features | Cases (n) | Positive | Negative | P-value |
|---|
| Gender |
| Male | 68 | 27 | 31 | 0.01 |
| Female | 16 | 3 | 13 | |
| Age (years) |
| >50 | 37 | 19 | 18 | 0.543 |
| ≤50 | 47 | 21 | 26 | |
| Histology |
| Well | 12 | 9 | 3 | 0.121 |
| Moderately | 54 | 23 | 31 | |
| Poor | 18 | 8 | 10 | |
| Tumor stage |
| I+II | 76 | 36 | 40 | 0.887 |
| III+IV | 8 | 4 | 4 | |
| Node number (NI,
3) |
| Single | 70 | 31 | 39 | 0.232 |
| Multiple | 11 | 7 | 4 | |
| Tumor size (cm; NI,
3) |
| >5 | 30 | 7 | 23 | 0.001 |
| ≤5 | 51 | 31 | 20 | |
| Capsule
invasion |
| Yes | 41 | 19 | 22 | 0.819 |
| No | 43 | 21 | 22 | |
| Microvascular
invasion |
| Yes | 41 | 20 | 21 | 0.835 |
| No | 43 | 20 | 23 | |
| Liver
cirrhosis |
| Yes | 49 | 27 | 22 | 0.104 |
| No | 35 | 13 | 22 | |
Discussion
Plexins are receptors for multiple classes of
semaphorins that mediate the function of semaphorins, alone or in
combination with neuropilins, including roles in cell repulsion,
integrin function, cell migration and cell survival (6,10,24).
In recent years, there has been increasing evidence indicating the
important roles that plexins play in a variety of tumor initiation
and progression processes (9,11).
In the present study, the expression of plexin-B3 was analyzed in
HCC samples and corresponding adjacent non-cancerous tissue using
quantitative PCR and western blot analysis. The results revealed
that the mRNA and protein expression levels of plexin-B3 were
significantly downregulated in the HCC samples when compared with
the corresponding adjacent non-cancerous tissue. There is a
possible tendency for plexin-B3 to function as a putative tumor
suppressor in HCC. The spatial distribution and expression levels
of plexin-B3 were further confirmed by IHC staining. Plexin-B3
positive staining was observed in the membrane and cytoplasm of the
tumor cells and hepatocytes. Plexin-B3 immunoreactivity in the HCC
samples was significantly lower compared with the corresponding
adjacent non-cancerous tissue, and the loss of plexin-B3 expression
was found to correlate with the patient gender and tumor size. In
addition, plexin-B3 expression levels in female HCC patients were
significantly lower compared with those in male HCC patients, and
the positive rate of plexin-B3 staining was significantly decreased
in tumors of a large size (>5 cm in diameter) compared with
tumors of a small size (≤5 cm in diameter). These results indicate
that plexin-B3 may be used as a potential biological target for the
diagnosis, progression and prognosis of HCC.
Semaphorins and their receptors, including plexins
and neuropilins, are aberrantly expressed in human tumors, and can
promote or inhibit cancer progression, with certain receptors
exerting a dual role (11). Kantor
et al (25) reported that
Sema5A is a bifunctional guidance cue, which exerts both attractive
and inhibitory effects on developing axons of the fasciculus
retroflexus. The neuronal responses to Sema5A are regulated by
heparin and chondroitin sulfate proteoglycans. Recently, several
studies have reported that Sema5A has a dual effect on cell
migration (26–28). Li and Lee found that Sema5A
inhibited the Rac1 GTPase through stimulation of plexin-B3, which
resulted in the inhibition of glioma cell migration and invasion
(16). However, overexpression of
Sema5A in pancreatic cancer has been shown to correlate with
invasion, metastasis and increased endothelial cell proliferation
(28,29), while overexpression of Sema5A and
plexin-B3 are associated with the invasion and metastasis of
gastric carcinoma (20). In the
present study, in order to improve the understanding into the
expression and role of plexin-B3 in HCC as a specific Sema5A
receptor, a number of assays were performed. Plexin-B3 expression
in HCC cases was shown to be downregulated, indicating that
plexin-B3 may exert a suppressive effect on HCC tumors. Therefore,
it is possible that the Sema5A/plexin-B3 signaling pathway may
exert tumor promoting and suppressive effects depending on the type
of malignancy.
In conclusion, to the best of our knowledge, the
present study is the first to investigate the expression levels of
plexin-B3 in HCC samples and the associations with
clinicopathological data. The results indicated that plexin-B3
expression is downregulated in HCC, and the expression levels
correlate with gender and tumor size. However, future studies are
required to investigate the exact mechanisms underlying the role of
plexin-B3 in the progression of HCC. Although the current results
are not able to describe the full properties of plexin-B3 in HCC,
the observations indicate that plexin-B3 plays an important role in
the development and occurrence of HCC; thus, the receptor may be
used as a predictive and therapeutic biomarker.
Acknowledgements
The study was supported by grants from the National
Natural Science Foundation of China (no. 81270501) and the
Exploring Program of Central South University (no.
2012QNZT080).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perz JF, Armstrong GL, Farrington LA,
Hutin YJ and Bell BP: The contributions of hepatitis B virus and
hepatitis C virus infections to cirrhosis and primary liver cancer
worldwide. J Hepatol. 45:529–538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shin JW and Chung YH: Molecular targeted
therapy for hepatocellular carcinoma: current and future. World J
Gastroenterol. 19:6144–6155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trevisani F, Cantarini MC, Wands JR and
Bernardi M: Recent advances in the natural history of
hepatocellular carcinoma. Carcinogenesis. 29:1299–1305. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Y, Nagano H, Ota H, et al: Patterns
and clinicopathologic features of extrahepatic recurrence of
hepatocellular carcinoma after curative resection. Surgery.
141:196–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kruger RP, Aurandt J and Guan KL:
Semaphorins command cells to move. Nat Rev Mol Cell Biol.
6:789–800. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yazdani U and Terman JR: The semaphorins.
Genome Biol. 7:2112006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mann F, Chauvet S and Rougon G:
Semaphorins in development and adult brain: Implication for
neurological diseases. Prog Neurobiol. 82:57–79. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tamagnone L: Emerging role of semaphorins
as major regulatory signals and potential therapeutic targets in
cancer. Cancer Cell. 22:145–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tamagnone L, Artigiani S, Chen H, et al:
Plexins are a large family of receptors for transmembrane,
secreted, and GPI-anchored semaphorins in vertebrates. Cell.
99:71–80. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rehman M and Tamagnone L: Semaphorins in
cancer: biological mechanisms and therapeutic approaches. Semin
Cell Dev Biol. 24:179–189. 2013. View Article : Google Scholar
|
|
12
|
Zhou Y, Gunput RA and Pasterkamp RJ:
Semaphorin signaling: progress made and promises ahead. Trends
Biochem Sci. 33:161–170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sadanandam A, Varney ML and Singh RK:
Identification of semaphorin 5A interacting protein by applying
apriori knowledge and peptide complementarity related to protein
evolution and structure. Genomics Proteomics Bioinformatics.
6:163–174. 2008. View Article : Google Scholar
|
|
14
|
Artigiani S, Conrotto P, Fazzari P, et al:
Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep.
5:710–714. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hartwig C, Veske A, Krejcova S,
Rosenberger G and Finckh U: Plexin B3 promotes neurite outgrowth,
interacts homophilically, and interacts with Rin. BMC Neurosci.
6:532005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X and Lee AY: Semaphorin 5A and
plexin-B3 inhibit human glioma cell motility through
RhoGDIalpha-mediated inactivation of Rac1 GTPase. J Biol Chem.
285:32436–32445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong OG, Nitkunan T, Oinuma I, et al:
Plexin-B1 mutations in prostate cancer. Proc Natl Acad Sci USA.
104:19040–19045. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tong Y, Hota PK, Hamaneh MB and Buck M:
Insights into oncogenic mutations of plexin-B1 based on the
solution structure of the Rho GTPase binding domain. Structure.
16:246–258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Balakrishnan A, Penachioni JY, Lamba S, et
al: Molecular profiling of the ‘plexinome’ in melanoma and
pancreatic cancer. Hum Mutat. 30:1167–1174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan GQ, Ren HZ, Zhang SF, Wang XM and Wen
JF: Expression of semaphorin 5A and its receptor plexin B3
contributes to invasion and metastasis of gastric carcinoma. World
J Gastroenterol. 15:2800–2804. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: a study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wittekind C: Pitfalls in the
classification of liver tumors. Pathologe. 27:289–293. 2006.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Greene FL, Page DL, Fleming ID, Fritz AG,
Balch CM, Haller DG and Morrow M: TNM classification of Malignant
Tumors. American Joint Committee on Cancer: AJCC Cancer Staging
Manual. 6th ed. Springer; New York, NY: pp. 1332002
|
|
24
|
Casazza A, Fazzari P and Tamagnone L:
Semaphorin signals in cell adhesion and cell migration: functional
role and molecular mechanisms. Adv Exp Med Biol. 600:90–108. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kantor DB, Chivatakarn O, Peer KL, et al:
Semaphorin 5A is a bifunctional axon guidance cue regulated by
heparan and chondroitin sulfate proteoglycans. Neuron. 44:961–975.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan G, Zhu Z, Huang J, et al: Semaphorin
5A promotes gastric cancer invasion/metastasis via urokinase-type
plasminogen activator/phosphoinositide 3-kinase/protein kinase B.
Dig Dis Sci. 58:2197–2204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan G, Zhang X, Ren J, et al: Semaphorin
5A, an axon guidance molecule, enhances the invasion and metastasis
of human gastric cancer through activation of MMP9. Pathol Oncol
Res. 19:11–18. 2013. View Article : Google Scholar
|
|
28
|
Sadanandam A, Sidhu SS, Wullschleger S, et
al: Secreted semaphorin 5A suppressed pancreatic tumour burden but
increased metastasis and endothelial cell proliferation. Br J
Cancer. 107:501–507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sadanandam A, Varney ML, Singh S, et al:
High gene expression of semaphorin 5A in pancreatic cancer is
associated with tumor growth, invasion and metastasis. Int J
Cancer. 127:1373–1383. 2010. View Article : Google Scholar : PubMed/NCBI
|