Introduction
Peripheral nerve injury often leads to various
degrees of permanent dysfunction. Although peripheral nerve injury
has been extensively studied, the biochemical, immunological and
neurological pathology following nerve injury has not been fully
elucidated. The primary factor in peripheral nerve injury is
primary axonal injury caused by physical mechanics. The secondary
injury is caused by a cascade of inflammatory reactions, including
vascular permeability changes and macrophage invasion (1). The internal environment of nerve
fibers changes with peripheral nerve injury and is affected by a
variety of local factors (including cytokines and humoral factors),
which directly or indirectly impact the injury and regeneration
reactions of peripheral nerves. Nerve tissues are particularly
sensitive to oxidative stress [excessive reactive oxygen species
(ROS) and reactive nitrogen radicals], and excessive quantities of
free radicals affect functional recovery following nerve injury
(2,3). It has been suggested that the
expression of nitric oxide synthase (NOS)-1 in the spinal cord
might be responsible for the maintenance of chronic peripheral
neuropathic pain in mice (4).
There is evidence that an increase in the level of inducible NOS
(iNOS) expression following sciatic nerve injury in rats may play a
role in nerve regeneration and that excessive iNOS expression
following nerve injury may hinder nerve regeneration (5). Lipid peroxidation caused by free
radicals plays an important role in tissue damage following
peripheral nerve injury (6,7).
Therefore, diseases associated with oxidative stress, drugs or
environmental factors can affect functional recovery after nerve
injury (8).
Compared with body fluids, seawater provides a
hypertonic, high-sodium and high-alkali environment. With a low
temperature, a high osmotic pressure and many kinds of bacteria,
seawater entering a body cavity or seawater immersion of a wound
may result in more serious problems that need to be urgently
addressed during trauma treatment. Few researchers have studied on
open injury combined with seawater immersion. There have been
studies on dermatoses caused by marine organisms and the impact of
seawater drowning injury (9,10),
as well as the effects of various rewarming methods on seawater
immersion-induced hypothermia (11). Until now, some studies on injuries
with seawater immersion have involved superficial soft tissue
injury, limbs injury, traumatic brain injury and open chest and
abdominal injuries (12–14). Pan et al studied topical
dorsal skin immersion in seawater in mice and reported that
immersion can cause time-dependent apoptosis and proliferation in
the epidermis (15). Chen et
al (16) found in a rabbit
model of a firearm-induced limb wound combined with seawater
immersion that lipid peroxidation in the firearm injury immersed in
seawater was strengthened, which thereby increased peroxidation of
the injury (15). In a study of
gunshot wounds of rabbits, Liu et al found that with
concomitant seawater immersion of the femoral arteries there was
marked swelling of cells as well as of the intercellular space in
the wound tract and area with contusion (17). Nitric oxide (NO) levels in skeletal
muscle tissues with firearm injury in limbs immersed in seawater
have been found to be significantly higher than those before
immersion, which may be associated with NOS activation, leading to
secondary injury in skeletal muscle tissues (18).
Although studies have shown that seawater immersion
aggravates damage to a variety of human organs and tissues, it
remains unknown whether it increases peripheral nerve injury or
delays neuronal recovery. Open hip or thigh injuries often cause
sciatic nerve injury. Many previous studies of sciatic nerve injury
are based on the terrestrial environment, but there are huge
differences, both in physical and chemical properties and
biological components, compared with those in the marine
environment. The nerve crush injury model is used to study the
degeneration and regeneration process of nerve fibers following
peripheral nerve injury (19,20).
In crush injury, in which there is contusion and laceration caused
by open injuries, nerve tissues can be directly in contact with
seawater and this immersion in seawater may participate in and
influence the development of pathophysiological processes following
nerve injury. The conditions may be more serious in sciatic nerve
injury. In this study, a rat sciatic nerve crush injury model with
seawater immersion was established and the impact of seawater
immersion was observed on neuronal recovery and pathological
changes following sciatic nerve injury. On this basis, changes in
the contents of ROS, malondialdehyde (MDA) and iNOS in injured
nerve tissues were detected to explore the possible mechanism of
secondary sciatic nerve injury caused by seawater immersion and
provide an important theoretical basis for further clinical
treatment.
Materials and methods
Preparation of artificial seawater
Artificial seawater (ASW) was prepared according to
Iannacone et al (21) [460
mM NaCl, 10 mM KCl, 10 mM CaCl2, 22 mM MgCl2,
26 mM MgSO4 and 10 mM
2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (HEPES; pH
7.8)].
Animals and grouping
A total of 234 specific pathogen-free Sprague-Dawley
(SD) male rats that were 8 weeks old and weighed 200–250 g were
provided by the Experimental Animal Center of Anhui Medical
University (Hefei, China). The animals were randomly divided into
three groups with 78 rats in each group, namely the sham group,
injury control group and seawater immersion + injury group. In the
injury control group, the sciatic nerve was crushed with an artery
clip. In the seawater immersion + injury group, after the sciatic
nerve was crushed, rats were immersed in ASW for 1 h followed by
wound debridement and suturing. In the sham group, the rats
received a surgical incision similar to that in the other two
groups but without nerve injury or seawater immersion. Animal
feeding and management were performed according to the guidelines
for animal experiments at Anhui Medical University. All rats were
fed in dedicated cages with 6 rats in each cage. Under consistent
feeding and management conditions, the rats were fed dedicated rat
food and drank water freely and had 12 h of illumination time per
day. All experimental procedures involving animals were approved by
the Institutional Animal Care and Use Committee of Anhui Medical
University.
Surgical treatment
Rat sciatic nerve crush injury was performed
according to the procedure used by Pan et al (22). Rats were intraperitoneally injected
with 3% sodium pentobarbital (30 mg/kg) for anesthesia and were
fixed in the prone position. An incision was made in the middle of
the rear left femoral area and 3 cm sciatic nerve was exposed from
the lower edge of the piriformis to above the knee. One centimeter
under the piriformis, the sciatic nerve was clamped with an artery
clip and was crushed from the opposite direction twice, for 30 sec
each time. Microsutures (l0-0) were used to mark the distal injury.
In the seawater immersion + injury group, after the sciatic nerve
was crushed, the rats were immersed in ASW for 1 h followed by
wound debridement and suturing. Among the three groups, two rats
died from an overdose of anesthesia. Also, four rats in the
seawater immersion group died within 2 h after immersion. The
timely replenishment of rats was carried out.
Tissue preparation and slicing
Sciatic nerve tissues were drawn prior to and at 2,
6, 24 and 48 h, and 1 and 6 weeks after surgery. Rats were
anesthetized with 3% sodium pentobarbital and the injured side of
the sciatic nerve was exposed, after which 1 cm sciatic nerve was
removed from the distal injury and was frozen at −70°C. For each
group, six samples were preserved and the other six were fixed in
4% paraformaldehyde followed by dipping in wax, embedding and
sectioning (thickness, 4 μm).
Sciatic nerve function assessment
The Sciatic Functional Index (SFI) was used for the
assessment according to Pan et al (22). A 50×10-cm walking track was
prepared with a piece of white paper of equal length and width
placed at the bottom of the track. The rat plantar was dipped into
carbon ink and three or four bilateral hind foot prints were
clearly recorded. The SFI value of each group was calculated by the
Bain formula as follows: SFI = −38.3(EPL-NPL)/NPL +
109.5(ETS-NTS)/NTS + 13.3(EIT-NIT)/NIT − 8.8, where EPL is the
experimental print length; NPL is the normal print length; ETS is
the experimental toe spread; NTS is the normal toe spread; EIT is
the experimental intermediary toe spread; and NIT is the normal
intermediary toe spread. The print length, the toe spread and the
intermediary toe spread were obtained by measuring the prints of
experimental and normal feet. The data were accurate to the
millimeter. SFI = 0 corresponds with normal function and 100 with
complete dysfunction. All groups were assessed prior to surgery,
and at 24 h and each weekend of weeks 1–6 after surgery. At each
time-point, six rabbits were randomly selected for each group.
Electrophysiological testing
Electrophysiological testing was performed using the
evoked potential/electromyography measuring system Neuropack M1
MEB-9200K (Nihon Kohden Tomioka Corporation, Tomioka, Japan).
Following exposure of the sciatic nerve, two bipolar-protected
electrodes were placed 0.5 cm from both the far and near ends of
the injured nerve as the stimulating electrodes. The recording
electrode was a monopolar center needle electrode obliquely
inserted into the middle of the muscle belly in the triceps surae
muscle to record the compound muscle action potential and calculate
the amplitude, latency and motor nerve conduction velocity. All
groups were assessed at 6 weeks after surgery and each time six
rabbits were randomly selected for each group.
Morphological pathology observation
At 6 weeks after surgery, nerve specimens were cut
into continuous cross-sections and the specimens were stained using
hematoxylin and eosin (H&E) to observe nerve fiber
regeneration.
Detection of ROS and MDA in rat nerve
tissues
Cryopreserved rat neural tissues were thawed at room
temperature and diluted to 1% homogenate. Following centrifugation,
the levels of ROS and MDA in the supernatant were detected using a
biotin double-antibody sandwich enzyme-linked immunosorbent assay
(ELISA) according to the manufacturer’s instructions (E33106,
10417R; Nanjing Jiancheng Bioengineering Institute, Nanjing,
China).
Detection of iNOS
An immunohistochemistry assay was performed using a
two-step kit (cat no. PV-6000, Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd., Beijing, China). For each group, a section
was selected as the negative control to which no iNOS monoclonal
antibody was added. The rabbit anti-rat iNOS polyclonal antibody
(cat. no. BS-2072R; 1:200; 9 min incubation at 37°C),
biotin-labeled goat anti-rabbit IgG polyclonal antibody (1:300;
Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China; 30
min incubation at 37°C) and horseradish peroxidase-labeled
streptavidin working solution (1:300; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.) 30 min incubation at 37°C and were added
sequentially to the sections. Then, diaminobenzidine (DAB) was used
for chromogenic reaction (3 min incubation at 20°C) followed by
mounting, drying, transparency and mounting with neutral resin.
Brown granules were present only in positive cells. Under a
10×20-fold light microscope (BX-43; Olympus Corporation, Tokyo,
Japan), 10 non-overlapping fields of each section in the same area
were selected for analysis using a multimedia image analysis system
(Image-Pro Plus 7.0; Media Cybernetics, Inc., Rockville, MD, USA).
The absorbance value was measured, which indicated the relative
strength of iNOS expression.
iNOS mRNA expression was detected by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Total RNA was extracted from cryopreserved sciatic nerves using
TRIzol reagent (Thermo Fisher Scientific Inc., Waltham, MA, USA).
cDNA was synthesized according to the instructions of the
RevertAid™ First Strand cDNA Synthesis kit (Thermo Fisher
Scientific Inc.). The design and synthesis of primers used in this
study were completed by Life Technologies (Thermo Fisher Scientific
Inc.). Rat iNOS primer sequences for fluorescence qPCR were,
forward: 5′-GTTCTTTGCTTCTGTGCTAATGC-3′ and reverse:
5′-AGTTGTTCCTCTTCCAAGGTGTT-3′. β-actin was used as an internal
reference and its primer sequences were, forward:
5′-CCCATCTATGAGGGTTACGC-3′ and reverse:
5′-TTTAATGTCACGCACGATTTC-3′. qPCR analysis was performed using the
SYBR Green PCR kit (Qiagen GmbH, Hilden, Germany) on a real-time
PCR instrument (Thermo Scientific™ PikoReal™ Real-Time PCR system;
Thermo Fisher Scientific, Waltham, MA, USA). A total of 15 ng cDNA
of each sample was analyzed by PCR and the reaction conditions were
95°C for 5 min followed by 40 cycles of 95°C for 10 sec and 60°C
for 30 sec. Then, the melting curve was analyzed to ensure the
quality of the PCR products. The gene of interest in each tissue
was calibrated with its corresponding internal reference. The
analysis was repeated three times for each sample and the relative
quantitative analysis was performed using the 2−ΔΔCt
method.
Statistical analysis
Data are shown as mean ± standard error and were
analyzed using SPSS statistical software, version 13.0 (SPSS, Inc.,
Chicago, IL, USA). An α-value of P<0.05 was considered
statistically significant. Student’s t-tests and one-way analysis
of variance were used for pairwise comparison and multiple
comparisons, respectively.
Results
SFI values
No significant changes were found in the rat feet in
the sham group. In the injury and seawater immersion + injury
groups, the print length of the injured hind feet was longer and
the toe spread and intermediary toe spread were narrower than that
in the sham group. On the day following injury, the SFI values were
−71.21±3.13 and −85.35±2.78 for the injury and seawater immersion +
injury groups, respectively (P<0.05). At one week after injury,
rats in the two injury groups went through a slow and gradual
recovery process, which increased at 2–4 weeks and tended to be
stable at 5–6 weeks. The SFI values were significantly lower in the
seawater immersion + injury group compared with those in the injury
group (P<0.05, Fig. 1).
Electrophysiological testing
Results of nerve electrophysiological tests carried
out at 6 weeks after injury are shown in Table I. Compound muscle action potentials
of the seawater immersion + injury group are shown in Fig. 2. The latency was prolonged and the
amplitude and nerve conduction velocity decreased in the seawater
immersion + injury group compared with those in the sham group.
Comparisons between the three groups are shown in Table I. Differences between the groups
were statistically significant (P<0.05).
 | Table IComparison of electrophysiological
test results 6 weeks after injury. |
Table I
Comparison of electrophysiological
test results 6 weeks after injury.
| Groups | n | Latency (msec) | Amplitude (mV) | Conduction velocity
(m/sec) |
|---|
| Sham | 6 | 2.13±0.24 | 33.21±1.59 | 60.45±3.29 |
| Injury | 6 | 4.21±0.75 | 26.10±1.47 | 20.71±2.67 |
| Seawater immersion +
injury | 6 | 4.86±0.43a | 3.62±1.12a | 16.45±2.35a |
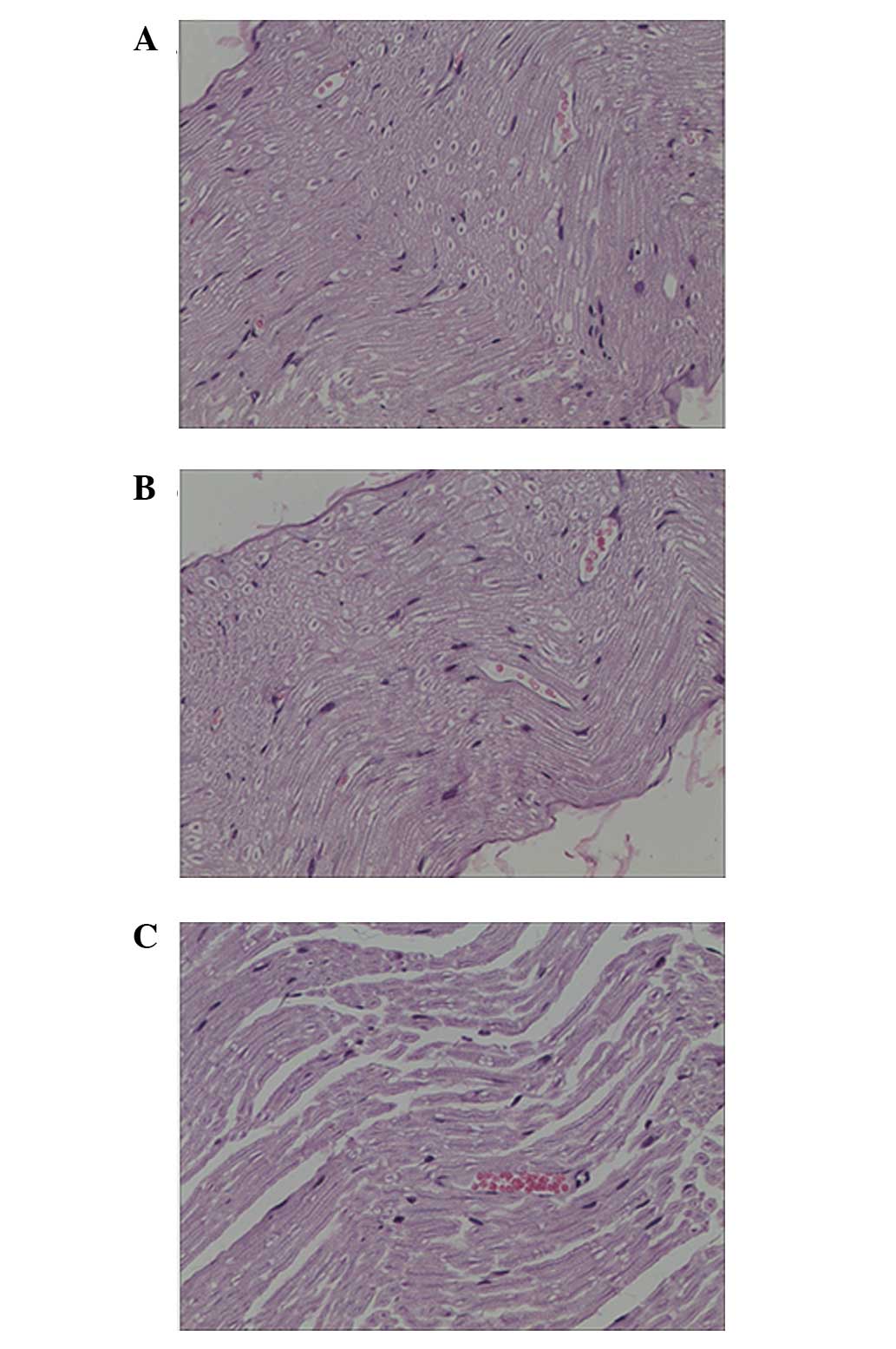
Pathological results
At 6 weeks after surgery, it was observed under the
optical microscope in the injury and seawater immersion + injury
groups that irregular annular regenerated nerve fibers grew with
different thickness, density and number. Following H&E
staining, numerous wispy tissues were visible in the injury group
and thick nerve fibers were arranged in neat rows. New capillaries
were observed between tissues. Wavy wispy tissues were also seen in
the seawater immersion + injury group. The nerve fibers were
thinner in the seawater immersion + injury group and new
capillaries were present between tissues that were mainly collagen
tissues (Fig. 3).
Levels of ROS and MDA in rat nerve
tissues
It is shown in Figs.
4 and 5 that the level of ROS
and MDA in the sham group did not significantly change at each
time-point. In the injury and seawater immersion + injury groups,
the levels of ROS and MDA gradually increased, peaking at 48 h
after injury. The levels of ROS and MDA in the seawater immersion +
injury group were higher than those in the sham and injury groups
at each time-point.
Expression of iNOS protein and mRNA in
rat sciatic nerve
Small amounts of iNOS-positive products were
detected in the sciatic nerve of the sham group. Following nerve
surgery, iNOS positive products gradually increased in quantity in
the injury and seawater immersion + injury groups, peaking at 48 h
after surgery. iNOS-positive cells were mainly macrophages and
Schwann cells with cytoplasm that was brownish yellow or brown with
uneven depth (Fig. 6A). The
average absorbance (A value) increased gradually and peaked at 2
days after surgery, after which it gradually decreased.
The expression level of iNOS mRNA in the sciatic
nerve in the sham group was very low at all time points. In the
injury and seawater immersion + injury groups, the level of iNOS
mRNA expression gradually increased and peaked at 48 h after
surgery. There were significant differences in the levels of iNOS
mRNA expression between each time-point in the injury and seawater
immersion + injury groups. The difference in mRNA expression level
was significant between the injury group and seawater immersion +
injury group at each time-point (P<0.01, Fig. 6B).
Discussion
Sciatic nerve crush injury is a relatively mild and
irreversible nerve injury that is frequently used in studies of
nerve regeneration (23,24). Motor function, mainly measured by
the SFI and Basso, Beattie and Bresnahan scores (22,25),
can directly reflect nerve function following peripheral nerve
injury. In the present study, severe limb dysfunction in rats was
found following both pure sciatic nerve crush injury and sciatic
nerve crush injury combined with seawater immersion; however, in
the seawater immersion + injury group the dysfunction was more
serious. In the first 2–4 weeks after injury, a fast functional
recovery was observed, which remained at a relatively stable status
at 5–6 weeks. Previous studies have shown that nerve function
returns to a stable state 2–4 weeks after sciatic nerve crush
injury (19,26), which is consistent with the results
of the present study. In this study, the SFI value in the seawater
immersion + injury group was lower than that in the injury group
and the difference was statistically significant, suggesting that
seawater immersion aggravated sciatic nerve injury and hindered the
recovery of neurological motor function.
The main function of peripheral nerves is to conduct
nerve impulses to the spinal cord and brain to form somatic or
visceral sensory sensations and conduct nerve impulses from the
brain and spinal cord to enable somatic or visceral movement.
Neuronal recovery is the key for determining the effects of nerve
regeneration after peripheral nerve injury. Nerve conduction, the
basic function of peripheral nerves, can be directly reflected by
the conduction velocity of the electrical activity of the neural
stem. The amplitude of the compound muscle action potential is
proportional to the number and size of regenerated axons (27). Nerve conduction velocity is
associated with the thickness and maturity of the myelin sheath. A
fast nerve conduction velocity can indirectly indicate a thick and
matured myelin sheath of regenerated nerve fibers (28). In the present study, it was shown
in Table I that the amplitude and
nerve conduction velocity of rat sciatic nerve action potentials in
the seawater immersion + injury group were lower than those of the
sham and injury groups (P<0.05), suggesting that seawater
immersion hindered axon regeneration and that the thickness and
maturity of the myelin sheath were less than that of the control
groups. These results indicate that seawater immersion weakened the
recovery of nerve conduction following sciatic nerve injury.
The response to and regeneration of the peripheral
nervous system following injury varies with the cause and extent of
injury. Pathological changes following peripheral nerve injury also
depend on the degree of injury. Wallerian degeneration following
peripheral nerve injury consists of a series of processes,
including axonal degeneration, myelin degeneration and
disintegration, Schwann cell proliferation, infiltration of
macrophages and mast cells, and axonal and myelin debris clearance
(29). Nerve fiber degeneration
after peripheral nerve injury is the response to injury and the
process of preparing for nerve regeneration. Peripheral nerve
regeneration is the process that occurs after the fracture of
peripheral nerve axons, when degenerated axons and myelin are
cleared with the establishment of a regenerated micro-environment,
after which nascent buds grow from axons proximal to the injury and
extend along the regenerative channel to target organs and contact
with them to achieve reinnervation of target organs (30). Histology is a traditional method to
evaluate the recovery of regenerated nerves. Nerve regeneration can
be indirectly reflected by histomorphological parameters, including
myelinated nerve fibers, regenerated axon diameter and myelin
sheath thickness. In the present study, histological examination of
regenerated nerves was conducted by conventional staining. At 6
weeks after surgery, relatively larger number of nerve fibers were
observed in the injury group, as well as axons with a long
diameter, whereas few nerve fibers were visible in the seawater
immersion + injury group. The pathological findings were consistent
with the neural electrophysiological results, indicating that nerve
regeneration in the injury group was better than that in the
seawater immersion + injury group, which strongly suggests that
seawater immersion inhibited peripheral nerve regeneration.
Hyperosmosis, high alkalinity, low temperature and
bacteria are generally accepted injury factors. Compared with body
fluids (including intracellular fluid and extracellular fluid),
seawater provides an environment of high sodium levels,
hyperosmosis and high alkalinity. Shapiro and Dinarello (31) demonstrated that peripheral blood
mononuclear cells expressed interleukin-8 (IL-8) mRNA and
synthesized IL-8 in hypertonic fluid. The synergistic effect of
IL-8 with bacterial lipopolysaccharide increased the synthesis of
tumor necrosis factor (TNF), and this increased as the acting time
of the hypertonic stimulating factor was prolonged. TNF can
directly activate monocytes/macrophages and neutrophils, resulting
in increased superoxide anion levels and tissue injury. Local
exposure of injured tissues to hypertonic seawater will lead to
intracellular dehydration and edema between tissues, which in turn
can easily lead to changes in intracellular and extracellular ion
concentrations and increase the burden on the cell membrane ion
pump (32). Furthermore, the low
temperature of seawater causes depletion of adenosine triphosphate,
resulting in a more active metabolism with significantly increased
requirements of oxygen and glucose, after which the reduction
products are increased in quantity, inducing a redox reaction.
Subalkaline seawater causes an imbalance of intracellular and
extracellular ion concentrations and increases the cell response to
injury (33). Under subalkaline
conditions, damaged cells are more likely to disintegrate, leading
to the release of membrane phospholipids and to lipid peroxidation
(34). Furthermore, tissue damage
may result in the saponification of fats or soluble basic protein,
which further increases the damage to the tissues (35). However, until now, investigations
concerning the mechanism by which seawater immersion affects open
injury have been lacking.
Peripheral nerves undergo a process of nerve
ischemia and inflammation after injury, during which oxygen
radicals and many toxic substances gather at the injury site,
changing the membrane permeability and stimulating calcium influx.
The proteolytic pathway is then activated, which leads to cell
damage, including damage to neurofilaments and microtubules
(36). Sayan et al
(37) reported similar findings in
a sciatic nerve ischemia-reperfusion injury model, and hypothesized
that this may be attributable to the myelin being rich with lipids,
which are the main target in the free radical-mediated lipid
peroxidation process. Liao et al (38) found that after the sciatic nerve is
crushed or tied, large amounts of oxygen free radicals are
produced, which may have an impact on peripheral nerve
regeneration. Oxygen radicals not only damage phospholipids in
nerve membranes, but also make myelin protein more vulnerable to
attack by ROS. Oxygen free radical-induced lipid peroxidation is an
important factor in the degeneration of nerve tissue following
injury. MDA is a lipid product formed by the action of free
radicals on unsaturated fatty acids in the cytoplasm, where two or
more double bonds of the unsaturated fatty acid are fractured after
being damaged by oxygen free radicals in the body. The
determination of MDA content can reflect the degree of lipid
peroxidation in the body and indirectly reflect the severity of the
attack on cells by free radicals (39). The present study demonstrated that
the levels of ROS and MDA in nerve tissues were increased following
sciatic nerve injury, and the levels of ROS and MDA were higher in
the seawater immersion + injury group than those in the sham and
injury groups. Also, the levels of ROS and MDA peaked at 48 h after
injury and were maintained at a high level for one week, indicating
that seawater immersion aggravated oxidative stress of nerve
tissues, lipid oxidation and nerve injury.
Reactive nitrogen comes from nitric oxide (NO), and
NOS is a major rate-limiting enzyme in the synthesis of NO in the
body. NOS includes neuronal NOS, endothelial NOS and iNOS. iNOS
mainly exists in macrophages and neutrophils and can be activated
in a variety of ways following tissue damage when excess NO is
produced. After nerve injury, iNOS is highly expressed and
excessive NO is produced, causing cell toxicity and further damage
to the nerve, which is not conducive to nerve regeneration. Shin
et al (40) reported that
iNOS expression was significantly increased following nerve
ischemia and reperfusion, and that iNOS synthesis was inhibited by
an iNOS specific inhibitor; this reduced NO production and thus may
play a protective role in peripheral nerve ischemia-reperfusion
injury. In the present study, the expression of iNOS protein and
mRNA was detected. The results revealed that the content of iNOS in
nerve tissues was increased after sciatic nerve injury, and was
higher in the seawater immersion + injury group than in the sham
and injury groups, peaked at 48 h after injury and was maintained
at a high level for one week. This indicated that seawater
immersion stimulated iNOS expression and increased reactive
nitrogen, thereby aggravating the oxidative stress to nerve tissues
and increasing nerve injury.
There are few studies that have investigated the
effect of seawater immersion on peripheral nerve injury. The
present study demonstrated that seawater immersion aggravated nerve
injury, hampered the recovery of neurological function, and
increased the oxidative stress in nerve tissues, as indicated by
increased ROS and MDA production and high expression levels of
iNOS. Further studies will be carried out to elucidate how
oxidative stress mediates the aggravating role of seawater
immersion in sciatic nerve injury.
Acknowledgements
This study was supported by a grant from the
Innovation of Science and Technology Project, Nanjing Military
Region (grant no. 09Z008).
References
|
1
|
Heumann R, Korsching S, Bandtlow C and
Thoenen H: Changes of nerve growth factor synthesis in nonneuronal
cells in response to sciatic nerve transection. J Cell Biol.
104:1623–1631. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ress AM, Babovic S, Angel MF, Im MJ,
Dellon AL and Manson PN: Free radical damage in acute nerve
compression. Ann Plast Surg. 34:388–395. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Love A, Cotter MA and Cameron NE: Effects
of alpha-tocopherol on nerve conduction velocity and regeneration
following a freeze lesion in immature diabetic rats. Naunyn
Schmiedebergs Arch Pharmacol. 355:126–130. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hervera A, Negrete R, Leánez S,
Martin-Campos JM and Pol O: The spinal cord expression of neuronal
and inducible nitric oxide synthases and their contribution in the
maintenance of neuropathic pain in mice. PLoS One. 5:e143212010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin H, Hou C and Chen D: Altered
expression of inducible nitric oxide synthase after sciatic nerve
injury in rat. Cell Biochem Biophys. 61:261–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khalil Z and Khodr B: A role for free
radicals and nitric oxide in delayed recovery in aged rats with
chronic constriction nerve injury. Free Radic Biol Med. 31:430–439.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naik AK, Tandan SK, Dudhgaonkar SP, et al:
Role of oxidative stress in pathophysiology of peripheral
neuropathy and modulation by N-acetyl-L-cysteine in rats. Eur J
Pain. 10:573–579. 2006. View Article : Google Scholar
|
|
8
|
Tariq M, Arshaduddin M, Biary N, Al Deeb S
and Al Moutaery K: Diethyldithiocarbamate (DEDC) impairs neuronal
recovery following sciatic nerve injury in rats. Restor Neurol
Neurosci. 17:135–141. 2000.PubMed/NCBI
|
|
9
|
Haddad V Jr, Lupi O, Lonza JP and Tyring
SK: Tropical dermatology: Marine and aquatic dermatology. J Am Acad
Dermatol. 61:733–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gregorakos L, Markou N, Psalida V, et al:
Near-drowning: clinical course of lung injury in adults. Lung.
187:93–97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tobias SW, Matthew CB, Dubose DA and
Hamlet MP: Comparison of oxygenated perfluorocarbon and humidified
oxygen for rewarming hypothermic miniswine. Mil Med. 166:853–861.
2001.PubMed/NCBI
|
|
12
|
Rui M, Duan YY, Zhang XH, Wang HL and Wang
DP: Urinary trypsin inhibitor attenuates seawater-induced acute
lung injury by influencing the activities of nuclear factor-κB and
its related inflammatory mediators. Respiration. 83:335–343. 2012.
View Article : Google Scholar
|
|
13
|
Fan ZF, Wang JH, Li ZQ and Yi CH:
Influence of sea water immersion on inflammation and healing of the
wounds in scalded rats. Zhonghua Shao Shang Za Zhi. 22:215–217.
2006.(In Chinese). PubMed/NCBI
|
|
14
|
Hu XH, Duan YY, Li Y and Xue ZQ: Early
responses of VEGF during acute lung injury induced by seawater
immersion after open chest trauma. Respiration. 79:490–496. 2010.
View Article : Google Scholar
|
|
15
|
Pan MH, Jiang SJ, Liu XH, et al: Topical
dorsal skin immersion in seawater induces apoptosis and
proliferation in hairless mice. J Dermatol. 34:683–690. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen L, Lai X, Chen Z and Zhang L: The
influence of seawater on lipid peroxidation of the muscle tissue
wounded by firearm in rabbit limbs. Zhongguo Bing Li Sheng Li Za
Zhi. 17:556–558. 2001.(In Chinese).
|
|
17
|
Liu P, Liu JC, Lai XN, et al: Pathological
study of rabbits’ femoral arteries subjected to gunshot wounds
combining with seawater immersion. Chin J Traumatol. 8:186–190.
2005.PubMed/NCBI
|
|
18
|
Lai X, Wang L and Chen L: Nitric oxide
level in firearm wounded skeletal muscle of rabbits extremities
after seawater immersion and its significance. Chuang Shang Wai Ke
Za Zhi. 1:26–28. 1999.(In Chinese).
|
|
19
|
Brown TJ, Khan T and Jones KJ: Androgen
induced acceleration of functional recovery after rat sciatic nerve
injury. Restor Neurol Neurosci. 15:289–295. 1999.
|
|
20
|
Kaya Y, Sarikcioğlu L, Aslan M, et al:
Comparison of the beneficial effect of melatonin on recovery after
cut and crush sciatic nerve injury: a combined study using
functional, electrophysiological, biochemical and electron
microscopic analyses. Childs Nerv Syst. 29:389–401. 2013.
View Article : Google Scholar
|
|
21
|
Iannacone JM, Ren S, Hatcher NG and
Sweedler JV: Collecting peptide release from the brain using porous
polymer monolith-based solid phase extraction capillaries. Anal
Chem. 81:5433–5438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan HC, Wu HT, Cheng FC, Chen CH, Sheu ML
and Chen CJ: Potentiation of angiogenesis and regeneration by G-CSF
after sciatic nerve crush injury. Biochem Biophys Res Commun.
382:177–182. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang M, Zhuge X, Yang Y, Gu X and Ding F:
The promotion of peripheral nerve regeneration by
chitooligosaccharides in the rat nerve crush injury model. Neurosci
Lett. 454:239–243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gong Y, Gong L, Gu X and Ding F:
Chitooligosaccharides promote peripheral nerve regeneration in a
rabbit common peroneal nerve crush injury model. Microsurgery.
29:650–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schiaveto de Souza A, da Silva CA and Del
Bel EA: Methodological evaluation to analyze functional recovery
after sciatic nerve injury. J Neurotrauma. 21:627–635. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tariq M, Arshaduddin M, Biary N, Al Deep S
and Al Moutaery K: Effect of aminoguanidine, a nitric oxide
synthase inhibitor on sciatic nerve crush injury in rats. Med Sci
Res. 25:815–818. 1997.
|
|
27
|
Yang Y, Ding F, Wu J, et al: Development
and evaluation of silk fibroin-based nerve grafts used for
peripheral nerve regeneration. Biomaterials. 28:5526–5535. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y, Ding F, Wu J, et al: Development
and evaluation of silk fibroin-based nerve grafts used for
peripheral nerve regeneration. Biomaterials. 28:5526–5535. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dubovy P: Wallerian degeneration and
peripheral nerve conditions for both axonal regeneration and
neuropathic pain induction. Ann Anat. 193:267–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gu X, Ding F, Yang Y and Liu J:
Construction of tissue engineered nerve grafts and their
application in peripheral nerve regeneration. Prog Neurobiol.
93:204–230. 2011. View Article : Google Scholar
|
|
31
|
Shapiro L and Dinarello CA: Hyperosmotic
stress as a stimulant for proinflammatory cytokine production. Exp
Cell Res. 231:354–362. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan H, Ge HJ, Lai XN and Liu HQ: Effect of
seawater immersion on hemostatic function after burn in dog.
Zhonghua Ma Zui Xue Za Zhi. 24:688–770. 2004.(In Chinese).
|
|
33
|
Luo ZA, Pu T and Huang Q: Treatment
analysis of calf injury from the final hypertrophic alkaline
seawater. Zhongguo Jiao Xing Wai Ke Za Zhi. 5:4671998.(In
Chinese).
|
|
34
|
Wei X and Hou SS: The characteristics and
treatment principle of seawater immersion firearm injury. Jie Fang
Jun Yu Fang Yi Xue Za Zhi. 21:76–78. 2003.(In Chinese).
|
|
35
|
Tikuisis P: Prediction of survival time at
sea based on observed body cooling rates. Aviat Space Environ Med.
68:441–448. 1997.PubMed/NCBI
|
|
36
|
Atik B, Erkutlu I, Tercan M,
Buyukhatipoglu H, Bekerecioglu M and Pence S: The effects of
exogenous melatonin on peripheral nerve regeneration and collagen
formation in rats. J Surg Res. 166:330–336. 2011. View Article : Google Scholar
|
|
37
|
Sayan H, Ozacmak VH, Ozen OA, et al:
Beneficial effects of melatonin on reperfusion injury in rat
sciatic nerve. J Pineal Res. 37:143–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liao W, Yang W and Tian J: Experimental
study on the effects of free peroxidation of neurolipid following
crushing injury of peripheral nerve. Zhongguo Xiu Fu Chong Jian Wai
Ke Za Zhi. 10:20–22. 1996.(In Chinese).
|
|
39
|
Baldus S, Rudolph V, Roiss M, et al:
Heparins increase endothelial nitric oxide bioavailability by
liberating vessel-immobilized myeloperoxidase. Circulation.
113:1871–1878. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shin SJ, Qi WN, Cai YT, et al: Inhibition
of inducible nitric oxide synthase promotes recovery of motor
function in rats after sciatic nerve ischemia and reperfusion. J
Hand Surg Am. 30:826–835. 2005. View Article : Google Scholar : PubMed/NCBI
|