Introduction
Lung cancer is the leading cause of cancer mortality
worldwide. It accounts for 22% of total cancer mortalities with
only a 7% 5-year survival expectancy in the United Kingdom
(1). Non-small cell lung cancer
(NSCLC) accounts for ~80% of all diagnosed cases of lung cancer
(2). Chemotherapy plays an
important role in the treatment of NSCLC. Anticancer drugs exert
their therapeutic action mainly by inducing tumor cell apoptosis.
However, the non-selective killing and toxicity of traditional
chemotherapy drugs limit their clinical application. In recent
years, Chinese herbal medicines have attracted attention for use as
anti-neoplastic medicines and adjuvant chemotherapeutic agents.
Accordingly, new therapeutic methods are worthy of development.
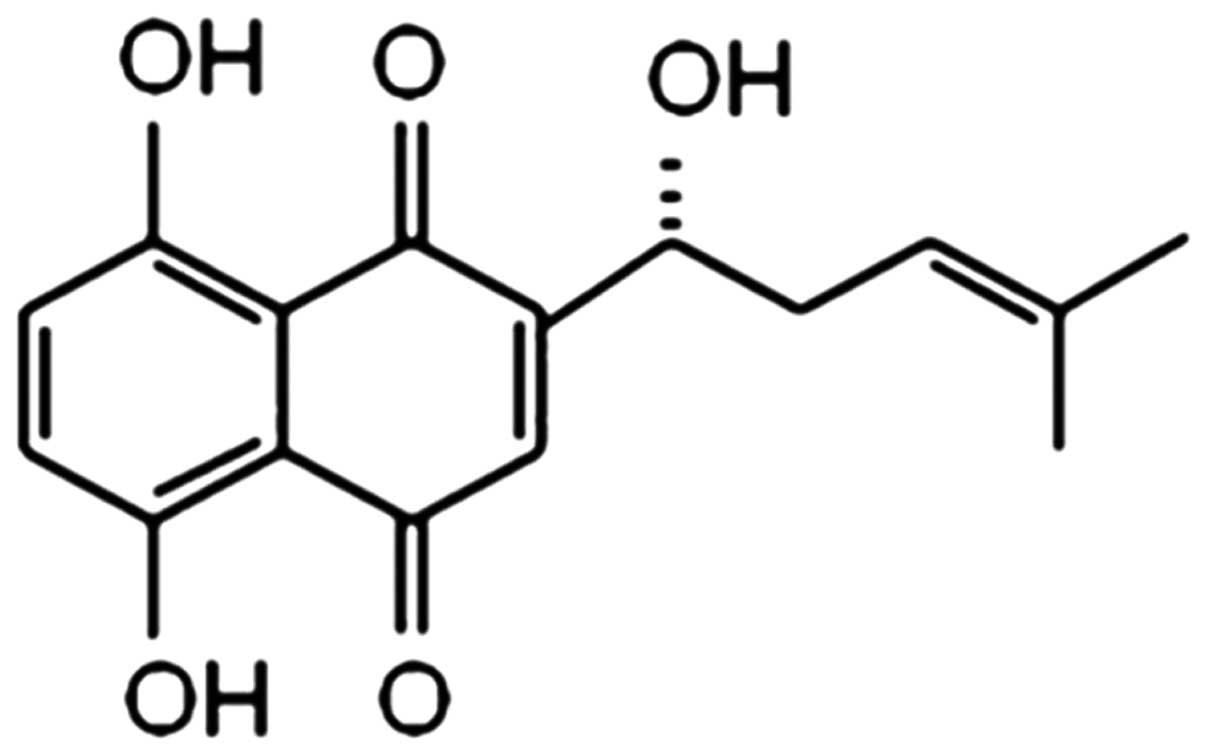
Shikonin (SK) is the major active ingredient
isolated from the dried roots of Lithospermum erythrorhizon,
with a molecular weight of 288 (Fig.
1). As a naphthoquinone pigment, SK exhibits multiple
biological activities such as anti-inflammatory, antimicrobial and
antitumor effects, as well as antagonism of the human
immunodeficiency virus (HIV) and the promotion of wound-healing
(3–5). Previous studies have demonstrated
that SK can induce apoptosis in various tumor cells, suggesting
that it could provide a novel option for antineoplastic therapy
(6,7).
Cell survival depends on the balance of apoptosis
and proliferation signals. The phosphoinositide 3-kinase (PI3K)/Akt
and extracellular signal-regulated kinase (ERK) signaling pathways
are the two major pro-proliferative and anti-apoptotic pathways; by
affecting the activation of downstream apoptosis-related proteins
and cell cycle regulatory proteins, they play important roles in
tumor cell proliferation, angiogenesis and metastasis as well as in
antagonistic chemotherapy. Studies have shown that components of
the PI3K/Akt and ERK signaling pathways are usually overexpressed
or activated excessively in numerous types of cancer, including
gastric, colon, lung and breast cancer (8–10).
Inhibition of the two pathways may increase the sensitivity of
tumor cells to cytotoxic drugs. However, whether SK induces
apoptosis in NCI-H460 lung cancer cells by affecting the PI3K/Akt
and/or ERK pathways is not known.
Cbl-b is one of the key members of the Cbl family of
ubiquitin ligases; it can induce the ubiquitination and degradation
of a variety of proteins, and participate in receptor tyrosine
kinase signaling (11). A number
of studies have found that Cbl-b can negatively regulate the
PI3K/Akt and ERK pathways by the ubiquitination of PI3K and ERK
(12–14). However, it remains unclear whether
Cbl-b is involved in SK-induced apoptosis. In the present study,
whether Cbl-b potentiates the apoptotic action of SK by inhibiting
the ERK and/or PI3K/Akt pathways was investigated in human NCI-H460
large-cell lung carcinoma cells.
Materials and methods
Reagents and antibodies
Mouse anti-human monoclonal anti-phospho (p)-ERK
(cat. no. sc-7383) and rabbit anti-human polyclonal anti-Cbl-b
(cat. no. sc-1704) antibodies were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). Rabbit anti-human monoclonal
anti-p-Akt (Ser-473) (cat. no. 3787s), rabbit anti-human polyclonal
anti-Akt (cat. no. 9272) and rabbit anti-human polyclonal
anti-ERK1/2 (cat. no. 9102s) antibodies were purchased from Cell
Signaling Technology (Beverly, MA, USA). Rabbit anti-human
polyclonal anti-β-actin antibodies (cat. no. ab16039) were
purchased from Abcam (Cambridge, UK). SK (>98% pure) was
purchased from the National Institute for the Control of
Pharmaceutical and Biological Products (Beijing, China). SK was
dissolved in dimethyl sulfoxide (DMSO) at a stock concentration of
100 mM, aliquoted, and stored at −20°C. Fetal bovine serum (FBS)
was purchased from Solarbio Science & Technology Co., Ltd.
(Beijing, China).
3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT), DMSO and Hoechst 33342 were purchased from Sigma-Aldrich
(St. Louis, MO, USA). The annexin V-fluorescein isothiocyanate
(FITC) and propidium iodide (PI) double staining kit was purchased
from Biosea Biotechnology Co., Ltd. (Beijing, China).
Cell culture
The NCI-H460 human large-cell lung carcinoma cells
were purchased from the Department of Cell Biology, China Medical
University (Shenyang, China). NCI-H460 cells were cultured in
RPMI-1640 medium Hyclone; Thermo Fisher Scientific, Rockford, IL,
USA) containing 10% FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin at 37°C under an atmosphere of 95% air and 5%
CO2. Cells were routinely subcultured every 2–3 days and
the cell samples used were all in the logarithmic growth phase.
Cell viability assay
Cells were seeded at 0.8×104 cells/well
in 96-well plates and incubated overnight. Different concentrations
(0.312–10 μM) of SK were then added and the cells were further
incubated for 24 and 48 h. Thereafter, 20 μl MTT solution (5 mg/ml)
was added to each well and the cells were incubated for another 4 h
at 37°C. Following the removal of culture medium, the cells were
lysed in 150 μl DMSO and the optical density (OD) was measured at
570 nm using a microplate reader (Model 550; Bio-Rad, Hercules, CA,
USA).
Analysis of cell apoptosis
NCI-H460 cells were plated at a density of
0.8×106 cells per well in 6-well plates overnight and
then treated with 2.1 or 2.6 μM SK for 24 h. The cells were then
collected. In addition, NCI-H460 cells were plated in 6-well plates
(0.8×106cells/well) overnight and were pretreated with
10 nM Ps341 (Active Biochem, Maplewood, NJ, USA), an inhibitor of
proteasome and Cbl, for 1 h. A total of 2.6 μM SK was then added to
the cells for a further 24 h, and the cells were collected. All of
the cells were then washed twice with PBS, and incubated with 10 μl
annexin V and 5 μl PI for 15 min in the dark. Samples were then
evaluated using a FACScan flow cytometer (Becton Dickinson, San
Jose, CA, USA).
Fluorescence microscopy
NCI-H460 cells were treated with 2.1 or 2.6 μM of SK
for 24 h. Then, the cells were collected, washed twice with PBS,
and fixed in a mixture of cold methanol and acetic acid (3/1, v/v)
prior to staining with Hoechst 33342 (1 mg/ml) for 30 min at 37°C.
Stained cells were observed with a fluorescence microscope
(magnification, ×400; Eclipse 90i, Nikon, Tokyo, Japan).
Western blot analysis
The expression levels of cellular proteins were
evaluated by western blotting. Following treatment with 2.6 μM SK
for 24 h, and pretreatment with 10 nM Ps341 or not for 1 h, the
cells were washed twice with ice-cold PBS and total proteins were
solubilized and extracted with lysis buffer (20 mM HEPES pH 7.9,
20% glycerol, 200 mM KCl, 0.5 mM EDTA, 0.5% NP-40, 0.5 mM DTT and
1% protease inhibitor cocktail). Protein concentration was
determined by the bicinchoninic acid (BCA) protein assay. Equal
amounts of protein (50 μg) from each sample were separated by
SDS-PAGE. Following electrophoresis, proteins were electroblotted
to polyvinylidene difluoride membranes. The membranes were blocked
with 5% nonfat dry milk at room temperature for 1 h and then
independently incubated at 4°C overnight with the primary
antibodies against ERK (1:1,000), p-ERK (1:1,000), Akt (1:1,000),
p-Akt (1:1,000), or Cbl-b (1:250), and β-actin (1:1,000) was used
as a control. After washing three times with TBST (20 mM Tris-Cl pH
7.5, 150 mM NaCl and 1 g/l Tween 20), the membranes were incubated
with horseradish peroxidase-conjugated goat anti-mouse (cat. no.
A0216) and goat anti-rabbit (cat. no. A0208) secondary antibodies
(1:800 dilutions; Beyotime Institute of Biotechnology, Shanghai,
China), followed by a further three washes with TBST. Proteins
bands were visualized using an enhanced chemiluminescent reagent
(Thermo Fisher Scientific).
Statistical analysis
The data are expressed as the mean ± standard
deviation. Statistical correlation of data was checked for
significance by two-way analysis of variance and Student’s t-test,
using SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
SK inhibits NCI-H460 cell growth
To determine whether SK inhibits the proliferation
of lung cancer cells, NCI-H460 cells were treated with different
concentrations of SK (0.312–10 μM) for 24 or 48 h. A significant
concentration- and time-dependent reduction of cell viability was
observed (Fig. 2). The 50%
inhibitory concentration (IC50) of SK for the NCI-H460
cells at 24 and 48 h was 2.64±0.52 and 1.75±0.28 μM,
respectively.
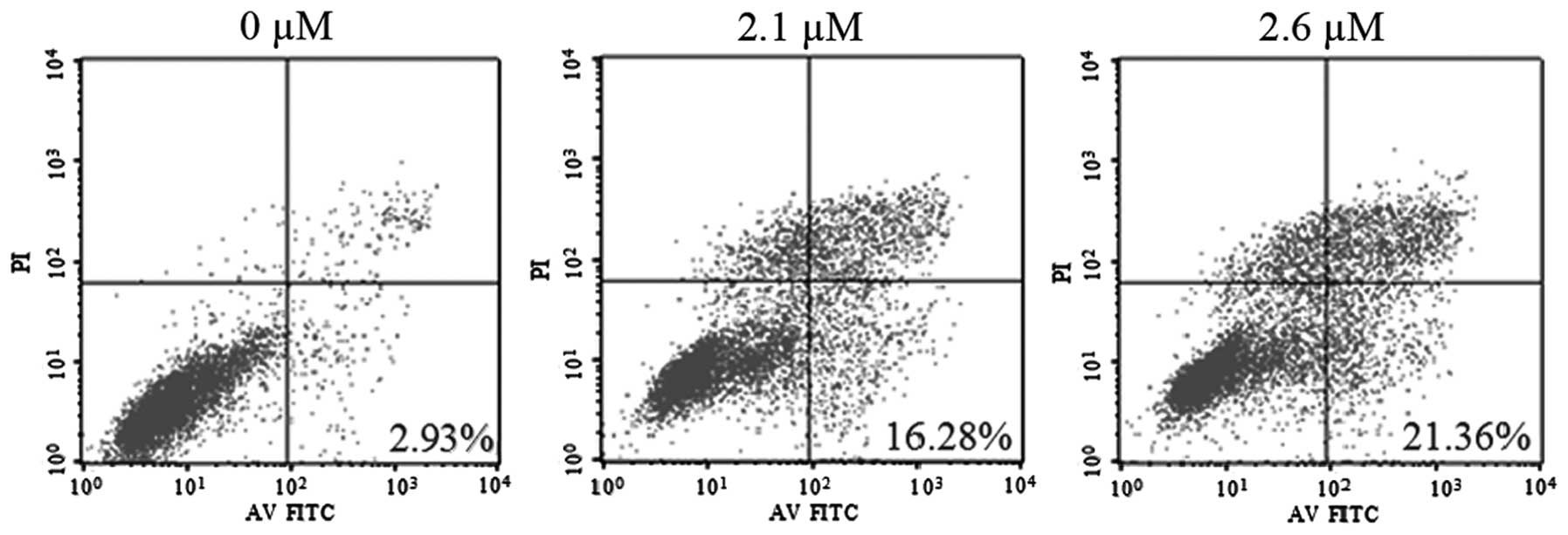
SK induces NCI-H460 cell apoptosis
To determine whether SK induces apoptosis in
NCI-H460 cells, SK-induced cytotoxicity was examined. Flow
cytometric analysis with Annexin V and PI demonstrated that SK
induced significant apoptosis in NCI-H460 cells. The percentages of
double-stained cells, indicative of apoptosis, were 16.28±2.18%
(P<0.01) and 21.36±2.67% (P<0.01) following treatment with
2.1 and 2.6 μM SK, respectively, compared with 2.93±0.23% in the
non-treated control (Fig. 3).
Consistent with this, confocal fluorescence microscopy revealed
clear morphological changes typical of apoptosis, such as
condensation of chromatin and nuclear fragmentation, following
treatment with SK (Fig. 4).
SK induces NCI-H460 cell apoptosis by
inhibiting ERK signaling
To further determine the mechanism of SK-induced
apoptosis in NCI-H460 cells, the levels of p-Akt, total Akt, p-ERK
and total ERK were investigated following treatment with 2.6 μM SK
for 4–24 h. It was found that the expression of p-ERK protein began
to decrease at 4 h, and reached a minimum expression level at 24 h
(Fig. 5), while the expression
level of p-Akt was not changed (Fig.
6). This indicates that SK induced NCI-H460 cell apoptosis by
inhibiting the ERK signaling pathway but not the PI3K/Akt
pathway.
Cbl-b contributes to the SK-induced
apoptosis of NCI-H460 cells by negatively regulating ERK
signaling
To explore whether the inactivation of ERK is
associated with Cbl-b, the protein expression level of Cbl-b was
evaluated by western blotting (Fig.
7). The expression of Cbl-b was significantly increased by
treatment with SK for 4 h, and gradually increased to a maximal
level at 24 h; the time taken for the upregulation of Cbl-b protein
was in accordance with that required for the downregulation of
p-ERK protein. This indicates that the inactivation of ERK is
probably associated with the upregulation of Cbl-b. The results
indicate that there is a correlation between Cbl-b and p-ERK. To
confirm this hypothesis, NCI-H460 cells were pretreated with Ps341,
a proteasome and Cbl inhibitor for 1 h; then, 2.6 μM SK was added
for a further 24 h. It was found that Ps341 reversed the SK-induced
downregulation of p-ERK and apoptosis of NCI-H460 cells (Fig. 6). These data suggest that Cbl-b
potentiates the apoptotic action of SK by inhibiting the ERK
pathway in lung cancer cells.
Discussion
SK, one of the major naphthoquinone pigments
isolated from the traditional Chinese herb Lithospermum
erythrorhizon, has numerous biological functions. Recent
studies have shown that SK can induce apoptosis in liver cancer,
osteosarcoma and prostate cancer cells (15–17).
SK exerts antitumor effects by upregulating the expression of p53
protein, regulating the expression of Bcl-2 family proteins,
inducing reactive oxygen species (ROS) production, promoting ERK
and c-Jun N-terminal kinase (JNK) phosphorylation, inhibiting
epidermal growth factor receptor (EGFR) and protein tyrosine kinase
(PTK) phosphorylation and the activity of topoisomerase, telomerase
and matrix metalloproteinase-9 (MMP) (18–20).
In the present study, it was confirmed that SK inhibits cell
proliferation in a time- and concentration-dependent manner and
induces the apoptosis of NCI-H460 cells.
The PI3K/Akt pathway plays a key role in maintaining
cell survival and inhibiting apoptosis. Activated Akt exerts
anti-apoptotic activity by phosphorylating Bad and caspase 9 and
activating the transcription factor NF-kB (21). Numerous cytotoxic drugs induce cell
apoptosis by inhibiting the PI3K/Akt pathway. ERK is associated
with the mitogen-activated protein kinase (MAPK) pathway; it plays
an important role in tumor incidence and development by inducing
tumor cell differentiation and proliferation. Indeed, PI3K/Akt and
ERK signaling pathways are excessively activated in some cancer
cells, while their inhibition can increase the sensitivity of tumor
cells to cytotoxic drugs (22–24).
SK has been shown to increase ROS generation and ERK activation,
and induce apoptosis in osteosarcoma cells (16). Phosphorylated ERK has been shown to
upregulate p53 expression in SK-induced HeLa cell apoptosis
(20). In the present study, the
treatment of cells with 2.6 μM SK for 4–24 h resulted in the
gradually decreased expression of p-ERK protein, which reached a
minimum at 24 h, while the expression of p-Akt did not change.
Typical apoptosis was observed at 24 h, suggesting that SK first
inhibited the ERK pathway, which in turn, blocked its regulation of
downstream factors, resulting in the loss of ERK pathway-mediated
anti-apoptotic function the and induction of NCI-H460 cell
apoptosis. This indicates that inhibition of the ERK pathway is
probably one of the mechanisms by which SK induces the apoptosis of
NCI-H460 cells.
The Cbl family of ubiquitin ligases comprises
adaptor proteins and E3 ligases that play both positive and
negative roles in several signaling pathways and affect various
cellular functions (25–27). The results of the present study
showed that the expression of Cbl-b began to increase when the
NCI-H460 cells had been exposed to 2.6 μM SK for 4 h, and reached
maximal expression at 24 h. The kinetics of the upregulation of
Cbl-b corresponded with those of ERK inhibition, suggesting that SK
promotes ERK ubiquitination by upregulating the expression of
Cbl-b, which in turn inhibited ERK activity and finally induced
NCI-H460 cell apoptosis. Ps341, a Cbl inhibitor, reversed both
SK-induced cell apoptosis and inhibition of p-ERK activity. These
findings confirm that Cbl-b is involved in SK-induced NCI-H460 cell
apoptosis by negatively regulating ERK activity.
In summary, the results of the present study
indicate that SK induces apoptosis in NCI-H460 cells and inhibits
their proliferation. Cbl-b-regulated ERK signals are involved in
SK-induced cell apoptosis in vitro. This study indicates
that SK may serve as an important potential chemotheraputic agent
in human lung cancer.
References
|
1
|
Dean EJ, Ward T, Pinilla C, Houghton R,
Welsh K, Makin G, Ranson M and Dive C: A small molecule inhibitor
of XIAP induces apoptosis and synergises vinorelbine and cisplatin
in NSCLC. Br J Cancer. 102:97–103. 2010. View Article : Google Scholar :
|
|
2
|
Janet WT and Elizabeth HB: Drug resistance
mechanism in non-small cell lung carcinoma. J Can Res Updates.
2:265–282. 2013.
|
|
3
|
Chen X, Yang L, Zhang N, Turpin JA,
Buckheit RW, Osterling C, Oppenheim JJ and Howard OM: Shikonin, a
component of Chinese herbal medicine, inhibits chemokine receptor
function and suppresses human immunodeficiency virus type 1.
Antimicrob Agents Chemother. 47:2810–2816. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yeh CC, Kuo HM, Li TM, Lin JP, Yu FS, Lu
HF, Chung JG and Yang JS: Shikonin-induced apoptosis involves
caspase-3 activity in a human bladder cancer cell line (T24). In
Vivo. 21:1011–1019. 2007.
|
|
5
|
Wiench B, Eichhorn T, Paulsen M and
Efferth T: Shikonin directly targets mitochondria and causes
mitochondrial dysfunction in cancer cells. Evid Based Complement
Alternat Med. 2012:7260252012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee MJ, Kao SH, Huang JE, Sheu GT, Yeh CW,
Hseu YC, Wang CJ and Hsu LS: Shikonin time-dependently induced
necrosis or apoptosis in gastric cancer cells via generation of
reactive oxygen species. Chem Biol Interact. 211:44–53. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu C, Yin L and Chen J and Chen J: The
apoptotic effect of shikonin in human papillary thyroid carcinoma
cells through mitochondrial pathway. Tumour Biol. 35:1791–1798.
2014. View Article : Google Scholar
|
|
8
|
Murakami D, Tsujitani S, Osaki T, Saito H,
Katano K, Tatebe S and Ikeguchi M: Expression of phosphorylated Akt
(pAkt) in gastric carcinoma predicts prognosis and efficacy of
chemotherapy. Gastric Cancer. 10:45–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roy HK, Olusola BF, Clemens DL, Karolski
WJ, Ratashak A, Lynch HT and Smyrk TC: AKT proto-oncogene
overexpression is an early event during sporadic colon
carcinogenesis. Carcinogenesis. 23:210–205. 2002. View Article : Google Scholar
|
|
10
|
Zinda MJ, Johoson MA, Paul JD, Horn C,
Konicek BW, Lu ZH, Sandusky G, Thomas JE, Neubauer BL, Lai MT and
Graff JR: Akt-1, -2 and -3 are expressed in both normal and tumor
tissues of the lung, breast, prostate, and colon. Clin Cancer Res.
7:2475–2479. 2001.PubMed/NCBI
|
|
11
|
Pennock S and Wang Z: A tale of two Cbls:
Interplay of c-Cbl and Cbl-b in epidermal growth factor receptor
downregulation. Mol Cell Biol. 28:3020–3037. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qu J, Zhao M, Teng Y, Zhang Y, Hou K,
Jiang Y, Yang X, Shang H, Qu X and Liu Y: Interferon-α sensitizes
human gastric cancer cells to TRAIL-induced apoptosis via
activation of the c-CBL-dependent MAPK/ERK pathway. Cancer Biol
Ther. 12:494–502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Xu L, Qu X, Zhao M, Yu P, Kang J,
Liu Y and Hu X: Cbl-regulated Akt and ERK signals are involved in
β-elemene-induced cell apoptosis in lung cancer cells. Mol Med Rep.
4:1243–1246. 2011.PubMed/NCBI
|
|
14
|
Li Y, Qu X, Qu J, Zhang Y, Liu J, Teng Y,
Hu X, Hou K and Liu Y: Arsenic trioxide induces apoptosis and G2/M
phase arrest by inducing Cbl to inhibit PI3K/Akt signaling and
thereby regulate p53 activation. Cancer Lett. 284:208–215. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nie YK, Zhu LS and Yu HM: Shikonin
inhibits the proliferation and induces the apoptosis of human HepG2
cells. Can J Physiol Pharmacol. 88:1138–1146. 2010. View Article : Google Scholar
|
|
16
|
Chang IC, Huang YJ, Chiang TI, Yeh CW and
Hsu LS: Shikonin induces apoptosis through reactive oxygen
species/extracellular signal-regulated kinase pathway in
osteosarcoma cells. Biol Pharm Bull. 33:816–824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang H, Zhou P, Huang H, Chen D, Ma N, Cui
QC, Shen S, Dong W, Zhang X, Lian W, Wang X, Dou QP and Liu J:
Shikonin exerts antitumor activity via proteasome inhibition and
cell death induction in vitro and in vivo. Int J Cancer.
124:2450–2459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng HM, Qiu YK, Wu Z and Zhao YF: DNA
damage induced by shikonin in the presence of Cu(II) ions:
potential mechanism of its activity to apoptotic cell death. J
Asian Nat Prod Res. 13:12–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng YW, Chang CY, Lin KL, Hu CM, Lin CH
and Kang JJ: Shikonin derivatives inhibited LPS-induced NOS in RAW
264.7 cells via downregulation of MAPK/NF-kappaB signaling. J
Ethnopharmacol. 120:264–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Z, Wu LJ, Tashiro S, Onodera S and
Ikejima T: Phosphorylated extracellular signal-regulated kinase
up-regulated p53 expression in shikonin-induced HeLa cell
apoptosis. Chin Med J (Engl). 118:671–677. 2005.
|
|
21
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: a play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li T, Yan F, Wang R, Zhou H and Liu L:
Shikonin suppresses human T lymphocyte activation through
inhibition of IKK β activity and JNK phosphorylation. Evid Based
Complement Alternat Med. 2013:3795362013. View Article : Google Scholar
|
|
23
|
Tsao AS, McDonnell T, Lam S, Putnam JB,
Bekele N, Hong WK and Kurie JM: Increased phospho-AKT (Ser (473))
expression in bronchial dysplasia: implications for lung cancer
prevention studies. Cancer Epidemiol Biomarkers Prev. 12:664–666.
2003.
|
|
24
|
Brognard J, Clark AS, Ni Y and Dennis PA:
Akt/protein kinase B is constitutively active in non-small cell
lung cancer cells and promotes cellular survival and resistance to
chemotherapy and radiation. Cancer Res. 61:3986–3997.
2001.PubMed/NCBI
|
|
25
|
Swaminathan G and Tsygankov AY: The Cbl
family proteins: ringleaders in regulation of cell signaling. J
Cell Physiol. 209:21–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Naramura M, Band V and Band H:
Indispensable roles of mammalian Cbl family proteins as negative
regulators oprotein tyrosine kinase signaling: Insights from in
vivo models. Commun Integr Biol. 4:159–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thien CB, Dagger SA, Steer JH, Koentgen F,
Jansen ES, Scott CL and Langdon WY: c-Cbl promotes T cell
receptor-induced thymocyte apoptosis by activating the
phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem.
285:10969–10981. 2010. View Article : Google Scholar : PubMed/NCBI
|