Introduction
Juvenile idiopathic arthritis (JIA) is a very common
childhood autoimmune disease (1).
Its main features are chronic arthritis associated with
multi-system involvement (2). Its
clinical manifestations are diverse and early diagnosis is
challenging. The disease is chronic and persistent and tends to
involve repeated episodes. During later stages, it can cause
deformity and dysfunction of the joints and blindness, severely
impairing the physical and mental health of children (3). Studies have found that a variety of
immune injuries are involved in the pathogenesis of JIA (1,4,5). Clarification of the substances and
methods underlying these immune injuries should provide a valuable
basis for the treatment of JIA (6).
The production of various cytokines marks the occurrence of
abnormalities of lymphocyte function during the active period of
JIA (6). It has been confirmed that
dual signal stimuli are required for the activation of lymphocytes.
The interaction of B7 molecules expressed on antigen-presenting
cells (APCs) and the ligands cluster of differentiation (CD)28 and
cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) expressed on
T-cells. CD28 provides the most important co-stimulatory signal,
which plays an important role in initiating and maintaining the
activation and proliferation of T cells. CTLA-4 belongs to the
immunoglobulin (Ig) super-family, having 31% homology with CD28 at
the amino acid level. The function of CTLA-4 opposes that of CD28
in lymphocyte activation (7). On
combining with B7, CTLA-4 inhibits CD28+ T lymphocytes
and effectively terminates their activation and proliferation
(8). However, whether CTLA-4 is
expressed effectively during the active period of JIA and has the
same inhibitory effect on the CD28− T lymphocytes
predominant in JIA has never been reported. In the present study,
flow cytometry was used to measure the expression of CD28/CTLA-4 on
the surface of CD4+ and CD8+ T lymphocytes in
peripheral blood and to investigate the correlation with JIA
activity. The goal was to understand the role of abnormal
lymphocyte activity in the active phase of JIA and to provide a
theoretical basis for its clinical treatment and improvements in
prognosis.
Materials and methods
Clinical data
A total of 36 children with JIA admitted to the
Affiliated Hospital of Qingdao University Medical College, Qingdao
Children's Hospital (Qingdao, China) from October 2010 to July 2011
were included in the JIA group. All patients were diagnosed
according to diagnostic and classification standards specified by
the International Union of Rheumatology (9,10). The
patient group consisted of 16 males and 20 females aged 5–14 years
(mean, 7.5 years). The normal control group contained 39 healthy
children consisting of 17 males and 22 females aged 4–13 years
(mean, 6.9 years). The age and gender ratios between the two groups
were not significantly different. None of the enrolled subjects had
cancer, a family history of autoimmune disease, history of
infectious disease in the past 3 months, or received any
glucocorticoid or other immunosuppressive treatment within the past
6 months. Other autoimmune diseases were also excluded. The
experiments were performed with informed consent from all patients
and their families.
Ethical considerations
This study was conducted in compliance with the
Declaration of Helsinki (http://www.wma.net/en/30publications/10policies/b3/index.html).
It was approved by the Faculty of Medicine Ethics Committee of
Qingdao Women and Children's Hospital and Qingdao University
Medical College in July 2009. Before the study began, full details
were given to all participants or their guardians, who were assured
of confidentiality and gave their informed written consent. All
data were reported in a collective fashion, and participants were
able to withdraw at any time without affecting their children's
treatment.
Patient groupings
Among the 36 children in the JIA group, 7 had
oligoarticular-type JIA, 16 were polyarticular-rheumatoid-factor
negative, 12 had systemic JIA and one had JIA associated with
enthesitis. The longest follow-up was for 2 years and 7 months,
while the shortest was 3 months. For follow-ups, the patients
visited the hospital every 1–2 weeks to undergo routine blood tests
as well as C-reactive protein (CRP) and erythrocyte sedimentation
rate (ESR) tests. Patients showing severe symptoms were
hospitalized for treatment.
Patients were stratified into the following groups:
i) Initial active phase: Patients were positively diagnosed with
JIA, showing distinct clinical symptoms accompanied by increased
values in indicators for inflammation in peripheral blood, such as
increased white blood cell count, increased CRP and quickened ESR.
ii) Resting phase: Patients were positively diagnosed with JIA.
After treatment, however, clinical symptoms disappeared, and
results from routine blood tests as well as of CRP and ESR were all
normal throughout the follow-ups. iii) Controls: Concurrent
controls were physically examined in the hospital.
Collection of clinical data
Clinical information of all enrolled patients was
recorded in detail, including name, gender, age, treatment and
follow-ups. Laboratory tests included the blood routine tests as
well as the CRP and ESR tests.
Methods
Instruments and reagents
A BD FACSCalibur flow cytometer (BD Biosciences, San
Jose, CA, USA) and fluorescence-labeled mouse anti-human monoclonal
antibodies (mAbs; #FHP0041-100 and #11524-MM026; dilution, 1:10;
BioLegend, Inc., San Diego, CA, USA) were used in the flow
cytometric analysis.
Methods
i) Blood sampling. For all subjects, 3 ml venous
blood was collected using heparin as anticoagulant and diluted with
an equal volume of phosphate-buffered saline (PBS). Lymphocytes
were then isolated using Ficoll separation fluid (BioLegend Inc.)
to obtain a single-cell layer, rinsed with PBS and centrifuged at
1,000 × g for 15 min. The supernatant was discarded. The cells were
then resuspended in PBS, and the cell concentration was adjusted to
∼5×106 cells/ml.
ii) Cell staining. Cells were surface stained with
three-color labeling using antibodies against CD3, CD4, CD8 and
CTLA-4 (CD152). Specifically, the peripheral blood mononuclear cell
suspension was added to the bottom of a flow tube and evenly
divided into the three samples. The first sample was treated with
phycoerythrin (PE)-Cy5-labeled CD3, fluorescein isothiocyanate
(FITC)-labeled CD4 and PE-labeled CD8 mAbs to measure the
T-lymphocyte subset frequency in peripheral blood. The second
sample was treated with PE-Cy5-labeled CD4 and PE-labeled CD152
mAbs to measure CTLA-4 expression on CD4+ T cells. The
third sample was treated with PE-Cy5-labeled CD8 and PE-labeled
CD152 mAbs to measure CTLA-4 expression on CD8+ T cells.
Each sample was mixed with 20 µl of the appropriate mAbs, incubated
at room temperature in the dark for 20 min, and then centrifuged at
250 × g for 5 min; the supernatants were discarded. Then, 2 ml PBS
wash solution was added and mixed well to resuspend the cells. The
cells were centrifuged at 250 × g for 5 min, and the supernatant
was discarded. Flow cytometry wash solution (200 µl; BioLegend
Inc.) was added to resuspend the cell pellets, and the suspension
was loaded onto the cytometer for collection.
iii) Flow cytometric analysis. In a scatter plot,
forward scatter (FSC) and side scatter (SSC) parameters were used
to set the threshold and determine lymphocyte position. The
PE-Cy5-positive cell population was first selected, followed by
determination of the CD4 and CD8 cell subpopulation percentages
among PE-Cy5-positive cells. CTLA-4 expression levels in
CD4+ and CD8+ T cells were then calculated. A
total of 10,000 cells were analyzed per sample.
Statistical analysis
SPSS software, version 13.0 (SPSS, Inc., Chicago,
IL, USA) was used to analyze data. Data are presented as mean ±
standard deviation. A normality test was first performed on all
data; comparisons that passed the normality test were analyzed by a
Student's t-test, and those that did not were analyzed with an
approximate t-test. A value of P<0.05 was considered to be
statistically significant.
Results
CD4+CD28+ and
CD8+CD28+ T-cell frequency
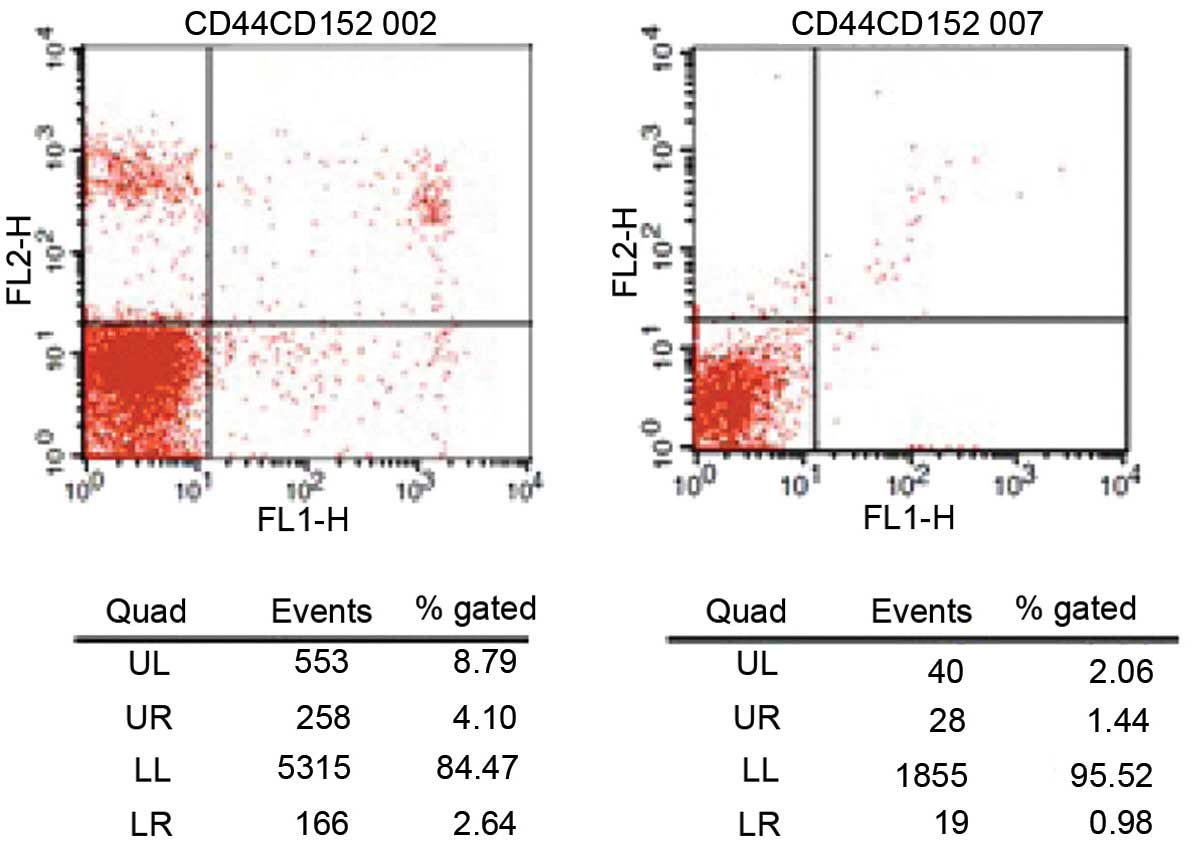
During the active phase of the disease, the
CD4+CD28+ and CD8+CD28+
T cell frequencies in the peripheral blood of children with JIA
were significantly lower compared with those in the normal control
group (P<0.01; Table I and
Fig. 1).
 | Table I.Frequency of CD28+ T cells
in the peripheral blood of patients with JIA during the active
phase of the disease. |
Table I.
Frequency of CD28+ T cells
in the peripheral blood of patients with JIA during the active
phase of the disease.
| Groups | No. of cases |
CD4+CD28+ (%) |
CD8+CD28+ (%) |
|---|
| JIA | 36 |
7.46±0.41a |
10.94±1.10a |
| Normal | 39 | 37.13±1.92 | 13.24±0.84 |
CD4+CD28−,
CD4+CTLA-4+ and
CD8+CTLA-4+ T-cell frequency
During the active phase of the disease, the
CD4+CD28− T-cell frequency in the peripheral
blood of children with JIA was significantly higher compared with
that in the normal control group (P<0.01; Fig. 2 and Table
II), whereas the frequencies of
CD4+CTLA-4+ and
CD8+CTLA-4+ T cells were significantly higher
compared with those in the normal control group (P<0.01;
Table III).
 | Table II.Frequency of CD28− T cells
in the peripheral blood of patients with JIA during the active
phase of disease. |
Table II.
Frequency of CD28− T cells
in the peripheral blood of patients with JIA during the active
phase of disease.
| Group | No. of cases |
CD4+CD28− (%) |
CD8+CD28− (%) |
|---|
| JIA | 36 |
7.81±1.89a | 20.30±10.15 |
| Normal | 39 | 1.91±0.69 | 20.03±10.02 |
 | Table III.Frequency of CTLA-4+ T
cells in the peripheral blood of patients with JIA during the
active phase. |
Table III.
Frequency of CTLA-4+ T
cells in the peripheral blood of patients with JIA during the
active phase.
| Group | No. of cases |
CD4+CTLA-4+ (%) |
CD8+CTLA-4+ (%) |
|---|
| JIA | 36 |
5.15±0.52a |
1.80±0.20a |
| Normal | 39 | 2.35±0.29 | 0.73±0.20 |
CD4+ and CD8+
T-cell counts
During the active phase of the disease, the
CD4+ T-cell count in the peripheral blood of children
with JIA was significantly higher compared with that in the normal
control group (P<0.01), where CD4+CD28−
cells mostly accounted for the increase in CD4+ T-cell
frequency. By contrast, CD8+ T-cell counts were
significantly reduced in the JIA group (P<0.01; Table IV).
 | Table IV.Changes in T-cell subsets in patients
with JIA during the active phase. |
Table IV.
Changes in T-cell subsets in patients
with JIA during the active phase.
| Group | No. of cases |
CD3+CD4+ (%) |
CD3+CD8+ (%) |
|---|
| JIA | 36 |
40.63±4.14a |
23.27±3.37a |
| Normal | 39 | 31.82±3.12 | 27.72±3.23 |
During the resting phase, the CD4+ and
CD8+ T-cell counts in the peripheral blood of children
with JIA were not significantly different from those in normal
controls (P>0.05; Table V).
 | Table V.Changes in T cell subsets in patients
with JIA during the resting phase. |
Table V.
Changes in T cell subsets in patients
with JIA during the resting phase.
| Group | No. of cases |
CD3+CD4+ (%) |
CD3+CD8+ (%) |
|---|
| JIA | 36 | 33.31±2.58 | 26.27±0.40 |
| Normal | 39 | 31.82±3.12 | 27.72±3.23 |
CD28+ and
CTLA-4+ frequency during the resting phase
During the resting phase of the disease, the
CD28+ and CTLA-4+ frequencies in the
CD4+ or CD8+ T-cell populations in the
peripheral blood of children with JIA were not significantly
different from those in the normal control group (P>0.05;
Tables VI and VII).
 | Table VI.Frequency of CD28+ T cells
in the peripheral blood of patients with JIA during the resting
phase. |
Table VI.
Frequency of CD28+ T cells
in the peripheral blood of patients with JIA during the resting
phase.
| Group | No. of cases |
CD4+CD28+ (%) |
CD8+CD28+ (%) |
|---|
| JIA | 36 | 36.43±1.52 | 12.97±0.53 |
| Normal | 39 | 37.13±1.67 | 13.24±0.84 |
 | Table VII.Frequency of CTLA-4+ T
0;cells in the peripheral blood of patients with JIA during the
resting phase. |
Table VII.
Frequency of CTLA-4+ T
0;cells in the peripheral blood of patients with JIA during the
resting phase.
| Group | No. of cases |
CD4+CTLA-4+ (%) |
CD8+CTLA-4+ (%) |
|---|
| JIA | 36 | 2.41±0.25 | 0.78±0.11 |
| Normal | 39 | 2.35±0.29 | 0.73±0.20 |
Discussion
JIA is the most common connective-tissue disease
that occurs in children and is a leading cause of disability and
blindness (6). Its main features are
chronic synovitis and symmetrically destructive joint disease
accompanied by involvement of multiple other systems in the body.
In 2001, the International League of Associations for Rheumatology
named the condition defined by joint swelling that persists for
more than 6 weeks due to an unknown cause in children under 16
years old as juvenile idiopathic arthritis, which was further
divided into seven subtypes (10).
There is significant heterogeneity among these subtypes, including
modes of onset and numerous clinical manifestations that can
sometimes make diagnosis rather challenging. While symptoms can be
alleviated and joint function restored to normal following
treatment in 75% of patients, lifelong symptoms, significant
disability, or even mortality due to concurrent infection or
starch-like degeneration may occur in some children (11). Hence, the pathogenesis underlying JIA
has become a popular research topic worldwide, with the aim of
finding more effective methods for early diagnosis and treatment to
reduce the morbidity and mortality of this disease. Currently,
research into the underlying causes of JIA is mainly focused on
aspects such as genetics, environment, and infection. In terms of
JIA pathogenesis, the majority of scholars believe that JIA is a
type of autoimmune disease and that abnormal immune function,
particularly abnormal cellular immune function, is an important
mechanism underlying JIA. Inflammatory cells infiltrating synovial
tissues in JIA are mainly activated T cells, suggesting that the
pathologic autoimmune response may be activated by antigens and
mediated by T cells (12). Since
these activated T cells play an important role in the pathogenesis
of JIA by affecting cell-cell contact and producing cytokines
(13), inhibiting the function of
excessively activated T cells may theoretically inhibit
inflammatory processes and improve disease outcome in patients with
JIA.
In addition to stimulating primary signals provided
by T-cell receptor (TCR)-CD3-major histocompatibility complex (MHC)
interaction, T-lymphocyte activation also requires secondary
signals for co-stimulation. During an immune response, secondary
signal expression depends on the normal transfer of primary
signals. If the latter is hindered, the immune response cannot
proceed. T lymphocytes cannot be effectively stimulated without
secondary signals, resulting in clonal anergy or programmed cell
death (PCD). Excessive co-stimulatory signaling may lead to T-cell
overactivation and, ultimately, autoimmune disease (13). CD28 and CTLA-4 were the first
costimulatory molecules confirmed to play a key role in T-cell
activation by interaction with B7 (14) during an immune response, where CD28
transfers co-stimulatory signals, enhances T-cell activation, and
upregulates downstream signal transmission, while CTLA-4 transfers
inhibitory signals, downregulates the activation of B cells and
macrophages, reduces pro-inflammatory factor secretion and
ultimately leads to anergy (15). In
a rat adjuvant arthritis (AA) model, Rodriguez-Palmero et al
(16) found that stimulating CD28
with a monoclonal antibody promoted Th2 function and regulatory
T-cell proliferation, thus providing effective protection against
AA. Clinically, immune function disorder correlates with abnormal
T-lymphocyte activation and abnormal co-stimulatory molecule
expression in adult patients with rheumatoid arthritis (RA) and
rheumatoid inflammation (17).
Therefore, investigating co-stimulatory molecule expression levels
and abnormal T-cell activation in children with JIA should help in
further understanding the pathogenesis underlying JIA and provide a
theoretical basis for the development of biological treatments
against JIA.
CTLA-4, discovered by screening mouse
CD8+ T-cell cDNA (18),
belongs to the Ig superfamily and shares 31% homology with CD28 at
the amino acid level (19). CTLA-4
contains an IgV-like extramembranous functional domain, a
transmembrane domain, and a short, conserved functional
intracellular domain. Complete CTLA-4 molecules mainly exist on the
surface of T cells in dimeric form and are only expressed on
activated CD4+ and CD8+ T cells. While the
level of CTLA-4 expression is only 2–3% of the CD28 expression
level, its affinity for B7 is 10-100-fold greater than that for
CD28 (7). As a co-stimulatory signal
for lymphocyte activation, the function of CTLA-4 opposes that of
CD28. At the beginning of the T-cell response, CD28 expression
mediates T-cell activation and clonal expansion by binding to B7 on
APCs. Activated T cells gradually increase CTLA-4 expression, which
then competes against CD28 for binding to B7 and functions as a
strong inhibitory signal to terminate excessive T-cell
proliferation and activation. This maintains the immune response in
a relatively balanced state. When antigen and co-stimulatory
effects are weak, CTLA-4 maintains T cells in a resting state to
avoid unnecessary T-cell response. Such CTLA-4-mediated mechanisms
play a positive role in immune tolerance to autoantigens (8).
The ligands for CTLA-4 and CD28 on the surface of T
lymphocytes are B7-1 (CD80) and B7-2 (CD86), both of which are
expressed on the surface of APCs and activated T cells. The genes
for both molecules are located on human chromosome 3, and the
structural features of these proteins indicate that they belong to
the Ig superfamily (19). While
examining adhesion molecules, Linsley et al (20) first demonstrated that CD28 binds to
B7-1 to co-stimulate cells. CTLA-4 was later found to bind B7-1 as
well, although the effect was the opposite of that of B7-1 binding
to CD28. CD28 overexpression can lead to T-cell overactivation; if
this cannot be inhibited by CTLA-4, immune disorders can develop.
Following an excess of CD28 expression, CD28 molecules on cells
that have been repeatedly stimulated by antigens fall off the
surface to form CD28− T cells. If CD28 function is
normal, it can upregulate the expression of anti-apoptotic gene
Bcl-XL in target cells, block T-cell activation, and induce
apoptosis, thus promoting T-cell proliferation that sustains
auto-responses (21). Otherwise,
immunosenescence occurs, and apoptosis is impaired. A number of
studies have confirmed that CTLA-4 is involved in autoimmunity, and
its expression is significantly increased in many types of
autoimmune diseases, including RA, systemic lupus erythematosus,
systemic sclerosis, Behcet's disease (22), and ankylosing spondylitis (23). CTLA-4-Ig, a soluble fusion protein
formed by fusing the extramembrane domain of human CTLA-4 and the C
domain of Ig, blocks the interaction between B7 and its receptor
molecule CTLA-4 by binding to B7. In fact, CTLA-4-Ig has shown some
therapeutic effect in treating autoimmune diseases.
In the present study, CD28/CTLA-4 on the surface of
T lymphocytes was examined in the peripheral blood of 36 children
with JIA during the active and resting phases of the disease by
flow cytometry. CD4+ T-cell counts increased during the
active phase of JIA, suggesting that CD4+ T-cell
apoptosis is significantly impaired at disease onset. The
immune-active T cells within the CD4+CD28−
compartment that persistently survive, then induce
inflammation-mediated pathological damage that is characteristic of
JIA, producing a series of clinical symptoms within the patient. It
may, therefore, be speculated that inhibiting the immune activity
or increasing the rate of apoptosis of these cells may alleviate
the inflammatory responses in JIA and improve the condition of the
patients.
The present study also demonstrated that CTLA-4
expression on the surface of CD4+ T cells in JIA
patients was significantly higher than that in normal controls
during the active phase of the disease. In addition, the majority
of CD4+ T cells in children with JIA were
CD28−. Therefore, CTLA-4 is highly expressed, mainly on
the surface of CD4+CD28− T cells. As CTLA-4
expressed on the surface of CD4+CD28− T cells
cannot effectively bind to B7, it cannot effectively inhibit the
immune activity of CD4+CD28− T cells or
accelerate their apoptosis. Hence, the number of
CD4+CD28− T cells is substantially increased.
These increased CD4+CD28− T lymphocytes
remain immunologically active, and their function changes. For
example, their ability to activate Th1 cells is enhanced (24); they can secrete IFN-γ and IL-1,
stimulate macrophages to release matrix-degrading protease, and
directly cause damage to blood vessels, tissues and organs in
patients with RA (25). Thus, these
changes in CD28/CTLA-4 occurring during the active phase of disease
are key factors contributing to the pathogenesis of JIA. By
contrast, CD28/CTLA-4 expression on the surface of T cells during
the resting phase of the disease is restored to normal levels.
Therefore, the excessive expression of ineffective CTLA-4 together
with low CD28 expression levels can serve as an indicator for the
active phase of JIA. In clinical practice, the data from the
present study also suggest that intervening treatments that promote
CD4+CD28− T-lymphocyte apoptosis may stop the
development of JIA.
In the present study, CD4+ T-cell counts
were significantly higher during the active phase of the disease in
patients with JIA compared with those in normal controls,
suggesting that CD4+ T-lymphocyte apoptosis is impaired.
Hence, abnormally activated T lymphocytes, particularly
CD4+ T lymphocytes, are the main cell subsets inducing
immune damage in JIA. High CTLA-4 expression levels cannot reduce
the activity of these cells, and the increased number
CD4+ T lymphocytes is mainly accounted for by
CD4+CD28− T cells. Therefore, inhibiting the
activation of these cells may alleviate JIA. Studies have confirmed
that CD4+CD28− is an optimal biological
marker for an aging immune system (26–28). T
cells enter cellular senescence following multiple cell-division
cycles, which is characterized by three main features (29) as follows: i) functional changes
(e.g., production of a large amount of inflammatory cytokines); ii)
telomere shortening and eventual cessation of proliferation; and
iii) tolerance to apoptosis. The loss of CD28 is a well-known
phenotypic change associated with cellular senescence. It has been
suggested that CD28− T cells represent aging lymphocytes
(30), and the results of the
present study are consistent with this hypothesis.
The present study used flow cytometry to examine
co-signaling molecules CD28/CTLA-4 on the surface of T cells in the
peripheral blood of children with JIA to study the association
between CD28/CTLA-4 and the pathogenesis and activity of JIA, and
the results provide a basis for the development of new treatments
for JIA.
The present study was limited by its relatively
small sample size; clinical studies with larger sample sizes are
required to further validate the results. Future studies will focus
on further investigating CD28/CTLA-4 expression in different JIA
subtypes and the correlation between their gene polymorphisms and
JIA.
Acknowledgements
The study was funded by the Science and Technology
Fund, Qingdao China. The authors are grateful to the Qingdao Women
and Children's Hospital Authority, particularly Peng Zeng, and
Qingdao University Medical College. The authors thank the
departmental operations managers, nurse specialists and all the
registered nurses in the pediatric departments of the two hospitals
who participated in and facilitated this study.
References
|
1
|
Ravelli A and Martini A: Juvenile
idiopathic arthritis. Lancet. 369:767–778. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thierry S, Fautrel B, Lemelle I and
Guillemin F: Prevalence and incidence of juvenile idiopathic
arthritis: a systematic review. Joint Bone Spine. 81:112–117. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tugal-Tutkun I, Quartier P and Bodaghi B:
Disease of the year: juvenile idiopathic arthritis-associated
uveitis – classification and diagnostic approach. Ocul Immunol
Inflamm. 22:56–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vastert SJ, Kuis W and Grom AA: Systemic
JIA: new developments in the understanding of the pathophysiology
and therapy. Best Pract Res Clin Rheumatol. 23:655–664. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nusman CM, van den Berg JM,
Schonenberg-Meinema D, et al: Juvenile idiopathic arthritis: from
biomarker to treatment. Ned Tijdschr Geneeskd. 157:A63912013.[(In
Dutch)]. PubMed/NCBI
|
|
6
|
Goldzweig O and Hashkes PJ: Abatacept in
the treatment of polyarticular JIA: development, clinical utility,
and place in therapy. Drug Des Devel Ther. 5:61–70. 2011.PubMed/NCBI
|
|
7
|
Carreno BM and Collins M: The B7 family of
ligands and its receptors: new pathways for costimulation and
inhibition of immune responses. Ann Rev Immunol. 20:29–53. 2002.
View Article : Google Scholar
|
|
8
|
McCoy KD and Le Gros G: The role of CTLA-4
in the regulation of T cell immune responses. Immunol Cell Biol.
77:1–10. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pediatric Rheumatology Collaborative Study
Group in China: Diagnosis and treatment of pediatric rheumatic
diseases: a consensus statement (I). Lin Chuang Er Ke Za Zhi.
28:984–991. 2010.[(In Chinese)].
|
|
10
|
He XZ: Juvenile idiopathic arthritis.
Edmonton, Canada, 2001 (Draft for new classification standard for
International Society of Rheumatology). Zhonghua Feng Shi Bing Xue
Za Zhi. 6:62–63. 2002.[(In Chinese)].
|
|
11
|
Hu YM and Jiang ZF: Zhu Futang Practical
Pediatrics. 7th. People's Medical Publishing House; Beijing: pp.
667–672. 2002
|
|
12
|
Grom AA and Hirsch R: T-cell and T-cell
receptor abnormalities in the immunopathogenesis of juvenile
rheumatoid arthritis. Curr Opin Rheumatol. 12:420–424. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang LL, Li LX, Liu Z and Feng WH:
Relationship between disequilibrium of T lymphocyte subgroups and
inflammatory adhesion molecules in patients with rheumatoid
arthritis. Mian Yi Xue Za Zhi. 18:378–380. 2002.[(In Chinese)].
|
|
14
|
Li YJ and Dong WL: The last advances of
study on the B7 family members. Guo Ji Mian Yi Xue Za Zhi.
34:221–226. 2011.[(In Chinese)].
|
|
15
|
Goëb V, Buch MH, Vital EM and Emery P:
Costimulation blockade in rheumatic diseases: where we are? Curr
Opin Rheumatol. 21:244–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rodríguez-Palmero M, Franch A, Castell M,
et al: Effective treatment of adjuvant arthritis with a stimulatory
CD28-specific monoclonal antibody. J Rheumatol. 33:110–118.
2006.PubMed/NCBI
|
|
17
|
Qi JJ, Zhang P and Xiong XH: Relationship
between abnormality of costimulative molecular expression and
imbalanced immune function in patients with rheumatoid arthritis.
Lin Chuang Hui Cui. 22:174–176. 2007.[(In Chinese)].
|
|
18
|
Brunet JF, Denizot F, Luciani MF, et al: A
new member of the immunoglobulin superfamily – CTLA-4. Nature.
328:267–270. 1987. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang TT, Kuchroo VK and Sharp AH: Role of
the B7-CD28/CTLA-4 pathway in autoimmune disease. Curr Dir
Autoimmun. 5:113–130. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Linsley PS, Greene JL, Brady W, et al:
Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but
distinct kinetics to CD28 and CTLA-4 receptors. Immunity.
1:793–801. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Colucci F, Bergman ML, Penha-Gonçalves C,
et al: Apoptosis resistance of nonobese diabetic peripheral
lymphocytes linked to the Idd5 diabetes susceptibility region. Proc
Natl Acad Sci USA. 94:8670–8674. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsui T, Kurokawa M, Kobata T, et al:
Autoantibodies to T cell costimulatory molecules in systemic
autoimmune diseases. J Immunol. 162:4328–4335. 1999.PubMed/NCBI
|
|
23
|
Zhang SH, Han YX and Wu JB: Expression of
co-stimulatory molecules CD28/CTLA-4:B7 in peripheral blood
lymphocytes of patients with ankylosing spondylitis. Zhejiang Yi
Xu. 28:800–802. 2006.[(In Chinese)].
|
|
24
|
Duftner C, Dejaco C, Kullich W, et al:
Preferential type 1 chemokine receptors and cytokine production of
CD 28-T cells in ankylosing spondylitis. Ann Rheum Dis. 65:647–653.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang R, Jiang L, Li SF, et al: Expression
of 4-1BB on T lymphocytes and relationship with TH1/TH2 cytokines
from patients with rheumatoid arthritis. Zhonghua Wei Sheng Wu Xue
He Mian Yi Xue Za Zhi. 27:285–288. 2007.[(In Chinese)].
|
|
26
|
Vallejo AN, Nestel AR, Schirmer M, et al:
Aging-related deficiency of CD28 expression in CD4+ T cells is
associated with the loss of gene-specific nuclear factor binding
activity. J Biol Chem. 273:8119–8129. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Posnett DN, Sinha R, Kabak S and Russo C:
Clonal populations of T cells in normal elderly humans: the T cell
equivalent to “benign monoclonal gammapathy”. J Exp Med.
179:609–618. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Effros RB: Loss of CD28 expression on T
lymphocytes: a marker of replicative senescence. Dev Comp Immunol.
21:471–478. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weyand CM, Fulbright JW and Goronzy JJ:
Immunosenescence, autoimmunity, and rheumatoid arthritis. Exp
Gerontol. 38:833–841. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vallejo AN, Weyand CM and Goronzy JJ:
T-cell senescence: a culprit of immune abnormalities in chronic
inflammation and persistent infection. Trends Mol Med. 10:119–124.
2004. View Article : Google Scholar : PubMed/NCBI
|