Introduction
Fracture not only directly destroys bone integrity,
but also causes damage to local soft tissues and interrupts blood
flow, which is followed by the onset of ischemic-hypoxia in the
local bone tissue. The poor oxygen supply and nutrient deficiency
at the fracture site affects the fracture healing, particularly
without timely treatment, as the ischemic-hypoxia deteriorates the
physiological status of local osteoblast cells and inhibits bone
repair (1). Oxygen deprivation under
ischemic conditions causes functional impairment of the cells and
often structural tissue damage (2).
Furthermore, ischemia at fracture sites is the key cause of delayed
union or non-union fracture healing, and it is rarely a solitary
factor affecting fracture repair (3). Studies have shown that the early stages
of fracture in humans are characterized by inflammation and
hypoxia, and the initial inflammatory phase of fracture represents
a critical step for the outcome of the healing process (4–6).
Hypoxia-inducible factor-1α (HIF-1α) has a regulatory function
during inflammation resolution in vivo (7,8).
HIF-1 is a transcription factor that acts as a
master regulator in oxygen homeostasis, existing as a heterodimer
composed of α and β subunits. HIF-1β, an aryl hydrocarbon receptor
nuclear translocator, is expressed in normoxic cells
constitutively; HIF-1α is continuously synthesized and only present
in hypoxic cells, due to rapid degradation by the
ubiquitin-proteasome system under normoxic conditions (9). HIF-1α plays a key role in the cellular
response to hypoxia and is involved in glucose metabolism, vascular
remodeling and erythropoiesis via gene activation (10), in addition to being required for
solid tumor formation and embryonic vascularization (11). When cells are exposed to hypoxia,
HIF-1α initiates the protective and adaptive mechanism; if this is
not sufficient to rescue cells from the severe hypoxia, the cells
die via apoptosis and even necrosis (12).
Apoptosis, which is also called programmed cell
death, is induced by hypoxic conditions, which cause decreases in
the mitochondrial membrane potential and the release of cytochrome
c (13). The released
cytochrome c then stimulates the protein caspase 9, which
activates the apoptosis executioner caspase 3, thus leading to cell
death (14). It has been reported
that the activation of HIF-1α delays inflammation resolution by
reducing neutrophil apoptosis (7).
It has also been demonstrated that HIF-1α may act as a protective
factor in the apoptotic process of cardiac fibroblasts and
represent a potential therapeutic target for heart remodeling
following injury due to hypoxia (15). HIF-1α plays a role in hypoxia-induced
apoptosis and does not only stimulate, but may also prevent
apoptosis (16).
In the present study, the viability of the
osteoblast cell line MC3T3-E1 was investigated following exposure
to hypoxia, and HIF-1α protein expression was determined. The
HIF-1α level was then manipulated and the reduction in the
viability of the MC3T3-E1 cells in response to the hypoxia was
re-evaluated.
Materials and methods
Cell culture and treatment
Osteoblastic MC3T3-E1 cells were purchased from the
American Type Culture Collection (Manassas, VA, USA) and cultured
in α-Minimum Essential Media (αMEM; Invitrogen Life Technologies,
Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; Invitrogen
Life Technologies) at 37°C in 5% CO2. Subsequent to
reaching 85–95% confluence, the MC3T3-E1 cells were washed with
0.1% phosphate-buffered saline (PBS) and detached with 0.25%
trypsin (dissolved in 0.1% PBS; Ameresco Inc., Framingham, MA, USA)
with 0.025% EDTA and subcultured. To upregulate the HIF-1α, a
murine HIF-1α coding sequence was amplified and cloned into a
eukaryotic expression vector, pcDNA3.1 (+) (Invitrogen Life
Technologies), and confirmed by sequencing. HIF-1α-pcDNA3.1 (+), or
chloramphenicol acetyl transferase (CAT)-pcDNA3.1 (+) vectors were
then transfected into MC3T3-E1 cells to upregulate the HIF-1α level
or act as a control, respectively. The positive clone, MC3T3-E1
(HIF-1α), and MC3T3-E1 (Con) were selected in the presence of 800
µg/ml G418 and maintained in medium containing G418 (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 400 µg/ml. To suppress
HIF-1α expression, HIF-1α-specific small interfering (si)RNAs and
siRNA control (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
were utilized at a concentration of 40 nM. Each siRNA was
transfected into the MC3T3-E1 cells using Lipofectamine® 2000
(Invitrogen Life Technologies).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total mRNA was extracted from the MC3T3-E1 or
MC3T3-E1 (HIF-1α) cells with the RNeasy® Mini kit (Qiagen,
Valencia, CA, USA), and an RNase inhibitor (Promega Corp., Madison,
WI, USA) was then added. A SYBR® Green RT-qPCR kit (Takara, Tokyo,
Japan) was used for the RT-qPCR analysis of HIF-1α mRNA, and
tubulin was used as a reference gene. The ∆∆Ct method was used for
relative quantification (17).
Protein sample isolation and western
blot analysis
Whole MC3T3-E1 or MC3T3-E1 (HIF-1α) cells were
collected and lyzed with a cell lysis reagent (Pierce, Rockford,
IL, USA). Protein samples were then treated with a protease
inhibitor cocktail kit (Roche Biochemicals, Basel, Switzerland) and
quantified with a bicinchoninic acid assay kit (Thermo Fisher
Scientific, Inc., Rockford, IL, USA). SDS-PAGE gel (8–12%) was used
to separate the protein samples, which were then transferred to a
polyvinylidene difluoride membrane. HIF-1α and tubulin protein
levels were detected by immunoblot analysis using rabbit polyclonal
antibodies against mouse HIF-1α (#ab82832) or tubulin (#ab18251;
1:500; Abcam, Cambridge, UK). Goat anti-rabbit immunoglobulin G
conjugated to horseradish peroxidase (Pierce) and an enhanced
chemiluminescence detection system (SuperSignal® West Femto;
Pierce) were used for detection. The HIF-1α level was expressed as
a percentage relative to tubulin expression.
Cell viability determination by MTT
assay
MC3T3-E1 cells with overexpression of HIF-1α or CAT
were seeded in 96-well plates. Upon reaching 85% confluence, the
medium was substituted with αMEM containing 2% FBS. At different
time-points post-normoxia or -hypoxia treatment, with or without
siRNA transfection, the MTT assay (Invitrogen Life Technologies)
was conducted according to the manufacturer's instructions. The
optical density was then measured at 570 nm using a
spectrophotometer.
Determination of caspase
activation
MC3T3-E1 or MC3T3-E1 (HIF-1α) cells were seeded on
six-well plates and treated with hypoxia for 24 or 48 h. The
activity of caspase 3 was determined as previously described
(18). Briefly, MC3T3-E1 or MC3T3-E1
(HIF-1α) cells were pelleted and resuspended in lysis buffer, prior
to being incubated with Ac-DEVD-AMC fluorogenic peptide substrates
(BD Pharmingen, San Diego, CA, USA) for caspase 3 for 30 to 60 min
at 37°C. The yellow-green fluorescence of the reaction product was
monitored on a spectrofluorometer by setting the excitation and
emission wavelengths to 380 and 440 nm, respectively. The amount of
yellow-green fluorescence was proportional to the amount of active
caspase 3 present in the samples. The increase in caspase activity
was expressed as a relative value to the control group.
Detection of apoptotic cells
MC3T3-E1 or MC3T3-E1 (HIF-1α) cells were seeded into
Nunc™ LabTek™ II chamber slides (Nalge Nunc International Corp.,
Rochester, NY, USA) and subjected to hypoxia with or without siRNA
transfection. The cells were then fixed, washed and stained with 1
µg/ml Hoechst 33528 (Invitrogen Life Technologies) using standard
procedures (19). Apoptotic cells
were screened and counted under a fluorescence microscope (Carl
Zeiss, Oberkochen, Germany) using a 4,6-diamidino-2-phenylindole
filter set.
Statistical analysis
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analyses. The Student's t-test was used to
analyze the difference between two groups. Data are presented as
the mean ± standard error of the mean, and P<0.05 was considered
to indicate a statistically significant difference.
Results
Viability and HIF-1α expression of
MC3T3-E1 cells under normoxic and hypoxic conditions
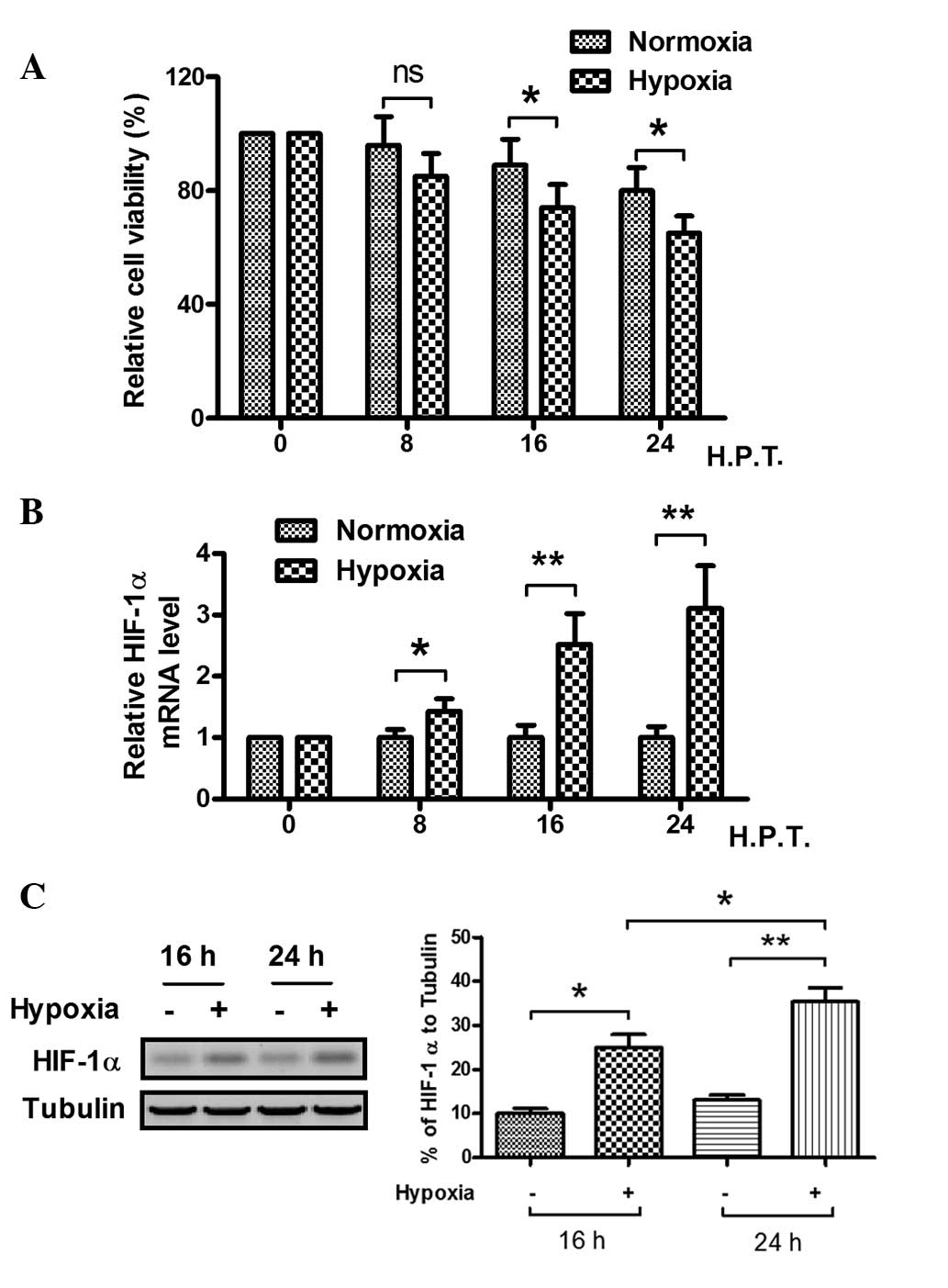
To explore the effect of hypoxia on the MC3T3-E1
cell line, the viability and HIF-1α protein levels of the cells
were examined. In hypoxic and normoxic conditions, the viability of
the cells was observed by MTT assay. The relative cell viability
decreased significantly after 16 h in hypoxia, as compared with the
cell viability in the normoxic condition (Fig. 1A). When the MC3T3-E1 cells were
cultured in 1% O2 conditions for 8 h or longer, the
relative HIF-1α mRNA level became higher than that of cells
cultured in 20% O2 conditions, particularly when the
cells were cultured for >16 h, as demonstrated by fluorescence
qPCR (Fig. 1B). Western blot
analysis was also conducted to analyze HIF-1α expression at the
protein level, as shown in Fig. 1C.
The HIF-1α expression in the MC3T3-E1 cells was significantly
higher when the cells were cultured under hypoxic conditions for 16
and 24 h. These results suggest that the hypoxic condition reduces
the viability of MC3T3-E1 cells and induces HIF-1α protein
expression.
Effect of upregulated HIF-1α
expression on the hypoxia-induced decrease in cell viability
As stated previously, the viability of cells was
decreased and the expression of HIF-1α was increased by the hypoxic
condition. In order to elucidate the effect of HIF-1α expression on
the viability decrease in the MC3T3-E1 cell line caused by hypoxia,
the viability of cells with forced expression of HIF-1α was
investigated using an MTT assay. As shown in Fig. 2A and B, significantly high levels of
HIF-1α expression were confirmed in the HIF-1α-pcDNA3.1-transfected
cells, as compared with the hypoxic and control groups. In
addition, as shown in Fig. 2C, the
MTT assay demonstrated that the viability of the MC3T3-E1 cells was
increased by the forced expression of HIF-1α. These results showed
that the effect of the forced HIF-1α expression was in contrast to
the effect of hypoxia on the viability of MC3T3-E1 cells.
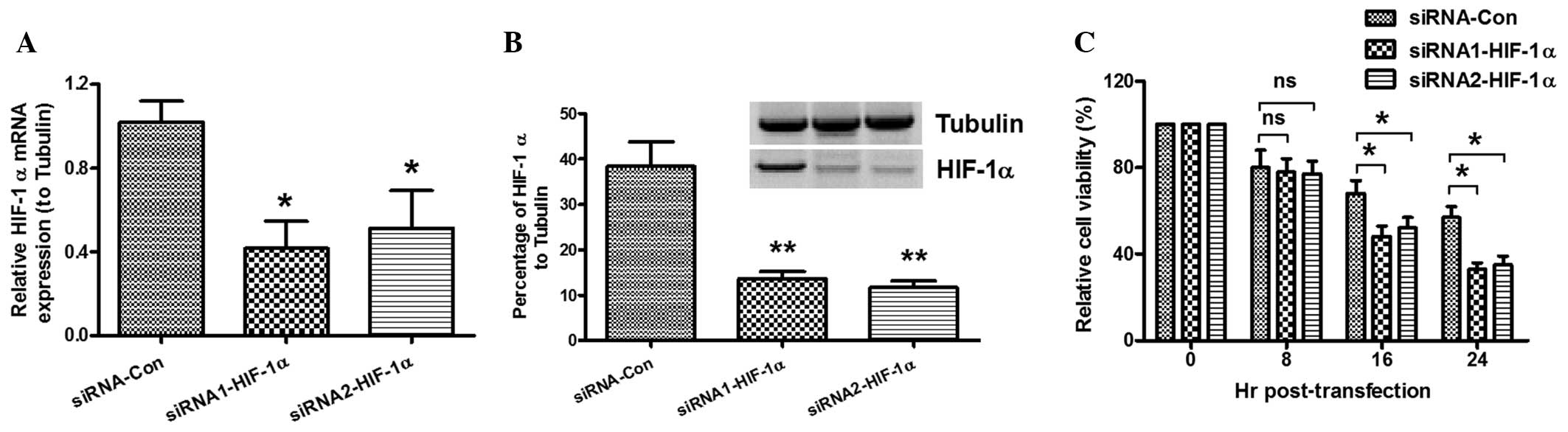
Effect of HIF-1α-knockdown on the
hypoxia-induced decrease in cell viability
To detect the role of HIF-1α in the hypoxia-induced
decrease in cell viability, MC3T3-E1 cells were cultured under
hypoxic conditions and transfected with siRNA. The MTT assay and
western blot analysis were then conducted to confirm the relative
expression levels of HIF-1α to tubulin and determine cell
viability. As shown in Fig. 3A, low
levels of HIF-1α mRNA expression were found post-siRNA
transfection. The western blotting results also demonstrated that
the HIF-1α expression in the cells transfected with HIF-1α-siRNA
was significantly lower than that in the siRNA-control group
(Fig. 3B). The viability of the
MC3T3-E1 cells post-siRNA transfection under hypoxic conditions was
determined by MTT assay. Fig. 3C
shows that the viability of the cells was reduced by
HIF-1α-knockdown. These results suggest that HIF-1α-knockdown
enhances the hypoxia-induced decrease in cell viability.
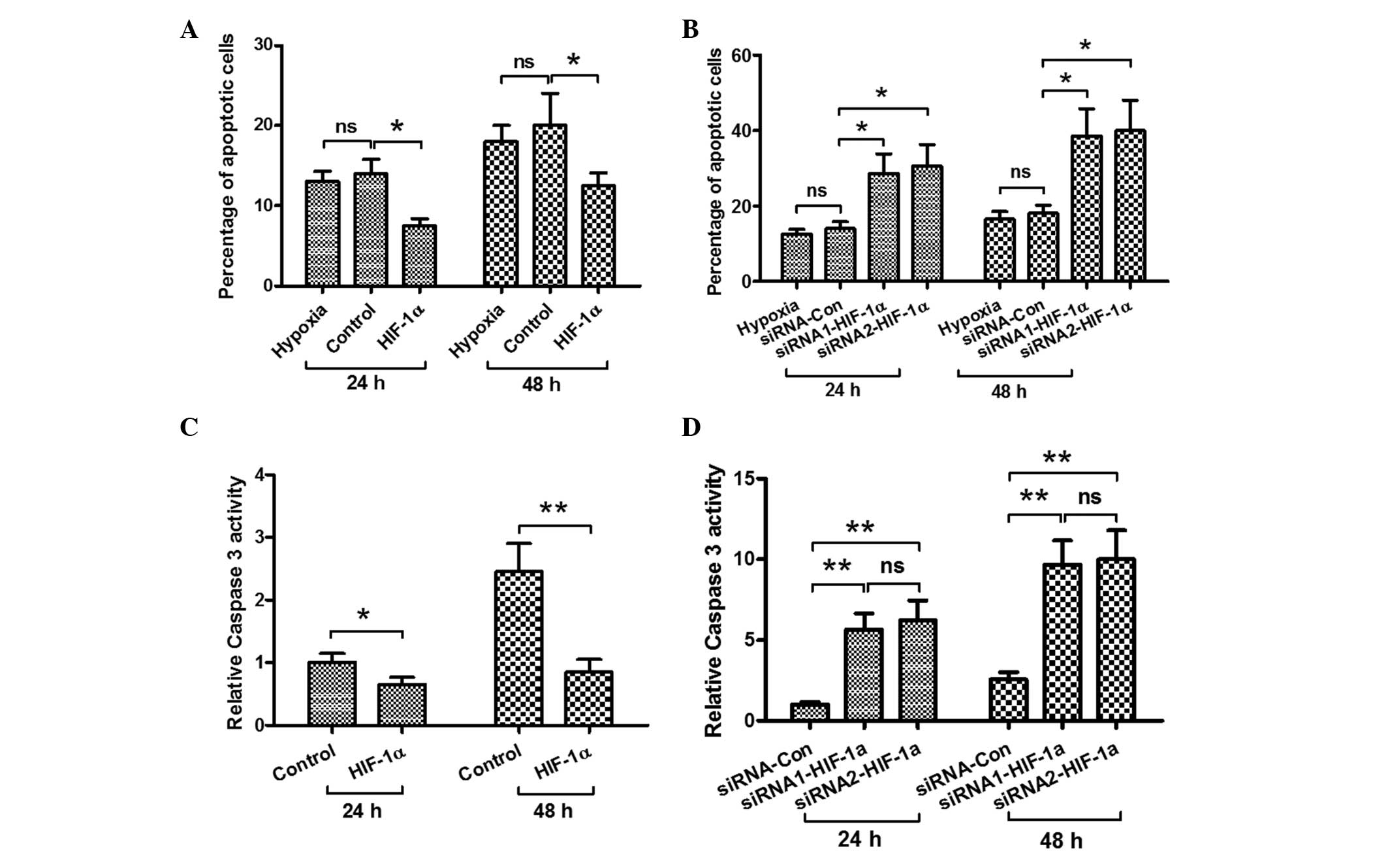
Effect of HIF-1α on the
hypoxia-induced osteoblast apoptosis and caspase 3 activity
In order to explore the possible mechanism by which
HIF-1α attenuates the hypoxia-induced decrease in MC3T3-E1 cell
viability, the effects of HIF-1α on the hypoxia-induced osteoblast
apoptosis and the activity of caspase 3 were investigated. The
cells were transfected with pcDNA3.1 (+) (control) and
HIF-1α-pcDNA3.1 (+) under hypoxic conditions. As shown in Fig. 4A, forced HIF-1α expression
significantly suppressed the hypoxia-induced apoptosis after 24 and
48 h. By contrast, when the cells were transfected with siRNA
(control) and two types of siRNA-HIF-1α during hypoxia, the levels
of HIF-1α in the siRNA-HIF-1α-transfected cell groups were
decreased and the percentage of cells undergoing apoptosis was
increased significantly (Fig. 4B).
In addition, the activity of caspase 3 was examined; as shown in
Fig. 4C, the activity of caspase 3
was inhibited in the cells with forced HIF-1α expression under
hypoxia after 24 and 48 h. By contrast, in the
siRNA-HIF-1α-transfected osteoblasts, the hypoxia-induced caspase 3
activity was enhanced (Fig. 4D).
These results show that HIF-1α inhibits hypoxia-induced osteoblast
apoptosis.
Discussion
Secondary or indirect bone healing typically
involves four phases, known as the inflammatory, soft callus, hard
callus and remodeling phases (20).
Numerous factors can affect fracture healing, including the
coordination of multiple cell types (such as osteoblasts and
chondrocytes); cytokines (such as transforming growth factor-β,
basic fibroblast growth factor and platelet-derived growth factor),
which have a regulatory effect on the initiation and development of
the fracture repair process (21–23); and
the oxygen level of the tissues at the fracture site. Since oxygen
plays a critical role as a participant in multiple basic cellular
processes, hyperbaric oxygen therapy is one of the methods used to
promote fracture healing by delivering 100% oxygen at pressures
greater than one atmosphere (24).
Low-intensity pulsed ultrasound (LIPUS) can also accelerate
fracture healing by inducing the homing of circulating osteogenic
progenitors to the fracture site (25); furthermore, LIPUS treatment combined
with functional electrical stimulation treatment has shown better
effects in accelerating new bone formation (26). In addition, improvements in the
adaptation of osteoblasts and chondrocytes to hypoxia ameliorate
the physiological status of these cells, which are subject to
hypoxia (27).
The protective role of HIF-1α has been confirmed in
various types of cells (28). Cells
with high HIF-1α levels showed more resistance to apoptosis caused
by hypoxia and glucose deprivation than did cell lines with low
HIF-1α expression under normoxia (28). It can thus be concluded that HIF-1α
plays a role in hypoxia-induced apoptosis, and acts as an
antiapoptotic factor (12). In the
present study, the viability of MC3T3-E1 cells decreased and the
expression of HIF-1α protein in the MC3T3-E1 cells increased under
hypoxic conditions. It was also found that the viability of
HIF-1α-transfected MC3T3-E1 cells was higher than that in cells
without forced expression of HIF-1α (Fig. 2C), whereas HIF-1α-knockdown by siRNA
in MC3T3-E1 cells enhanced the hypoxia-induced decrease in cell
viability (Fig. 3C). It was
ascertained that the forced expression of HIF-1α in the MC3T3-E1
cell line attenuated the hypoxia-induced decrease in cell viability
by inhibiting apoptosis. These results indicate that HIF-1α plays a
key role in the hypoxia-induced decrease in osteoblast
viability.
In conclusion, the viability of the MC3T3-E1 cell
line decreased under hypoxia and HIF-1α expression was upregulated.
The forced expression of HIF-1α in the MC3T3-E1 cell line
attenuated the hypoxia-induced decrease in osteoblast viability by
inhibiting apoptosis. These present findings provide novel insight
into the mechanism underlying the hypoxia-induced decrease in cell
viability, and indicate that HIF-1α expression affects cell
viability by inhibiting apoptosis.
References
|
1
|
Lu C, Wang X, Sinha A, et al: The role of
oxygen during fracture healing. Bone. 52:220–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scaringi R, Piccoli M, Papini N, et al:
NEU3 sialidase is activated under hypoxia and protects skeletal
muscle cells from apoptosis through the activation of the epidermal
growth factor receptor signaling pathway and the hypoxia-inducible
factor (HIF)-1α. J Biol Chem. 288:3153–3162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu C, Hu D, Miclau T and Marcucio RS:
Ischemia leads to delayed-union during fracture healing: a mouse
model. J Orthop Res. 25:51–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kolar P, Gaber T, Perka C, Duda GN and
Buttgereit F: Human early fracture hematoma is characterized by
inflammation and hypoxia. Clin Orthop Relat Res. 469:3118–3126.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoff P, Maschmeyer P, Gaber T, et al:
Human immune cells' behavior and survival under bioenergetically
restricted conditions in an in vitro fracture hematoma model. Cell
Mol Immunol. 10:151–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoff P, Gaber T, Schmidt-Bleek K, et al:
Immunologically restricted patients exhibit a pronounced
inflammation and inadequate response to hypoxia in fracture
hematomas. Immunol Res. 51:116–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elks PM, van Eeden FJ, Dixon G, et al:
Activation of hypoxia-inducible factor-1α (hif-1α) delays
inflammation resolution by reducing neutrophil apoptosis and
reverse migration in a zebrafish inflammation model. Blood.
118:712–722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eltzschig HK and Carmeliet P: Hypoxia and
inflammation. N Engl J Med. 364:656–665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salceda S and Caro J: Hypoxia-inducible
factor 1alpha (HIF-1alpha) protein is rapidly degraded by the
ubiquitin-proteasome system under normoxic conditions. Its
stabilization by hypoxia depends on redox-inducud changes. J Biol
Chem. 272:22642–22647. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Corn PG, Ricci MS, Scata KA, et al: Mxi1
is induced by hypoxia in a HIF-1-dependent manner and protects
cells from c-Myc-induced apoptosis. Cancer Biol Ther. 4:1285–1294.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ryan HE, Lo J and Johnson RS: HIF-1alpha
is required for solid tumor formation and embryonic
vascularization. EMBO J. 17:3005–3015. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Piret JP, Mottet D, Raes M and Michiels C:
Is HIF-1alpha a pro-or an anti-apoptotic protein? Biochem
Pharmacol. 64:889–892. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sinha K, Das J, Pal PB and Sil PC:
Oxidative stress: the mitochondria-dependent and
mitochondria-independent pathways of apoptosis. Arch Toxicol.
87:1157–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang TM, Qi SN, Zhao N, et al: Induction
of apoptosis through caspase-independent or caspase-9-dependent
pathway in mouse and human osteosarcoma cells by a new nitroxyl
spin-labeled derivative of podophyllotoxin. Apoptosis. 18:727–738.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang B, He K, Zheng F, et al:
Over-expression of hypoxia-inducible factor-1 alpha in vitro
protects the cardiac fibroblasts from hypoxia-induced apoptosis. J
Cardiovasc Med (Hagerstown). 15:579–586. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Greijer AE and van der Wall E: The role of
hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J
Clin Pathol. 57:1009–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(ΔΔC(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seong GJ, Park C, Kim CY, et al:
Mitomycin-C induces the apoptosis of human Tenon's capsule
fibroblast by activation of c-Jun N-terminal kinase 1 and caspase-3
protease. Invest Ophthalmol Vis Sci. 46:3545–3552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sareen D, van Ginkel PR, Takach JC, et al:
Mitochondria as the primary target of resveratrol-induced apoptosis
in human retinoblastoma cells. Invest Ophthalmol Vis Sci.
47:3708–3716. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar G and Narayan B: The biology of
fracture healing in long bonesClassic Papers in Orthopaedics.
Banaszkiewicz P and Kader D: Springer; London: pp. 531–533.
2014
|
|
21
|
Bolander ME: Regulation of fracture repair
by growth factors. Proc Soc Exp Biol Med. 200:165–170. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho TJ, Gerstenfeld LC and Einhorn TA:
Differential temporal expression of members of the transforming
growth factor beta superfamily during murine fracture healing. J
Bone Miner Res. 17:513–520. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakamura T, Hara Y, Tagawa M, et al:
Recombinant human basic fibroblast growth factor accelerates
fracture healing by enhancing callus remodeling in experimental dog
tibial fracture. J Bone Miner Res. 13:942–949. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bennett MH, Stanford RE and Turner R:
Hyperbaric oxygen therapy for promoting fracture healing and
treating fracture non-union. Cocbrane Database Syst Rev.
11:CD0047122012.
|
|
25
|
Kumagai K, Takeuchi R, Ishikawa H, et al:
Low-intensity pulsed ultrasound accelerates fracture healing by
stimulation of recruitment of both local and circulating osteogenic
progenitors. J Orthop Res. 30:1516–1521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu J, Qu J, Xu D, Zhang T, Qin L and Lu H:
Combined application of low-intensity pulsed ultrasound and
functional electrical stimulation accelerates bone-tendon junction
healing in a rabbit model. J Orthop Res. 32:204–209. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Steinbrech DS, Mehrara BJ, Saadeh PB, et
al: Hypoxia regulates VEGF expression and cellular proliferation by
osteoblasts in vitro. Plast Reconstr Surg. 104:738–747. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Akakura N, Kobayashi M, Horiuchi I, et al:
Constitutive expression of hypoxia-inducible factor-1alpha renders
pancreatic cancer cells resistant to apoptosis induced by hypoxia
and nutrient deprivation. Cancer Res. 61:6548–6554. 2001.PubMed/NCBI
|