Introduction
Chronic kidney disease (CKD) is an important public
health problem, affecting 10–15% of the adult general population.
CKD has a negative effect on cardiac function and frequently leads
to anomalies in left ventricular (LV) structure and function.
Cardiovascular disease-related mortality is the most common cause
of mortality in patients with CKD (1–5). LV
diastolic dysfunction, characterized by abnormalities of
ventricular filling, including decreased diastolic distensibility
and impaired relaxation, is a very common structural abnormality in
patients with CKD and is independently associated with morbidity
and mortality (2,3). Previous studies have shown that LV
function deterioration in CKD patients progresses in a predictable
fashion, with LV diastolic dysfunction usually preceding LV
systolic dysfunction (5–7). These findings suggest that maintaining
LV diastolic function may be critical for preventing cardiac
failure in CKD patients (6,7).
CKD frequently coexists with traditional
cardiovascular risk factors, hypertension (HP) being the most
common. HP can be either a cause or a consequence of CKD.
Specifically, HP is known to play an important role in causing LV
diastolic dysfunction. Several trials have demonstrated the benefit
of blood pressure (BP) control in slowing the progression of kidney
disease and improving clinical outcomes, particularly in patients
with CKD stage ≥3 (4,8); however, the mechanisms involved remain
unknown. Diastolic dysfunction is considered as an important
pathophysiological intermediate to heart failure and represents a
attractive target for cardiovascular disease prevention (4–7).
However, to the best of our knowledge, the impact of
antihypertensive treatment on LV diastolic function has never been
analyzed in CKD patients.
Newly developed two-dimensional speckle tracking
echocardiography (2DSTE) is based on tracking of the speckles
produced by the interaction of ultrasound with the ventricular
structures and quantitatively analyzes global and regional
myocardial motion. Global diastolic strain rate (SR) measurements,
averaged from all LV segmental SRs at the same time, have been
validated to be accurate in the assessment of diastolic function
and advantageous over the traditional tissue Doppler approach
(9,10). In light of this evidence, 2DSTE was
chosen for use in the current study to evaluate the effect of
antihypertensive treatment on LV diastolic function in patients
with CKD.
Materials and methods
Study population
Initially, 172 consecutive patients with newly
diagnosed and untreated hypertension with decreased estimated
glomerular filtration rate (eGFR) or elevated albuminuria levels
were recruited from the outpatient department of Wuxi People's
Hospital Affiliated to Nanjing Medical University (Wuxi, China),
among whom 152 patients met standard clinical criteria for CKD
(11). BP was measured in a sitting
position (measurements were made after a 5-min rest in a sitting
position with a certified mercury sphygmomanometer; an average of
three measurements made at an interval of ≥2 min was used in the
analysis). Patients underwent clinical assessment that included an
evaluation of their symptoms and physical condition prior to
enrollment. HP was diagnosed and treated in accordance with the
Seventh Report of the Joint National Committee on the Prevention,
Detection, Evaluation and Treatment of High Blood Pressure; the
goal of antihypertensive treatment is to attain a BP <130/80
mmHg (12). Antihypertensive agents,
namely β-blockers, diuretics, calcium channel blockers (CCBs),
angiotensin receptor blockers (ARBs) and angiotensin converting
enzyme inhibitors (ACEIs), were titrated to the maximal tolerated
dose and used in combination when the BP goal was not reached. BP
treatment was checked every 2 weeks and continued for 6 months. The
following indicators were used as exclusion criteria: clinically
significant coronary artery disease, any valvular diseases, atrial
fibrillation, anticipated dialysis initiation within 6 months, LV
ejection fraction (EF) <50% and poor-quality imaging on
echocardiography. A total of 18 patients were excluded on the basis
of these exclusion criteria; therefore, the final study population
consisted of 134 hypertensive patients with stable CKD (73 males
and 61 females; mean age, 50.8±11.5 years). Blood samples were
obtained from all participants and laboratory tests were performed
to determine the levels of hemoglobin, fasting glucose, total
cholesterol, low-density lipoprotein cholesterol (LDL),
triglyceride and B-type natriuretic peptide (BNP). eGFR was
calculated by the Modification of Diet in Renal Disease equation
(13). The final protocol was
approved by the Human Ethics Committee of Wuxi People's Hospital
Affiliated to Nanjing Medical University. Written informed consent
was obtained from all subjects. All patients were treated with
antihypertensive drugs and underwent two separate transthoracic
echocardiographic exams at baseline and after 6 months of
therapy.
Echocardiographic evaluation
Baseline and follow-up echocardiographic exams were
performed using a Vivid 7 ultrasound system (GE Medical Systems,
Milwaukee, WI, USA). Two-dimensional measurements were performed
according to the American Society of Echocardiography
recommendations (14) and included
LV ejection fraction (LVEF) by the biplane method of discs, maximal
left atrial (LA) volume by the method of discs and LV mass (LVM)
calculated using the Devereux formula. Maximal LA volume and LVM
were indexed to body surface area. Tissue Doppler imaging at the
septal side of the mitral annulus was used to specify early
diastolic (E') mitral annular velocity. Mitral inflow measurements
included peak early (E) and peak late (A) velocities and the
deceleration time (DT) of E (14).
The E/A and E/E' ratios were subsequently calculated. Three cardiac
cycles were measured and averaged for all Doppler measurements.
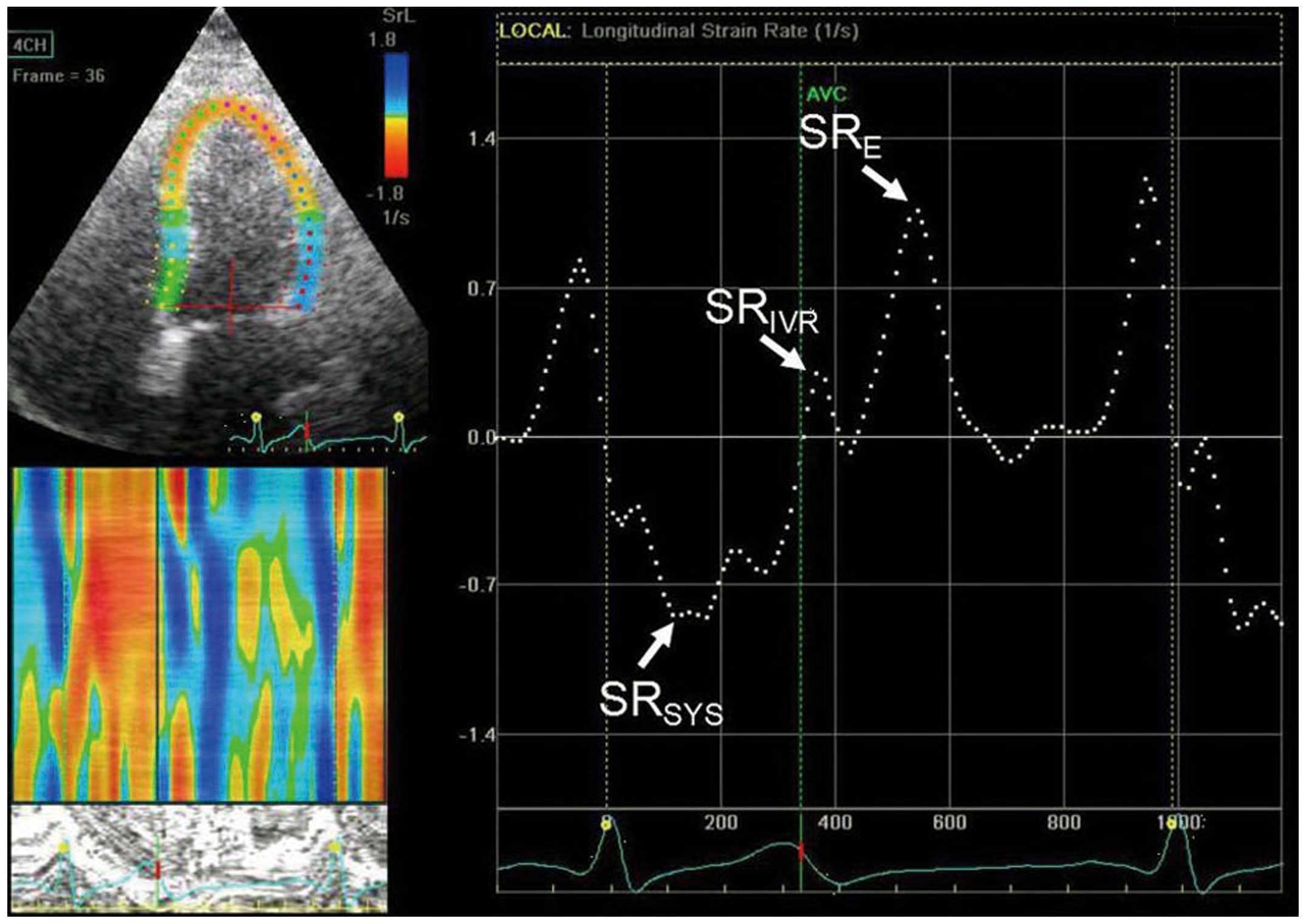
Grayscale images of apical views were obtained with
frame rates >80 Hz for strain analysis by 2DSTE (Fig. 1). Recordings were processed with
acoustic-tracking software (EchoPAC PC version 110.0.0, GE
Healthcare) allowing off-line semi-automated speckle-based strain
analyses. Longitudinal LV strain rates were measured by 2DSTE as
previously reported (9,10). Peak systolic strain rate
(SRSYS), peak strain rate during isovolumetric
relaxation period (SRIVR) and early diastole
(SRE) were calculated by averaging the values of each of
the 18 segments, which were derived from the 6 segments of each of
the 3 apical views (2-chamber, 4-chamber, and apical long-axis
views). The ratio of E and SRIVR was calculated. All
measurements of heart structure and performance were averaged over
3 cardiac cycles.
Intraobserver and interobserver
variation
A total of 15 randomly selected subjects were
independently assessed by two echocardiologists to identify
variability between and within observers in the measurement of
2DSTE parameters.
Statistical analysis
Distribution of data was assessed using a one-sample
Kolmogorov-Smirnov test. Data are reported as mean ± standard
deviation (SD) for normally distributed continuous variables,
median (quartile 1-quartile 3) for skew-distributed continuous
variables, and frequencies for categorical variables. For numerical
variables, an independent sample t-test and the Mann-Whitney U test
were used for inter-group comparisons. A comparison of the clinical
and echocardiographic variables before and after treatment was
performed by paired sample t-test or Wilcoxon signed-rank test.
Spearman's correlation analysis was used to assess the strength of
the association between variables with non-normal distributions.
Multivariate linear regression analyses (backward) were performed
with significant variables in univariate analysis to determine the
independence. Inter- and intra-observer agreements were assessed
with intra- and inter-class correlation coefficients. For all
tests, a P-value <0.05 was considered significant. All
statistical analyses were performed using SPSS statistical
software, version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of the study
population
A cohort of 134 non-dialyzed CKD patients was
included (73 males and 61 females; mean age, 50.8±11.5 years). Of
these participants, 36 patients (26.9%) had stage 1 CKD, 38
patients (28.4%) had stage 2 CKD, 44 patients (32.8%) had stage 4
CKD and 16 patients (11.9%) had stage 5 CKD. Subjects were
categorized into two groups based on the CKD stage. These were
group I (CKD stage 1 or 2; n=74) and group II (CKD stage ≥3; n=60).
Table I presents a detailed list of
demographic and clinical variables at baseline and follow-up.
Following antihypertensive treatment, 69 patients achieved their
target BP in group I (93.2%), and 53 patients in group II (88.3%).
Notably, participants in group II at study entry were older, had
higher systolic BP (SBP) and diastolic BP (DBP), as well as higher
BNP levels; however, SBP, DBP and the level of BNP declined from
baseline to follow-up in the two groups.
 | Table I.Baseline and follow-up demographic and
clinical variables. |
Table I.
Baseline and follow-up demographic and
clinical variables.
|
| Total (n=134) | Group I (n=74) | Group II (n=60) |
|---|
|
|
|
|
|
|---|
| Characteristics | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up |
|---|
| Age (years) | 50.8±11.5 |
| 48.8±11.6 |
| 52.9±11.2 |
| Male/female
(n/n) | 73/61 |
| 40/34 |
| 33/27 |
| BSA
(m2) | 1.77±0.35 | 1.77±0.35 | 1.76±0.37 | 1.76±0.36 | 1.78±0.33 | 1.77±0.34 |
| Systolic BP
(mmHg) | 154.6±7.6 |
124.0±7.3a | 150.4±5.6 |
122.9±6.5a |
159.8±6.4b |
125.4±8.0a,
b |
| Diastolic BP
(mmHg) | 92.6±10.2 |
74.6±10.4a | 90.5±9.3 | 73.8±9.3a |
94.9±10.7b |
75.4±11.5a |
| Heart rate (bpm) | 72.7±6.8 | 70.7±6.9 | 72.0±6.6 | 70.2±7.0 | 73.5±7.0 | 71.3±6.7 |
| Total cholesterol
(mmol/l) | 4.7±1.2 | 4.4±0.8 | 4.4±1.0 | 4.3±0.8 | 4.6±1.0 | 4.4±0.9 |
| LDL cholesterol
(mmol/l) | 2.3±0.7 | 2.2±0.6 | 2.3±0.6 | 2.2±0.7 | 2.4±0.7 | 2.2±0.6 |
| Hemoglobin
(g/l) | 116.7±23.9 | 117.7±22.5 | 120.0±21.5 | 118.2±21.9 | 113.0±25.9 | 117.1±23.3 |
| Glucose
(mmol/l) | 6.4±1.4 | 6.7±1.7 | 6.4±1.5 | 6.3±1.4 | 6.7±1.7 | 6.5±1.8 |
| eGFR (ml/min/1.73
m2) | 61.8±24.3 | 65.9±24.1 | 81.2±10.8 | 84.7±11.7 |
37.9±11.8b |
42.7±11.4a, b |
| BNP (pg/ml) | 205 (179,265) | 172
(155,200)a | 179 (172,190) | 158
(148,165)a | 272
(242,287)b | 202
(195,212)a, b |
| Smoking history, n
(%) | 25 (18.7) |
| 15 (20.2) |
| 10 (16.7) |
| Diabetes mellitus,
n (%) | 50 (37.3) |
| 27 (36.5) |
| 23 (38.3) |
| Dyslipidemia, n
(%) | 56 (41.8) |
| 30 (40.5) |
| 26 (43.3) |
| ARBs or ACEIs, n
(%) | 61 (45.5) |
| 38 (51.4) |
| 28 (38.3) |
| β-blockers, n
(%) | 58 (43.3) |
| 27 (36.5) |
| 31 (51.7) |
| CCBs, n (%) | 95 (70.9) |
| 48 (64.8) |
| 47 (73.4) |
| Diuretics, n
(%) | 49 (74.6) |
| 51 (68.9) |
| 49 (81.7) |
Baseline and follow-up
echocardiographic results
Table II lists
comprehensive list of echocardiographic measurements at baseline
and follow-up. LVEF and SRSYS at baseline and follow-up
were similar across the two groups. Compared with group I, higher
E/E' and E/SRIVR ratios, and lower SRIVR and
SRE indicated more severe baseline diastolic dysfunction
in group II. Over the study period, the E/E' ratio was similar
between baseline and follow-up in group I, but was decreased at
follow-up in group II; however, there were no significant
differences in E velocity, A velocity, E/A ratio or DT. Although
SRIVR and SRE improved from baseline to
follow-up in both groups, the ratio of E/SRIVR
decreased. As shown in Table III,
the patients in group II were more likely to demonstrate
improvements in diastolic speckle-tracking parameters following
treatment compared with those in group I. Plasma levels of BNP, as
a biomarker of diastolic function, decreased throughout the study;
however, the reduction was greater in group II. Therefore, these
analyses indicate an association between the plasma BNP levels and
echocardiographic parameters.
 | Table II.Baseline and follow-up
echocardiographic parameters in the study population. |
Table II.
Baseline and follow-up
echocardiographic parameters in the study population.
|
| Total (n=134) | Group I (n=74) | Group II
(n=60) |
|---|
|
|
|
|
|
|---|
| Parameters | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up |
|---|
| LA volume index
(ml/m2) | 36.2±5.9 |
33.1±5.9a | 35.7±5.7 |
32.8±5.9a | 36.8±6.0 |
33.5±5.8a |
| LV end-diastolic
dimension (mm) | 49.6±2.8 | 49.2±2.7 | 48.9±2.7 | 48.5±2.9 | 50.6±2.8 | 50.0±2.6 |
| LV MI
(g/m2) | 95.1±26.7 | 93.0±25.7 | 87.3±19.0 | 86.7±18.7 | 104.7±31.6 | 100.7±30.7 |
| LV ejection
fraction (%) | 68.0±8.9 | 68.7±11.4 | 68.5±7.5 | 69.4±10.0 | 67.2±10.3 | 67.8±12.9 |
| Mitral deceleration
time (msec) | 208.1±50.0 | 212.5±45.4 | 210.5±46.7 | 214.8±42.3 | 205.2±53.8 | 209.6±49.2 |
| E velocity
(m/sec) | 73.4±28.4 | 71.0±22.5 | 72.9±33.1 | 70.1±12.4 | 74.1±21.4 | 72.2±31.7 |
| A velocity
(m/sec) | 62.5±23.9 | 59.2±29.7 | 60.8±34.2 | 59.9±26.2 | 64.7±30.6 | 60.9±33.7 |
| E/A | 1.2±0.2 | 1.3±0.3 | 1.2±0.2 | 1.3±0.3 | 1.2±0.3 | 1.3±0.3 |
| E' velocity
(m/sec) | 8.4±3.3 |
9.9±3.1a | 8.7±3.8 |
10.0±2.0a | 8.0±2.4 |
9.9±4.0a |
| E/E' | 9.0±1.8 |
8.2±1.2a | 8.5±1.5 | 8.1±0.9 |
9.5±2.0b |
8.2±1.5a |
| SRSYS
(sec−1) | −0.80±0.06 | −0.82±0.07 | −0.81±0.06 | −0.83±0.06 | −0.80±0.06 | −0.81±0.09 |
| SRIVR
(sec−1) | 0.23±0.10 |
0.42±0.10a | 0.30±0.07 |
0.43±0.08a |
0.13±0.03b |
0.41±0.11a |
| SRE
(sec−1) | 0.58±0.25 |
1.07±0.24a | 0.76±0.17 |
1.08±0.20a |
0.34±0.13b |
1.04±0.27a |
|
E/SRIVR(m) | 3.8±1.8 |
1.7±0.4a | 2.3±0.7 |
1.7±0.2a |
5.6±1.0b |
1.8±0.5a |
 | Table III.Comparison of changes in diastolic
parameters and plasma BNP levels between the two groups. |
Table III.
Comparison of changes in diastolic
parameters and plasma BNP levels between the two groups.
| Parameters | Group I (n=74) | Group II
(n=60) | P-value |
|---|
| ΔLA volume index
(ml/m2) | −2.7 (−3.0,
−2.6) | −3.2 (−3.7,
−2.6) | 0.17 |
| Deceleration time
(msec) | −8.0 (−41.2,
27.2) | −6.0 (−34.8,
19.5) | 0.61 |
| ΔE/A | 0.04 (−0.13,
0.17) | −0.01 (−0.14,
0.17) | 0.81 |
| ΔE/E' | −0.01 (−0.78,
0.16) | −1.40 (−1.83,
−0.50) | <0.01 |
| ΔSRIVR
(sec−1) | 0.15 (0.10,
0.19) | 0.31 (0.27,
0.34) | <0.01 |
| ΔSRE
(sec−1) | 0.35 (0.25,
0.47) | 0.75 (0.69,
0.84) | <0.01 |
| ΔE/SRIVR
(m) | −0.08 (−0.10,
−0.04) | −0.39 (−0.45,
−0.35) | <0.01 |
| ΔBNP (pg/ml) | −21 (−28, −17) | −71 (−78, −48) | <0.01 |
Association between BNP levels and
echocardiographic parameters
In groups I and II, the BNP level was correlated
with SRIVR (r=-0.77, P<0.01), E/SRIVR
(r=0.69, P<0.01), SRE (r=-0.68, P<0.01), E/E'
(r=0.53, P<0.01) and LA volume index (LAVI) (r=0.47, P<0.01)
at baseline. The differences (Δ) of clinical and echocardiographic
parameters prior to and following treatment were determined by
subtracting the values at follow-up from the values at baseline.
There were significant correlations between ΔBNP and
ΔSRIVR (r=-0.73, P<0.01), ΔE/SRIVR
(r=0.64, P<0.01), ΔSRE (r=-0.66, P<0.01), ΔE/E'
(r=0.57, P<0.01) and ΔLAVI (r=0.51, P<0.01). Among these
parameters, SRIVR and ΔSRIVR presented the
highest correlation coefficient.
Predictors of ΔSRIVR
To control for potential confounding variables in
the data, univariate and multivariate linear regression analyses of
ΔSRIVR were used to evaluate potential predictive
factors for change of diastolic function. In univariate analyses,
it was found that age, baseline CKD stage, SBP, DBP, LVMI, ΔSBP and
ΔDBP each were significantly associated with ΔSRIVR
(Table IV). On multivariable
analysis, baseline CKD stage, SBP and ΔSBP were positively
associated with ΔSRIVR (P<0.01), and baseline
SRIVR was inversely associated with ΔSRIVR
(P<0.01).
 | Table IV.Clinical predictors for the
difference of SRIVR between the baseline and follow-up
using univariate and multivariate linear regression analyses. |
Table IV.
Clinical predictors for the
difference of SRIVR between the baseline and follow-up
using univariate and multivariate linear regression analyses.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | B | 95% CI | P-value | B | 95%CI | P-value |
|---|
| Age | 0.23 | 0.09, 0.37 | <0.01 |
|
|
|
| Baseline CKD
stage | 8.78 | 7.78, 9.78 | <0.01 | 4.11 | 1.79, 6.44 | <0.01 |
| ΔeGFR | 0.17 | −0.02, 0.35 | 0.04 |
|
|
|
| Baseline systolic
BP | 0.71 | 0.54, 0.89 | <0.01 | 0.15 | 0.02, 0.28 | 0.02 |
| ΔSystolic BP | 0.69 | 0.56, 0.82 | <0.01 | 0.20 | 0.08, 0.33 | <0.01 |
| Baseline diastolic
BP | 0.16 | −0.02, 0.33 | 0.03 |
|
|
|
| ΔDiastolic BP | 2.04 | 1,35, 2.73 | <0.01 |
|
|
|
| Baseline LVMI | 0.09 | 0.02, 0.15 | <0.01 |
|
|
|
| Baseline
SRIVR | −0.75 | −0.86, −0.64 | <0.01 | −0.26 | −0.44, −0.08 | <0.01 |
Reproducibility
The interobserver correlation coefficients [95%
confidence interval (CI)] were good for SRIVR [0.93
(0.93–0.99)] and SRE [0.97 (0.91–0.99)]. The
intraobserver correlation coefficients (95% CI) were also good for
SRIVR [0.98 (0.95–0.99)] and SRE [0.96
(0.89–0.98)].
Discussion
To the best of our knowledge, this is the first
study to demonstrate that LV diastolic function improves with
antihypertensive treatment in patients with CKD using 2DSTE.
Notably, antihypertensive treatment was particularly efficacious
among patients with CKD stage ≥3.
E/E' has been shown to be useful in predicting
elevated LV filling pressures in clinical studies; however, E/E'
has a significant gray zone and is less reliable in preserving LVEF
>50% due to several factors (9,15).
Firstly, there are well-known limitations of Doppler-based methods
such as the angle dependency, which has the potential for
significant errors with angulations >20°. Secondly, regional
wall motion abnormalities in the sampling region may lead to low
annular velocities on tissue Doppler despite near normal LV
relaxation. Thirdly, another important limitation of this approach
is the potential effect of LA pressure (9,15,16).
Since E' occurs during the early phase of LV filling, not only LV
relaxation, but also LA pressure has an important impact on its
value. Furthermore, Andersen et al found there was not a
strong correlation between changes in E/E' and changes in LV
filling pressure (17). Conversely,
the global LV diastolic SR collected using 2DSTE may overcome all
the aforementioned limitations and provide a more complete
reflection of overall LV diastolic function than E/E' (9,10,15–17).
Notably, this measurement reflects the total performance of all LV
segments. Therefore, in the present study, the E/E' ratio was
similar between baseline and follow-up in group I, However,
significant changes in LV diastolic performance over the study
period were revealed following 2DSTE evaluation in these
patients.
The BNP assessment used in this study has been
validated as a highly sensitive and accurate method for the
detection of LV diastolic dysfunction and applied in patients with
CKD (18,19). In previous studies concerning HP or
CKD, patients with an improvement in LV diastolic function
exhibited a reduction in BNP plasma concentration after treatment
(19–21). The present study found significant
changes in BNP concentrations and echocardiographic parameters in
patients with CKD following antihypertensive treatment, suggesting
an improvement of LV diastolic function. Among these
echocardiographic parameters, ΔSRIVR presented the
highest correlation with ΔBNP, suggesting that SRIVR
might have better diagnostic value than traditional echo indices in
detecting changes in LV diastolic function. SRIVR is
obtained directly from the ventricular myocardium during the
isovolumetric relaxation period (when the mitral valve is closed),
meaning that problems related to valvular pathology and LA
pressure, which undermine other echocardiographic parameters, are
circumvented (9,22).
Patients with CKD have a much greater cardiovascular
risk than the general population. Moreover, HP is common in these
patients and an important independent risk factor of LV diastolic
dysfunction (2,4,8).
Previous literature suggests that the mechanism underlying the
impairment of LV diastolic function in patients with CKD and HP may
be due to increased transmyocardial wall stress, which can produce
subendocardial ischemia, thus increasing myocardial stiffness and
reducing myocardial deformation in diastole. As BP decreases
following medical treatment, LV wall stress also decreases, thus
decreasing LV stiffness and improving myocardial diastolic function
(8,15). Antihypertensive agents reduce
endothelial dysfunction and microvascular disease, which has been
shown to contribute to a worsening of cardiovascular risk factors
and may also play a role in the pathophysiological process that
leads to accelerated cardiovascular disease in patients with CKD
(8). This effect may result in
greater benefit than that achieved by BP-lowering alone. Studies
have reported the benefits of antihypertensive treatment on
cardiovascular and renal outcomes in CKD patients, and absolute
risk reductions in people with CKD stage ≥3 have been found to be
greater than those in people with CKD of stages 1 and 2 (4,8). The
present study found significantly improved LV diastolic function in
patients with CKD following 6 months of antihypertensive treatment.
Notably, the patients with CKD stage ≥3 were more likely to
demonstrate improvements in diastolic parameters than the others.
On multivariate analysis, baseline CKD stage and BP were positively
associated with ΔSRIVR (P<0.01), and baseline
SRIVR was inversely associated with ΔSRIVR.
These results suggest the importance of BP reduction in this
population, particularly with CKD stage ≥3, higher BP and LV
diastolic dysfunction.
Several limitations of this study should be
considered. The sample was small (134 patients), but the careful
selection and recruitment of newly diagnosed and untreated
hypertensive individuals is a major challenge in this field.
Further confounding the recruitment efforts, patients with severe
renal dysfunction and LV systolic dysfunction were excluded from
this study. Consequently, the relatively small number of total
subjects that was analyzed lessened the power and interpretation of
the findings. The patients were not followed for clinical outcomes
(such as readmission for clinical events, mortality or stroke),
which meant that it was not possible to assess permit associations
between the speckle-tracking parameters and outcomes. Furthermore,
albuminuria was only assessed at some of the visits during the
course of the trial, making it impossible to assess the changes in
albuminuria and its interaction with echocardiographic parameters
(9). Although the results achieved
statistical significance, further follow-up investigation and
confirmation in a larger sample are required to validate the
findings.
Overall, in the present sample of individuals with
CKD, a significant improvement in LV diastolic function was
obtained following antihypertensive therapy, which was demonstrated
to be more effective in patients with CKD stage ≥3, higher baseline
SBP and worse LV diastolic function.
References
|
1
|
McAlister FA, Ezekowitz J, Tonelli M and
Armstrong PW: Renal insufficiency and heart failure: prognostic and
therapeutic implications from a prospective cohort study.
Circulation. 109:1004–1009. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nardi E, Cottone S, Mulè G, et al:
Influence of chronic renal insufficiency on left ventricular
diastolic function in hypertensives without left ventricular
hypertrophy. J Nephrol. 20:320–328. 2007.PubMed/NCBI
|
|
3
|
Bruch C, Rothenburger M, Gotzmann M, et
al: Chronic kidney disease in patients with chronic heart failure -
impact on intracardiac conduction, diastolic function and
prognosis. Int J Cardiol. 118:375–380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blood Pressure Lowering Treatment
Trialists' Collaboration, . Ninomiya T, Perkovic V, Turnbull F, et
al: Blood pressure lowering and major cardiovascular events in
people with and without chronic kidney disease: meta-analysis of
randomised controlled trials. BMJ. 347:f56802013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Foley RN, Parfrey PS and Sarnak MJ:
Clinical epidemiology of cardiovascular disease in chronic renal
disease. Am J Kidney Dis. 32:(Suppl 3). S112–S119. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fathi R, Isbel N, Haluska B, et al:
Correlates of subclinical left ventricular dysfunction in ESRD. Am
J Kidney Dis. 41:1016–1025. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Solomon SD, Janardhanan R, Verma A, et al:
Valsartan In Diastolic Dysfunction (VALIDD) Investigators: Effect
of angiotensin receptor blockade and antihypertensive drugs on
diastolic function in patients with hypertension and diastolic
dysfunction: a randomised trial. Lancet. 369:2079–2087. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heerspink HJ, Ninomiya T, Perkovic V, et
al: ADVANCE Collaborative Group: Effects of a fixed combination of
perindopril and indapamide in patients with type 2 diabetes and
chronic kidney disease. Eur Heart J. 31:2888–2896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Khoury DS, Thohan V, et al: Global
diastolic strain rate for the assessment of left ventricular
relaxation and filling pressures. Circulation. 115:1376–1383. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kasner M, Gaub R, Sinning D, et al: Global
strain rate imaging for the estimation of diastolic function in
HFNEF compared with pressure-volume loop analysis. Eur J
Echocardiogr. 11:743–751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
National Kidney Foundation, . K/DOQI
clinical practice guidelines for chronic kidney disease:
evaluation, classification and stratification. Am J Kidney Dis =
39. (Suppl 1). S1–S266. 2002.PubMed/NCBI
|
|
12
|
Lenfant C, Chobanian AV, Jones DW and
Roccella EJ: Joint National Committee on the Prevention, Detection,
Evaluation, and Treatment of High Blood Pressure: Seventh report of
the Joint National Committee on the Prevention, Detection,
Evaluation and Treatment of High Blood Pressure (JNC 7): resetting
the hypertension sails. Hypertension. 41:1178–1179. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma YC, Zuo L, Chen JH, et al: Modified
glomerular filtration rate estimating equation for Chinese patients
with chronic kidney disease. J Am Soc Nephrol. 17:2937–2944. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lang RM, Bierig M, Devereux RB, et al:
Chamber Quantification Writing Group; American Society of
Echocardiography's Guidelines and Standards Committee; European
Association of Echocardiography: Recommendations for chamber
quantification: a report from the American Society of
Echocardiography's Guidelines and Standards Committee and the
Chamber Quantification Writing Group, developed in conjunction with
the European Association of Echocardiography, a branch of the
European Society of Cardiology. J Am Soc Echocardiogr.
18:1440–1463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alam M, Zhang L, Stampehl M, et al:
Usefulness of speckle tracking echocardiography in hypertensive
crisis and the effect of medical treatment. Am J Cardiol.
112:260–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kimura K, Takenaka K, Ebihara A, et al:
Speckle tracking global strain rate E/E' predicts LV filling
pressure more accurately than traditional tissue Doppler E/E'.
Echocardiography. 29:404–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Andersen MJ, Ersbøll M, Gustafsson F, et
al: Exercise-induced changes in left ventricular filling pressure
after myocardial infarction assessed with simultaneous right heart
catheterization and Doppler echocardiography. Int J Cardiol.
168:2803–2810. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paulus WJ, Tschöpe C, Sanderson JE, et al:
How to diagnose diastolic heart failure: a consensus statement on
the diagnosis of heart failure with normal left ventricular
ejection fraction by the Heart Failure and Echocardiography
Associations of the European Society of Cardiology. Eur Heart J.
28:2539–2550. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tamez H, Zoccali C, Packham D, et al:
Vitamin D reduces left atrial volume in patients with left
ventricular hypertrophy and chronic kidney disease. Am Heart J.
164:902–909.e2. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tapp RJ, Sharp A, Stanton AV, et al: ASCOT
Investigators: Differential effects of antihypertensive treatment
on left ventricular diastolic function: an ASCOT
(Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll
Cardiol. 55:1875–1881. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aksoy H, Okutucu S, Kaya EB, et al:
Clinical and echocardiographic correlates of improvement in left
ventricular diastolic function after cardiac resynchronization
therapy. Europace. 12:1256–1261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Van Schinkel LD, Auger D, van Elderen SG,
et al: Aortic stiffness is related to left ventricular diastolic
function in patients with diabetes mellitus type 1: assessment with
MRI and speckle tracking strain analysis. Int J Cardiovasc Imaging.
29:633–641. 2013. View Article : Google Scholar : PubMed/NCBI
|