Introduction
Breast cancer is a common malignant tumor in
females, which has the second highest cancer mortality rate among
females worldwide (1). The morbidity
rate is increasing yearly; thus, breast cancer poses as a serious
threat to female health. The dissemination and proliferation of
tumor cells to other organs is the major factor causing difficulty
for clinical treatment, and is also the main cause of mortality in
patients with breast cancer. Almost 50% of breast cancer patients
already have distant metastases (2,3) at their
initial diagnosis. Therefore, it is particularly important to
prevent and control tumor metastasis in breast cancer.
SATB1 (special AT-rich sequence binding protein 1)
is a type of tissue specific nuclear matrix binding protein, which
has significant expression in thymus cells, progenitor cells and
the epithelial basal layer, with almost no expression in other
normal cells and tissues (4–6). As a global chromatin organizer and
transcription factor, SATB1 has emerged as a key factor integrating
higher-order chromatin architecture with gene regulation (7–9). The
protein plays an important role in T cell development, early
erythroid differentiation, cell homeostasis and responses to a
number of types of stimulation (4,5,7,10,11).
A previous study reported that SATB1 has an
abnormally high expression in a variety of tumor cells, and
controls >1,000 types of tumor-associated genes, affecting the
promotion of tumor growth and metastasis (12). Consequently, SATB1 is regarded as a
potentially important target molecule for antitumor drugs.
Baicalein (molecular formula,
C15H10O5; molecular weight, 270.24
gmol−1) is a monomer isolated from Scutellaria
baicalensis Georgi, which is the main effective component in
Radix Scutellariae. Baicalein has been reported to inhibit tumor
cell proliferation, invasiveness and metastasis in human breast
cancer, liver cancer and pancreatic cancer cell lines (13–15).
However, the specific molecular mechanism underlying the inhibition
of tumor growth and metastasis is yet to be elucidated. The present
study investigated a novel antitumor mechanism of baicalein in the
MDA-MB-231 human breast cancer cell line, where baicalein may exert
an antitumor, -invasion and -metastasis function by inhibiting the
protein expression of SATB1.
Materials and methods
Cell culture
An MDA-MB-231 cell line (Shanghai Institutes of
Biological Sciences, Shanghai, China) was cultured in RPMI-1640
medium (Hyclone; GE Healthcare, Logan, UT, USA), supplemented with
10% fetal bovine serum (Hyclone; GE Healthcare), in a humidified
atmosphere of 5% CO2 at 37°C. The medium was changed
every other day. Cells were passaged when 80% of the bottle wall
was covered.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay
MDA-MB-231 cells were seeded in a 96-well plate at a
density of 104 cells/well and treated with 200 µl
baicalein (99% purity; Sigma-Aldrich, St. Louis, MO, USA) at a
concentration of 0, 5, 10, 20, 40 or 80 µM. The cells were
incubated at 37°C for 24, 48 or 72 h. For the blank control group,
200 µl common cell culture fluid was added. Each concentration was
set as six parallel holes. Thereafter, the media was changed and
the cells were incubated with 20 µl MTT (5 mg/ml; Sigma-Aldrich)
for 4 h. The mixture was centrifuged at 12,000 × g at 4°C for 10
min. The supernatant was removed, 50 ul DMSO was added to each
well, and the solutions were agitated on a decolorization shaker
(ZHWY-100B; Zhicheng Analytical Instrument Manufacturing Co., Ltd.,
Shangghai, China) for 10 min. The optical density (OD) of each well
was measured using an enzyme immunoassay analyzer (PowerWave XS;
BioTek Instruments Inc., Winooski, VT, USA) at 490 nm. The
inhibitory rate of cell proliferation (inhibition ratio, IR) was
calculated as follows: IR = (1 - mean OD value of the experimental
group/mean OD value of the control group) × 100%. Using the cell
proliferation inhibitory rates with the different concentrations of
baicalein at 48 h, the 50% inhibitory concentration
(IC50) of baicalein was calculated.
Wound healing assay
MDA-MB-231 cells were seeded in a six-well plate at
a density of 2×105 cells/well with complete medium
overnight to obtain a full confluent monolayer. The cells were
scraped away vertically after 24 h using a plastic tip. Each well
was washed three times with phosphate-buffered saline (PBS) to
remove the cell debris, and then further incubated for 48 h in
serum-free RPMI-1640 medium with different concentrations of
baicalein (0, 10 and 20 µM). Two wells were used for each group,
and the experiment was repeated three times. The average distance
of cell migration to the injured area was determined under a TS100
inverted microscope (magnification, x40; Nikon Corporation, Tokyo,
Japan) at 0, 24 and 48 h.
Western blot analysis
MDA-MB-231 cells were lysed with
radioimmunoprecipitation assay lysis buffer (Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China), after which the total
cellular protein was extracted. The protein concentration in the
supernatants was determined using a bicinchoninic acid assay
(Beijing Biosynthesis Biotechnology Co., Ltd.) with a Varioskan
Flash Multimode microplate spectrophotometer (Thermo Fisher
Scientific, Waltham, MA, USA). An equal amount of protein was
separated using 10% SDS-polyacrylamide gel electrophoresis and
transferred onto polyvinylidene fluoride (PVDF) membranes (0.45 µm;
Millipore Corporation, Bedford, MA, USA), according to the semi-dry
protein transfer method. The membranes were blocked with 5% skimmed
milk powder for 90 min, and then incubated with primary antibodies
against SATB1 (#ab49061; rabbit polyclonal; 1.25:1,000 dilution;
Abcam, Cambridge, MA, USA) and β-actin (#sc-47778; rabbit
polyclonal; 1:1,000 dilution; #bs-0295G-HRP; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) for 4°C overnight.
Subsequently, the membranes were incubated with a horseradish
peroxidase-conjugated secondary antibody (1:1,000 dilution; Beijing
Biosynthesis Biotechnology Co., Ltd.) at 37°C for 1 h. Finally, the
PVDF membranes were washed with PBS and colored using enhanced
chemiluminescence (Thermo Fisher Scientific), after which detection
was performed using a gel imaging system (Syngene, Frederick, MD,
USA). The experiment was repeated three times.
Statistical analysis
Data are presented as the mean ± standard deviation.
The data were analyzed using SPSS 18.0 software (SPSS, Inc.,
Chicago, IL, USA) and GraphPad Prism software (GraphPad Software,
Inc., La Jolla, CA, USA). A one-way analysis of variance test was
used to compare the differences among groups. The RxC contingency
table and χ2 test were used to compare the quantitative
differences among groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
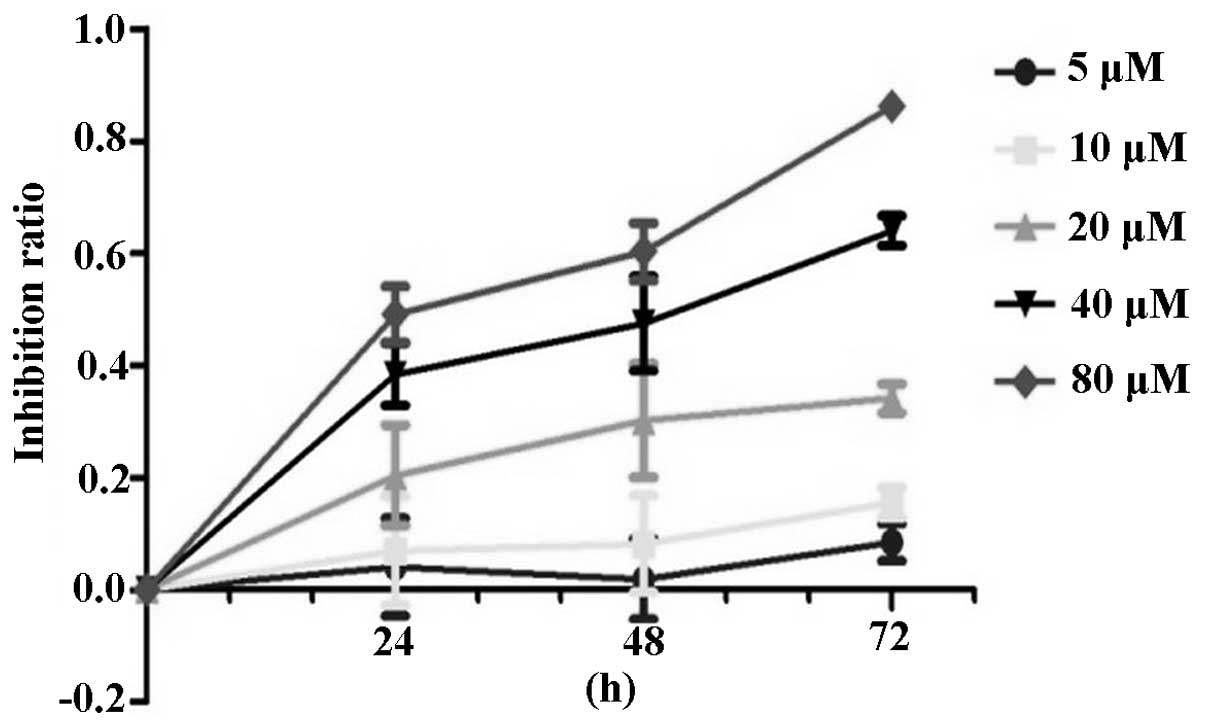
Baicalein suppresses the proliferation
of MDA-MB-231 cells
Baicalein administration suppressed the
proliferation of MDA-MB-231 cells. When compared with the control
group, baicalein administration was shown to significantly inhibit
MDA-MB-231 cell proliferation at various concentrations (5, 10, 20,
40 and 80 µM) for 48 and 72 h (P<0.05). At 24 h, the inhibition
rate increased with increased drug concentration but this was not
statistically significant. With the prolongation of administration
time and increase in drug concentration, the inhibitory effect of
baicalein on the proliferation of MDA-MB-231 cells gradually
increased in a time- and dose-dependent manner (P<0.05), as
shown in Table I and Fig. 1. The IC50 value was
determined to be 27.92 µM when treated with baicalein for 48 h.
 | Table I.Inhibitory effects of baicalein on
MDA-MB-231 cell proliferation. |
Table I.
Inhibitory effects of baicalein on
MDA-MB-231 cell proliferation.
|
| 24 h | 48 h | 72 h |
|---|
|
|
|
|
|
|---|
| Baicalein (µM) | OD | IR (%) | OD | IR (%) | OD | IR (%) |
|---|
| 0 | 0.406±0.031 | 0 | 0.603±0.036 | 0 | 0.981±0.027 | 0 |
| 5 | 0.482±0.013 | -22.84 | 0.533±0.051 | 13.20 | 0.841±0.063 | 14.27 |
| 10 | 0.408±0.052 | -6.53 | 0.507±0.033 | 18.18 | 0.824±0.025 | 20.08 |
| 20 | 0.338±0.029 | 20.63 | 0.356±0.025 | 46.90 | 0.465±0.011 | 52.60 |
| 40 | 0.316±0.016 | 27.26 | 0.283±0.022 | 60.49 | 0.212±0.030 | 78.39 |
| 80 | 0.264±0.014 | 42.67 | 0.207±0.023 | 74.76 | 0.193±0.014 | 80.33 |
Baicalein affects the migration of
MDA-MB-231 cells in vitro
Detection of the cell migration capacity is also a
method for analyzing the metastatic potential of tumor cells. Under
an inverted microscope, the cell morphology of MDA-MB-231 cells
exhibited no marked changes in the control group and 10 µM
baicalein treatment group. However, in the cells treated with 20 µM
baicalein for 48 h, the cell morphology had changed from the
original long spindle shape to a round shape. As shown in Fig. 2, the number of cells that had
migrated into the scratch area in the baicalein intervention group
(20 µM) was significantly lower when compared with the control
group for 24 and 48 h (P<0.05). With increasing concentrations
of baicalein, the scratch width decreased significantly when
compared with the control group at 24 and 48 h (Fig. 2A); this effect was time- and dose-
dependent (P<0.05; Fig. 2B). The
inhibition rate was ~10.8 and 88.7% at 48 h with 10 and 20 µM
baicalein, respectively. The scratch widths of the different groups
at each time point are shown in Fig.
2.
Baicalein inhibits SATB1 protein
expression in MDA-MB-231 cells
When compared with the control group, the protein
expression levels of SATB1 were shown to decrease significantly in
the MDA-MB-231 cells following treatment with the different
concentrations of baicalein (10, 20 and 40 µM) for 48 h
(P<0.01). The results demonstrated that baicalein reduced the
protein expression levels of SATB1 in MDA-MB-231 cells in a
dose-dependent manner (P<0.05). The results of the western blot
analysis are shown in Fig. 3.
Discussion
Abnormal growth and metastasis of cancer cells are
important biological properties of cancer. Invasion and metastasis
are the main reasons underlying the cancer morbidity and mortality
observed in millions of patients (16). There is an important theoretical and
clinical value to screening effective anti-invasive and
anti-metastatic drugs with few side-effects, as these drugs improve
the curative effect and prognosis of breast cancer, and the quality
of life of the patient. Baicalein, the aglycon compound of
baicalin, is extracted from the traditional Chinese medicine,
Scutellaria baicalensis Georgi. An increasing number of
studies have demonstrated that baicalein exhibits potent antitumor
properties (13,16,17). The
antitumor mechanism of baicalein primarily manifests through the
inhibition of tumor cell proliferation, tumor invasion and
metastasis. In addition, baicalein offers a synergistic effect to
chemotherapy drugs in tumor cells with multidrug resistance
(13,14,18).
Baicalein has been demonstrated to inhibit a number breast cancer
cell lines, including MCF-7, MDA-MB-231, BT549 and 4T-1, through
targeting their cell proliferation, invasion and migration ability,
distant metastasis and downregulating the protein expression levels
of matrix metalloproteinase (MMP)-2, MMP-9 and urinary plasminogen
activator (13,19–21).
However, the specific antitumor mechanism is yet to be
elucidated.
Dickinson et al identified SATB1 from the
human cDNA library by using the nuclear matrix-associating DNA
sequence, which is located on the 3′ end of the gene enhancer of µ
chain (an immunoglobulin heavy-chain), as a probe (22). SATB1 was revealed to be anchored on
chromatin with a unique ʻcage-likeʼ structure that was able to
provide binding sites for a number of transcription factors.
Therefore, SATB1 was hypothesized to play an important role in gene
transcription (10). Han et
al reported for the first time that SATB1 had abnormally high
expression levels in breast cancer (12), while almost no expression was
observed in the normal control tissues; thus, SATB1 can be regarded
as an independent adverse prognostic factor of breast cancer. SATB1
can promote the growth and metastasis of breast cancer cells, and
silencing SATB1 expression in breast cancer MDA-MB-231 cells has
been shown to change the invasive phenotype and inhibit tumor
growth. Therefore, SATB1 was hypothesized to play a key role in
tumor progression. Genomics research found that SATB1 can regulate
the expression of >1,000 genes that influence the occurrence and
development of tumors, including ERBB2, MMP-2, −3 and −9, ABL1 and
E-cadherin, and is involved in 61 types of biological activity,
such as cell proliferation, apoptosis, DNA synthesis/degradation,
electron transport, protein expression and receptor activities
(12). SATB1 has been shown to
positively correlate with the expression of a variety of biological
and genetic markers, including cyclin D1, MMP-2, nuclear factor-κB
and proliferating cell nuclear antigen, while negatively
correlating with the expression of APC and BRAF (V600E) (12,23).
Therefore, the expression of SATB1 may be used as a novel tool to
evaluate the gradation of breast cancer, and SATB1 is expected to
become a new therapeutic target in the future.
The MDA-MB-231 human breast cancer cell line has a
high level of invasiveness, and a previous study (12) reported that these cells also
expressed SATB1 at a high level. In the present study, baicalein
was demonstrated to exert a strong inhibitory effect on tumor cell
malignant proliferation in a time- and dose-dependent manner. The
wound healing assay confirmed that baicalein inhibited the cell
migration ability of the breast cancer MDA-MB-231 cells. In
addition, small doses of baicalein intervention were shown to
downregulate the expression of SATB1 protein, and inhibition was
significantly enhanced with increasing concentrations of baicalein
(P<0.01). These results indicate that the downregulation of
SATB1 protein expression may be one of the mechanism underlying the
inhibitory function of baicalein with regard to the invasion and
metastasis of breast cancer cells. However, the present study
utilized in vitro experiments only, and a positive control
group was not included. Therefore, further research is required,
including in vivo experiments.
In conclusion, the present study demonstrated that
baicalein strongly suppressed the proliferation and migration
ability of MDA-MB-231 cells, possibly through the inhibition of
SATB1 protein expression. However, the inhibitory mechanism of
baicalein on MDA-MB-231 breast cancer cells requires further
evaluation in future studies.
Acknowledgements
The study was supported by grants from the National
Natural Science Fund Surface Project (no. 81274136), the Xi'an
Jiaotong University Cross Project Fund (no. Xjj2012141) and the
talent fund of the Second Affiliated Hospital of Xi'an Jiaotong
University (no. RCCGG201105).
References
|
1
|
Friedenreich CM: Physical activity and
breast cancer: review of the epidemiologic evidence and biologic
mechanisms. Recent Results Cancer Res. 188:125–139. 2011.PubMed/NCBI
|
|
2
|
Ahmad A and Hart IR: Mechanisms of
metastasis. Crit Rev Oncol Hematol. 26:163–173. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sporn MB: The war on cancer. Lancet.
347:1377–1381. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alvarez JD, Yasui DH, Niida H, Joh T, Loh
DY and Kohwi-Shigematsu T: The mar-binding protein satb 1
orchestrates temporal and spatial expression of multiple genes
during t-cell development. Genes Dev. 14:521–535. 2000.PubMed/NCBI
|
|
5
|
Cai S, Han HJ and Kohwi-Shigematsu T:
Tissue-specific nuclear architecture and gene expression regulated
by satb1. Nat Genet. 34:42–51. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mai JC and Ellenbogen RG: SATB1: the
convergence of carcinogenesis and chromatin conformation.
Neurosurgery. 63:N62008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kohwi-Shigematsu T, Poterlowicz K,
Ordinario E, Han HJ, Botchkarev VA and Kohwi Y: Genome organizing
function of satb1 in tumor progression. Semin Cancer Biol.
23:72–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galande S, Purbey PK, Notani D and Kumar
PP: The third dimension of gene regulation: organization of dynamic
chromatin loopscape by satb1. Curr Opin Genet Dev. 17:408–414.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pavan Kumar P, Purbey PK, Sinha CK, Notani
D, Limaye A, Jayani RS and Galande S: Phosphorylation of satb1, a
global gene regulator, acts as a molecular switch regulating its
transcriptional activity in vivo. Mol Cell. 22:231–243. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wen J, Huang S, Rogers H, Dickinson LA,
Kohwi-Shigematsu T and Noguchi CT: Satb1 family protein expressed
during early erythroid differentiation modifies globin gene
expression. Blood. 105:3330–3339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai S, Lee CC and Kohwi-Shigematsu T:
Satb1 packages densely looped, transcriptionally active chromatin
for coordinated expression of cytokine genes. Nat Genet.
38:1278–1288. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Ling Y, Chen Y, et al: Flavonoid
baicalein suppresses adhesion, migration and invasion of mda-mb-231
human breast cancer cells. Cancer Lett. 297:42–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chiu YW, Lin TH, Huang WS, et al:
Baicalein inhibits the migration and invasive properties of human
hepatoma cells. Toxicol Appl Pharmacol. 255:316–326. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takahashi H, Chen MC, Pham H, et al:
Baicalein, a component of Scutellaria baicalensis, induces
apoptosis by m cl-1 down-regulation in human pancreatic cancer
cells. Biochim Biophys Acta = 1813. 1465–1474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee Y, Yeo H, Liu SH, Jiang Z, Savizky RM,
Austin DJ and Cheng YC: Increased anti-P-glycoprotein activity of
baicalein by alkylation on the a ring. J Med Chem. 47:5555–5566.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee WJ, Wu LF, Chen WK, Wang CJ and Tseng
TH: Inhibitory effect of luteolin on hepatocyte growth
factor/scatter factor-induced hep g2 cell invasion involving both
mapk/erks and pi3k - a kt pathways. Chem Biol Interact.
160:123–133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Po LS, Chen ZY, Tsang DS and Leung LK:
Baicalein and genistein display differential actions on estrogen
receptor (er) transactivation and apoptosis in MCF-7 cells. Cancer
Lett. 187:33–40. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu B, Li J, Huang D, et al: Baicalein
mediates inhibition of migration and invasiveness of skin carcinoma
through Ezrin in A431 cells. BMC Cancer. 11:5272011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang S, New L, Pan Z, Han J and Nemerow
GR: Urokinase plasminogen activator / urokinase-specific surface
receptor expression and matrix invasion by breast cancer cells
requires constitutive p38alpha mitogen-activated protein kinase
activity. J Biol Chem. 275:12266–12272. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gunther S, Ruhe C, Derikito MG, Böse G,
Sauer H and Wartenberg M: Polyphenols prevent cell shedding from
mouse mammary cancer spheroids and inhibit cancer cell invasion in
confrontation cultures derived from embryonic stem cells. Cancer
Lett. 250:25–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han HJ, Russo J, Kohwi Y and
Kohwi-Shigematsu T: Satb1 reprogrammes gene expression to promote
breast tumour growth and metastasis. Nature. 452:187–193. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dickinson LA, Joh T, Kohwi Y and
Kohwi-Shigematsu T: A tissue-specific MAR/SAR DNA-binding protein
with unusual binding site recognition. Cell. 70:631–645. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Zhang B, Zhang X, et al: Satb1
expression is associated with biologic behavior in colorectal
carcinoma in vitro and in vivo. PLoS One. 8:e479022013. View Article : Google Scholar : PubMed/NCBI
|