Introduction
The secretion of growth hormone (GH) is regulated by
a number of hypothalamic, pituitary and circulating factors, and
the classic hypothalamo-GH axis includes the GH secretagogues
(GHSs) and GH-releasing hormone (GHRH), as well as pituitary GH. It
is well established that both GHSs and GHRH can stimulate GH
secretion from the anterior pituitary through different mechanisms
(1). GHRH, which is synthesized by
neurons in the arcuate nucleus and secreted into the portal
vessels, binds to GHRH receptors (GHRH-Rs) in the anterior
pituitary, whilst GHS binds to GHS receptors (GHSRs) in the
hypothalamus and pituitary. These two receptors belong to the
G-protein-coupled receptor family (2). Human GHSR contains two subtypes, type
1a (GHSR-1a) and type 1b (GHSR-1b), with the latter being 77 amino
acids shorter than the former. Generally, GHRS-1a binds and
responds to GHS, while GHRS-1b is biologically inactive (3).
Ghrelin, a 28-amino acid peptide and an endogenous
ligand of GHSR, was originally isolated from the rat stomach
(4) and characterized, but both
ghrelin and GHSR are expressed in a number of other tissues,
including the hypothalamus, where the highest concentration of GHSR
has been described (5). GHRH acts to
induce the proliferation of somatotroph cells, as well as the
synthesis and secretion of GH. GHRH gene expression has been found
in normal pituitary and GH-secreting pituitary adenomas; since
concurrent GHRH-R expression can also be found in these adenomas,
it has been suggested that GHRH may be involved in the neoplastic
progression of GH-releasing adenoma cells (6). Ghrelin, by comparison, is believed to
induce GH secretion by binding to GHSR, and its expression, along
with that of GHSR, can be found in the normal pituitary as well as
in various types of pituitary adenomas (7). These results have raised the
possibility that ghrelin and GHSR are involved in the neoplastic
progression of pituitary adenomas.
Previous studies investigating ghrelin and GHSR
expression in pituitary adenomas have produced conflicting results
(3,6,8), and
data showing the correlation between ghrelin and GHSR expression
levels and the clinical features of pituitary adenomas are limited.
The aim of the present study, therefore, was to examine the mRNA
and protein expression levels of ghrelin and GHSR in a full
spectrum of human pituitary adenoma subtypes, as well as normal
pituitary tissue, and to analyze the correlation between the
expression and clinical characteristics in detail.
Materials and methods
Human pituitary tissues and
adenomas
Ethical approval was obtained from the Ethics
Committee of Wuhan Central Hospital (Wuhan, China) in accordance
with the Declaration of Helsinki, and written informed consent was
obtained from each individual patient. A total of 34 patients with
pituitary adenomas and a mean age of 39.9±11.1 years (19 women and
15 men; age range, 17–79 years) were enrolled in this study.
Pituitary adenoma tissues were obtained at the time of
transsphenoidal surgery. Normal human pituitary tissues (n=3) were
also collected at autopsy (4-24-h postmortem) from patients with no
evidence of endocrine abnormality. The tumor type was determined
according to clinical and biochemical findings and immunochemical
data prior to surgery. Specimens included 12 GH, 7 prolactin (PRL)
hormone, 4 adrenocorticotropin (ACTH), 3 thyroid-stimulating
hormone (TSH) and 8 non-functioning (NF) adenomas. Tumor
invasiveness was defined on the basis of preoperative radiological
investigation using the Knosp classification (9) and was confirmed during surgery. Basic
serum GH levels were also examined without any drug treatment prior
to surgery. Patient data regarding gender, age, basic serum GH
level and tumor type, diameter and invasiveness are summarized in
Table I.
 | Table I.Summary of the clinical and
histological characteristics of the patients and adenomas. |
Table I.
Summary of the clinical and
histological characteristics of the patients and adenomas.
| Case no. | Gender | Age (years) | Basic serum GH level
(µg/l) | Tumor diameter
(mm) | Immunohistochemical
staining | Invasiveness
(+/−) |
|---|
| GH adenomas |
|
| 1 | Male | 50 | 15.72 | 17 | GH (2+) PRL (1+) | − |
| 2 | Male | 29 | 101.44 | 14 | GH (3+) PRL (1+) | + |
| 3 | Female | 41 | 145.53 | 26 | GH (3+) PRL (2+) | + |
| 4 | Female | 37 | 12.39 | 12 | GH (1+) | + |
| 5 | Male | 17 | 141.78 | 17 | GH (3+) PRL (1+) | − |
| 6 | Female | 31 | 64.32 | 44 | GH (2+) PRL (2+) | + |
| 7 | Male | 40 | 57.43 | 25 | GH (2+) PRL (1+) | − |
| 8 | Male | 33 | 241.75 | 28 | GH (3+) PRL (2+) | + |
| 9 | Female | 20 | 201.76 | 18 | GH (2+) PRL (2+) | + |
| 10 | Female | 41 | 34.31 | 16 | GH (2+) PRL (1+) | − |
| 11 | Male | 30 | 110.91 | 23 | GH (2+) PRL (2+) | + |
| 12 | Male | 61 | 89.34 | 19 | GH (1+) | + |
| PRL adenomas |
|
| 13 | Male | 50 | 5.12 | 12 | PRL (2+) | − |
| 14 | Female | 30 | 1.78 | 21 | PRL (2+) | + |
| 15 | Female | 25 | 0.86 | 12 | PRL (1+) | − |
| 16 | Female | 47 | 1.52 | 38 | PRL (3+) | + |
| 17 | Female | 31 | 0.78 | 11 | PRL (1+) | − |
| 18 | Male | 48 | 2.42 | 21 | PRL (2+) | + |
| 19 | Female | 36 | 1.81 | 23 | PRL (3+) | + |
| ACTH adenomas |
|
| 20 | Female | 37 | 0.45 | 9 | ACTH (2+) | − |
| 21 | Male | 34 | 0.63 | 18 | ACTH (3+) | + |
| 22 | Female | 45 | 1.54 | 15 | ACTH (2+) | − |
| 23 | Female | 17 | 1.62 | 12 | ACTH (2+) | − |
| TSH adenomas |
|
| 24 | Male | 44 | 56.60 | 14 | TSH (2+) | + |
| 25 | Female | 39 | 8.47 | 38 | TSH (3+) | + |
| 26 | Female | 52 | 0.95 | 17 | TSH (2+) | − |
| NF adenomas |
|
| 27 | Female | 56 | 6.32 | 26 |
| + |
| 28 | Female | 53 | 1.54 | 33 |
| − |
| 29 | Male | 26 | 1.69 | 41 |
| − |
| 30 | Male | 41 | 2.13 | 22 |
| + |
| 31 | Female | 53 | 1.27 | 25 |
| − |
| 32 | Female | 50 | 1.61 | 18 |
| − |
| 33 | Male | 79 | 2.83 | 23 |
| + |
| 34 | Male | 35 | 0.81 | 28 |
| + |
RNA preparation and competitive
reverse transcription-polymerase chain reaction (RT-PCR)
analysis
Total RNA was extracted from tumor tissue using an
SV total RNA isolation kit (Promega Corp., Southampton, UK) with a
deoxyribonuclease treatment step. The quantification of the RNAs
was performed by Cecil CE5501 computing double-beam ultraviolet
spectrophotometry (Cecil Instruments Ltd., Cambridge, UK). cDNA was
generated from each sample using Moloney murine leukemia virus
reverse transcriptase (M-MLVRT) (Life Technologies, Paisley, UK).
Each reaction mixture contained 10 µl 5X first strand buffer, 2 µl
0.1 M dithiothreitol (DTT) (buffer and DTT supplied with M-MLVRT),
2.5 µl 20 µM deoxynucleotide triphosphate (Promega Corp.), 0.25 µl
20 mg/ml random hexamers (Boehriger Mannheim GmbH, Mannheim,
Germany), 1 µl 200 U/ml M-MLVRT, 0.1 µl rRNasin® (Promega Corp.),
RNA stock solution containing 5 µg RNA (heated to 65°C for 10 min)
and water to a final volume of 50 µl. The program for the thermal
cycler (Hybaid OmniGene, Teddington, UK) was 25°C for 10 min, 37°C
for 60 min and 92°C for 10 min. The integrity of the mRNA from each
specimen was verified by RT-PCR for the reference gene β-actin.
RT-PCR primers and probes were designed for human ghrelin, GHSR and
β-actin using GeneFisher® l.3 software (GeneFisher PCR Subtraction
System; Takara Shuzo Co., Ltd., Kyoto, Japan) based on the sequence
data of the genes available in GenBank (http://www.ncbi.nlm.nih.gov/genbank/) (Table II). The primers and probes were
purchased from Takara Shuzo Co., Ltd. For PCR, 5 µl cDNA, 2 µl 10
mM human ghrelin primers, 0.6 µl 10 mM β-actin primers, 25 µl 2X
Fast PCR Master Mix (Takara Shuzo Co., Ltd.) and water were used in
a 50-µl reaction volume. Thirty cycles were performed at 94°C for 1
min, 55°C for 45 sec and 72°C for 45 sec, following a denaturing
cycle of 95°C for 5 min. A final extension cycle of 5 min at 72°C
was then conducted. All amplifications were run on ethidium
bromide-stained 2% agarose gels and the results were documented
using a UV PCR cabinet and workstation (UVP Inc., Upland, CA,
USA).
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| Gene (GenBank
accession no.) | Primer sequence
(5′-3′) | Product size
(bp) |
|---|
| Ghrelin
(BO35700) | Sense:
GAGCCCTGAACACCAGAGAG | 237 |
|
| Antisense:
TCCCAGAGGATGTCCTGAAG |
|
| GHSR (U60179) | Sense:
GATCTGCTCATCTTCCTCTG | 105 |
|
| Antisense:
ACTGACGAATTGGAAGAGTT |
|
| β-actin
(BF000319) | Sense:
CCCAGAGCAAGAGAGGCATC | 247 |
|
| Antisense:
AGCACAGCCTGGATAGCAAC |
|
Protein extraction and western
blotting
Human pituitary and adenoma tissue samples (0.05
g/sample) were stored at −80°C and homogenized in 1 ml TRIzol®
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA), and the
total protein was isolated in accordance with the manufacturer's
instructions. Protein concentration assessment was performed using
a bicinchoninic acid assay (Beyotime Institute of Biotechnology,
Beijing, China). Equal quantities of sample extract were separated
using 10% SDS-PAGE, prior to the transferal of the proteins onto
nitrocellulose membranes. The membranes were blocked in 5% non-fat,
dried milk in 50 mM Tris (pH 7.5), 0.15 M NaCl and 0.05% Tween-20
at room temperature for 1 h and then incubated with primary
antibodies at 37°C for 2 h or 4°C overnight, depending on the
manufacturers' instructions. The primary antibodies were as
follows: goat polyclonal for ghrelin (1:1,000; cat# sc-10368; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), goat polyclonal for
GHSR (1:1,000; cat# sc-10362; Santa Cruz Biotechnology, Inc.) and
goat polyclonal for β-tubulin (1:1,000; cat# sc-31779; Santa Cruz
Biotechnology, Inc.). Following incubation with the primary
antibodies, the blots were washed and incubated with a donkey
anti-goat IgG horseradish peroxidase-conjugated secondary antibody
(1:1,000; cat# sc-2020; Santa Cruz Biotechnology, Inc.) at 37°C for
1 h. The peroxidase activity on the blots was determined using
SuperSignal chemiluminescent substrate (Pierce Co., Rockford, IL,
USA).
Statistical analysis
Data are expressed as the mean ± standard deviation
and were analyzed using the SPSS 10.0 software package (SPSS, Inc.,
Chicago, IL, USA). Analyses of variance with a protected t-test
were used for intergroup comparisons, and correlations were
analyzed using linear regression analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
Quantification of ghrelin and GHSR
mRNA expression in human pituitary tissues and adenomas
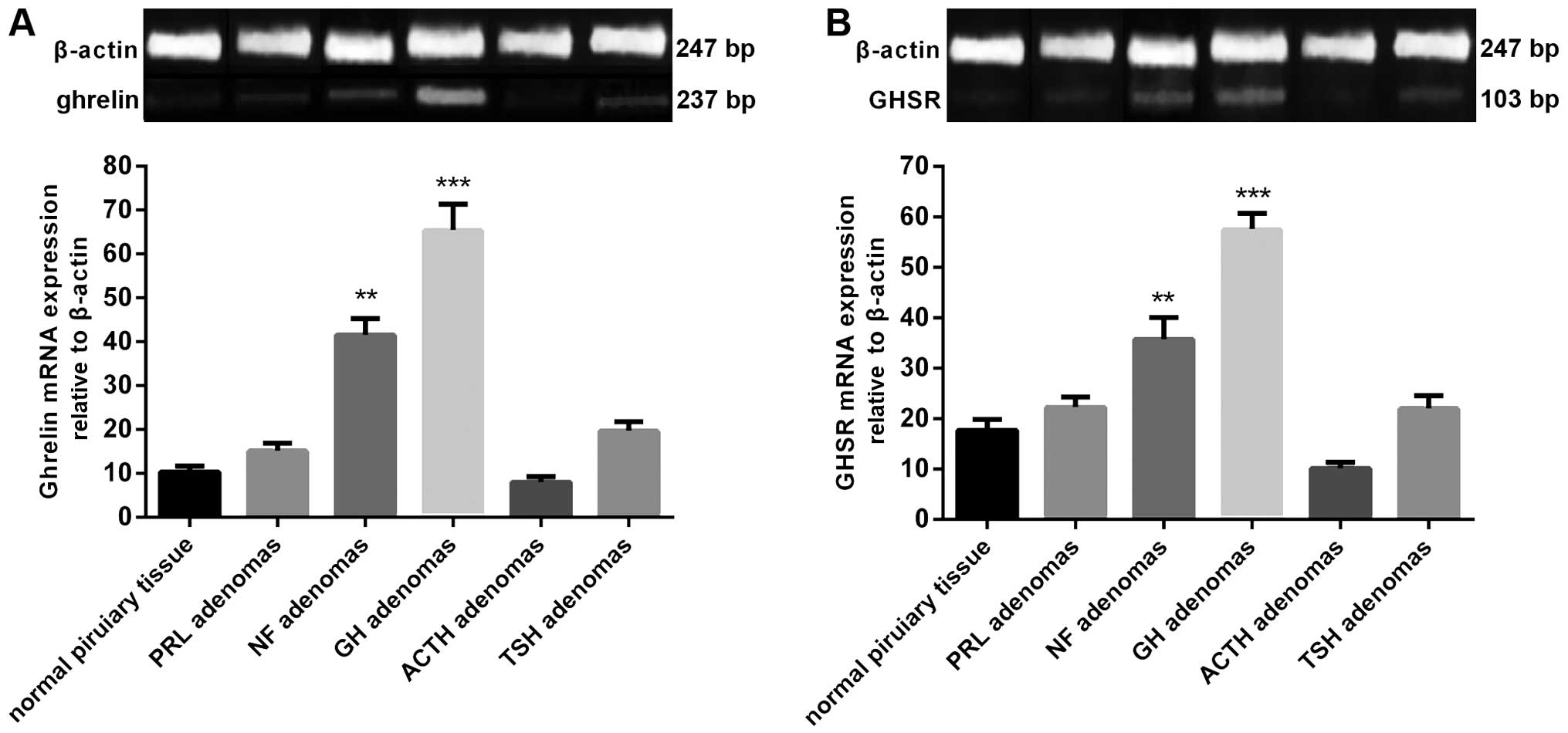
Ghrelin and GHSR mRNA expression was detected in all
human pituitary and adenomas samples. The highest mean levels of
the two mRNAs were found in the GH adenomas, while a moderate level
was found in NF adenomas. ACTH adenomas exhibited the lowest level
of ghrelin and GHSR mRNA expression. The mRNA expression levels in
the GH and NF adenomas were significantly higher than those in the
normal human pituitary (Fig. 1).
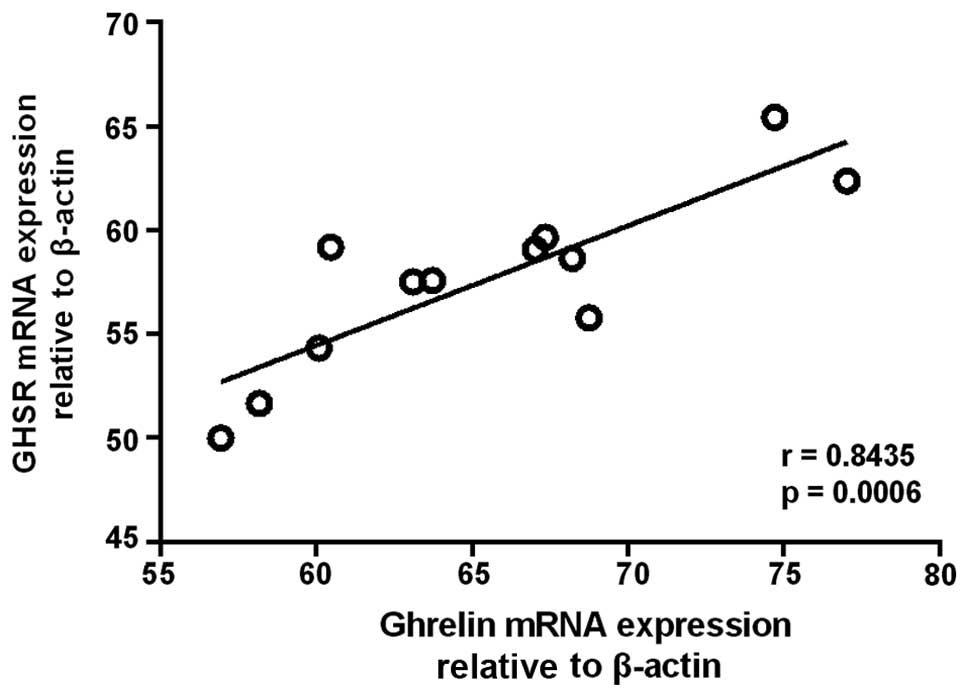
Furthermore, a significant correlation was observed between the
expression levels of ghrelin and GHSR mRNA in GH adenomas (n=12)
(r=0.8435, P=0.0006) (Fig. 2).
Quantification of ghrelin and GHSR
protein expression in human pituitary tissues and adenomas
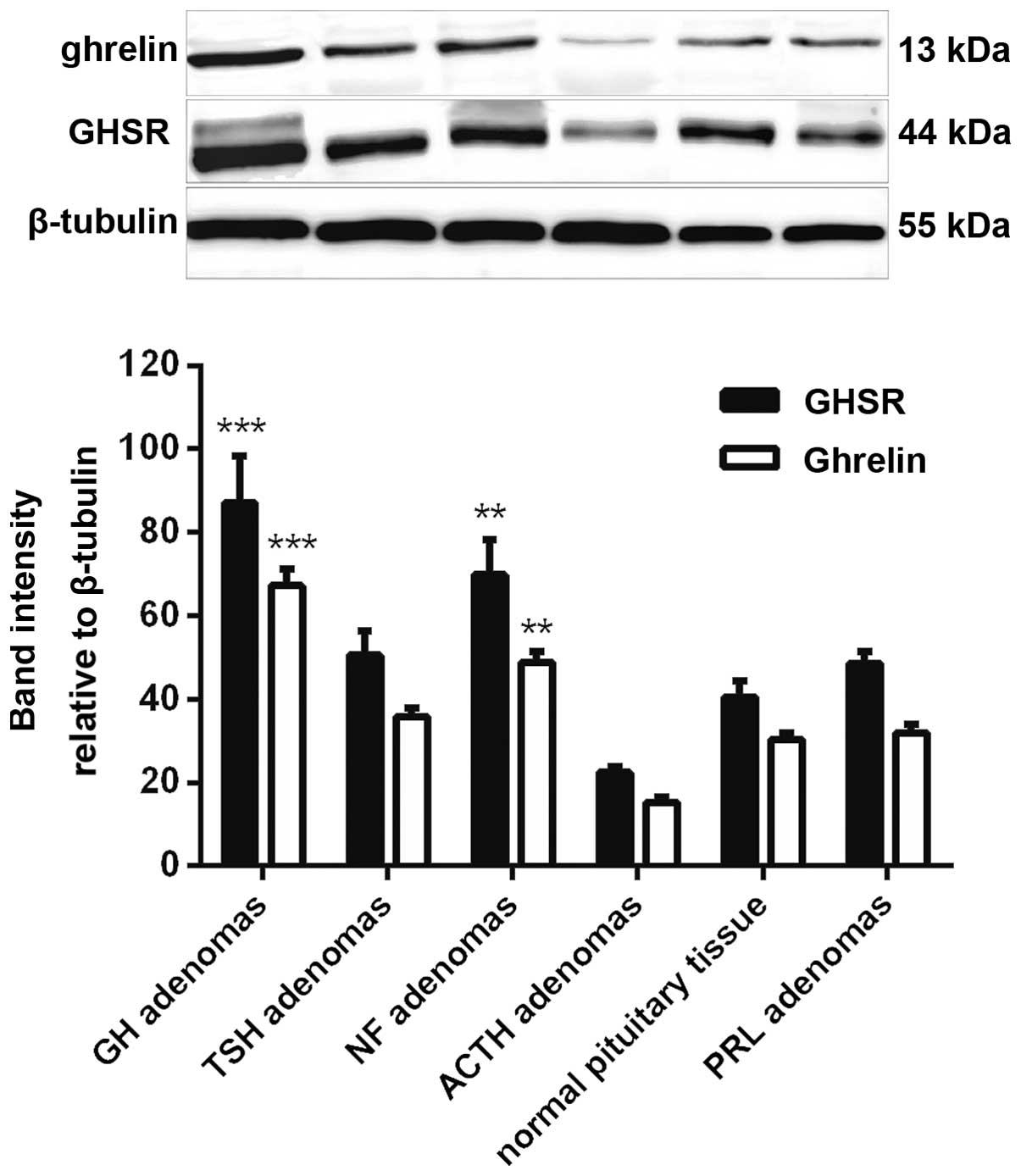
The detection of ghrelin and GHSR protein expression
was performed by western blotting, and the band intensity was
calculated using β-tubulin as a reference. The results showed that
ghrelin and GHSR protein expression correlated with the mRNA
expression. The strongest bands were from the GH adenomas, moderate
bands were from the NF adenomas and the weakest bands were from the
ACTH adenomas. The protein expression levels in the GH and NF
adenomas were also significantly higher than those in the normal
human pituitary (Fig. 3).
Comparison of the ghrelin mRNA
expression levels with clinical features in GH adenomas
The present study demonstrated i) specific
overexpression of ghrelin and GHSR in GH adenomas, and ii) a
significant correlation between ghrelin and GHSR mRNA expression
levels. The next part of the experiment therefore focused on GH
adenomas and compared the ghrelin mRNA expression levels with
various clinical features, including tumor diameter, tumor
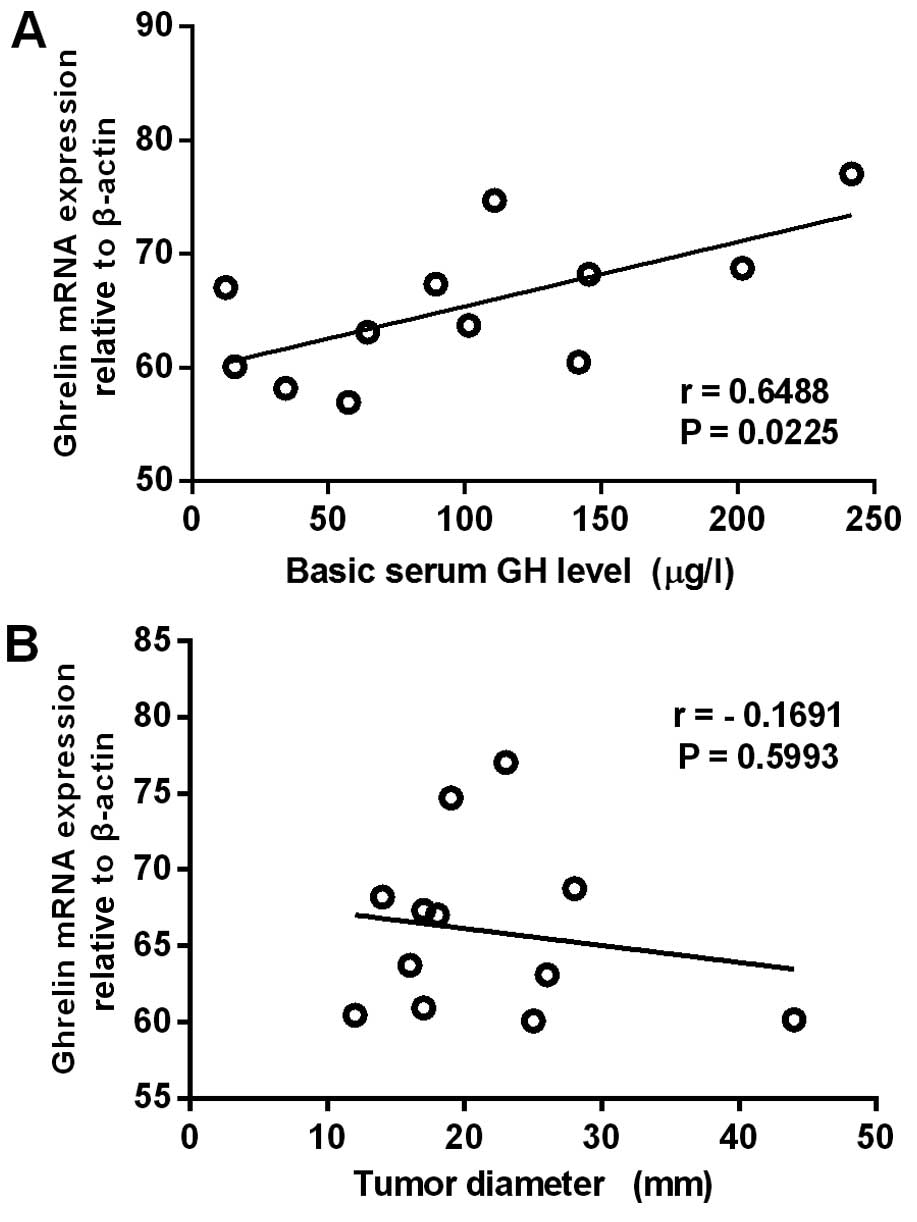
invasiveness and basic serum GH level. It was found that ghrelin
mRNA expression levels in the GH adenomas correlated positively
with basic serum GH levels (n=12) (r=0.6488, P=0.0225), but
negatively with tumor diameter (n=12) (r=-0.1691, P=0.5993)
(Fig. 4). In invasive GH adenomas,
the ghrelin mRNA expression levels were significantly higher than
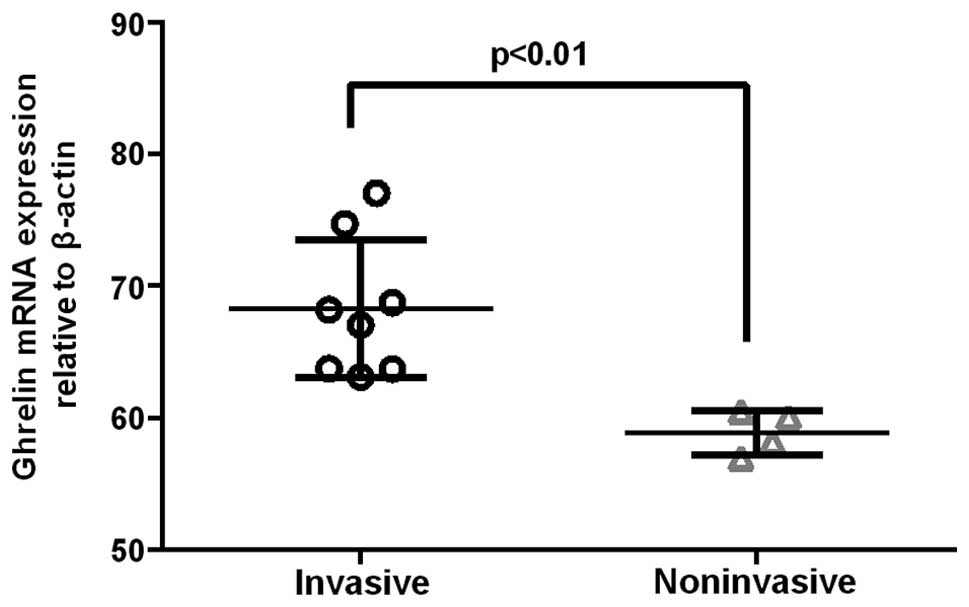
those in noninvasive GH adenomas (P<0.01) (Fig. 5).
Discussion
Ghrelin is a 28-amino acid peptide that was
originally isolated from the stomach (4) but has also been found in other tissues,
including the hypothalamic arcuate nucleus (10). As the natural ligand of GHSR, ghrelin
specifically activates the GHSR and stimulates GH release in
vitro while having no direct effect on other hormone subtypes,
such as ACTH, PRL and TSH (11,12).
Ghrelin has also been demonstrated to stimulate GH release in
anesthetized rats (13). An
n-octanoyl group on the third amino acid of ghrelin is
required for its biological activity, and the protein sequence has
been shown to be highly conserved between species, with rat and
human ghrelins only differing by two amino acids. Previous in
vitro and in vivo studies using synthetic GH
secretagogues (GHSs) have suggested that the effects of these GHSs
are mediated by the hypothalamus (14–16).
GHSs have also been shown to stimulate GH release directly from the
isolated rat or human pituitary. Previous studies using RT-PCR,
immunohistochemistry and double immunogold electron microscopy have
demonstrated the expression of ghrelin and GHSR in various
pituitary adenoma subtypes (3,6,7,8,17,18);
however, the results have been divergent. Kim et al
(3,8)
found that ghrelin mRNA was expressed in various pituitary adenoma
subtypes, the highest levels of expression being noted in NF
adenomas and the lowest in prolactinomas. Skinner et al
(6) observed the highest GHSR mRNA
expression in GH adenomas and the lowest in ACTH adenomas. No
correlation was found, however, between ghrelin or GHSR expression
levels and circulating GH levels in any studies (7,17,18).
In the present study, the ghrelin and GHSR
expression was assessed at the mRNA and protein levels using RT-PCR
and western blotting, respectively. The results showed that ghrelin
and GHSR mRNA were expressed in various pituitary adenomas and in
the normal pituitary, but that the expression levels varied among
different types of adenomas, with the highest level in GH adenomas,
a moderate level in NF adenomas and the lowest level in ACTH
adenomas. A significant correlation was found between the
expression levels of ghrelin and GHSR mRNA in GH adenomas. The same
pattern was observed in the protein expression levels using western
blotting. One notable finding was that the ghrelin mRNA expression
levels in the GH adenomas correlated positively with basic serum GH
level. These results are in accordance with the current hypothesis
that ghrelin could have a direct autocrine or paracrine effect via
GHSR, thereby promoting pituitary GH hormone synthesis and/or
release.
In addition to stimulating hormone secretion,
ghrelin, through its paracrine function, is believed to play an
important role in processes associated with tumor progression;
however, the molecular mechanisms are poorly understood (19,20).
Previous studies have investigated the effect of ghrelin on breast,
ovarian and gastric cancer cell lines, although contradictory
results were generated (21–23). An anti-proliferation effect has been
documented in certain studies (24,25),
whereas others have described a potential tumor-promoting role of
ghrelin (26,27). It should be taken into consideration,
however, that the use of cell lines and administered dose of
ghrelin in these studies may not be representative of physiological
conditions, underlying the necessity for further in vivo
studies to be conducted.
In the present study, it was confirmed that ghrelin
expression was associated with GH adenoma invasiveness, which is in
line with the negative correlation with tumor diameter. This
finding may suggest that ghrelin contributes to the development of
GH adenomas mainly by promoting adenoma cell invasion, but not
proliferation.
In conclusion, the present study found that ghrelin
and GHSR expression, at both the mRNA and protein levels, was
correlated with certain clinical features. The results showed that
the expression levels varied among the different adenoma subtypes,
with the highest mean level in GH adenomas, a moderate level in NF
adenomas and the lowest level in ACTH adenomas. The ghrelin mRNA
expression level in GH adenomas correlated positively with the
basic serum GH level, but negatively with tumor diameter.
Furthermore, the ghrelin mRNA expression levels in invasive GH
adenomas were significantly higher than those in noninvasive GH
adenomas. The present findings suggest that the binding of ghrelin
to GHSR promotes GH hormone secretion and has an important role in
the development of GH adenomas via an autocrine and/or paracrine
action.
Acknowledgements
The current study was partially supported by the
National Natural Science Foundation of China (grant no. 81101620)
and the Clinical Project of the Wuhan Health Bureau (grant no.
WX14B08). The authors would like to thank Miss Shunying Liu for her
support in this study.
References
|
1
|
Chopin L, Walpole C, Seim I, et al:
Ghrelin and cancer. Mol Cell Endocrinol. 340:65–69. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Honda K, Bailey AR, Bull PM, Macdonald LP,
Dickson SL and Leng G: An electrophysiological and morphological
investigation of the projections of growth hormone-releasing
peptide-6-responsive neurons in the rat arcuate nucleus to the
median eminence and to the paraventricular nucleus. Neuroscience.
90:875–883. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim K, Arai K, Sanno N, Osamura RY,
Teramoto A and Shibasaki T: Ghrelin and growth hormone (GH)
secretagogue receptor (GHSR) mRNA expression in human pituitary
adenomas. Clin Endocrinol. 54:759–768. 2001. View Article : Google Scholar
|
|
4
|
Howard AD, Feighner SD, Cully DF, et al: A
receptor in pituitary and hypothalamus that functions in growth
hormone release. Science. 273:974–977. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chu S and Schubert ML: Gastric secretion.
Curr Opin Gastroenterol. 29:636–641. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Skinner MM, Nass R, Lopes B, Laws ER and
Thorner MO: Growth hormone secretagogue receptor expression in
human pituitary tumors. J Clin Endocrinol Metab. 83:4314–4320.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Korbonits M, Bustin SA, Kojima M, et al:
The expression of the growth hormone secretagogue receptor ligand
ghrelin in normal and abnormal human pituitary and other
neuroendocrine tumors. J Clin Endocrinol Metab. 86:881–887. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim K, Sanno N, Arai K, et al: Ghrelin
mRNA and GH secretagogue receptor mRNA in human GH-producing
pituitary adenomas is affected by mutations in the alpha subunit of
G protein. Clin Endocrinol (Oxf). 59:630–636. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Knosp E, Steiner E, Kitz K and Matula C:
Pituitary adenomas with invasion of the cavernous sinus space: a
magnetic resonance imaging classification compared with surgical
findings. Neurosurgery. 33:610–617. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Korbonits M, Kojima M, Kangawa K and
Grossman AB: Presence of ghrelin in normal and adenomatous human
pituitary. Endocrine. 14:101–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stevanović D, Milosević V, Starcević VP
and Severs WB: The effect of centrally administered ghrelin on
pituitary ACTH cells and circulating ACTH and corticosterone in
rats. Life Sci. 80:867–872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giraldi Pecori F, Bucciarelli LG, Saccani
A, et al: Ghrelin stimulates adrenocorticotrophic hormone (ACTH)
secretion by human ACTH-secreting pituitary adenomas in vitro. J
Neuroendocrinology. 19:208–212. 2007. View Article : Google Scholar
|
|
13
|
Rau TT, Sonst A, Rogler A, et al: Gastrin
mediated down regulation of ghrelin and its pathophysiological role
in atrophic gastritis. J Physiol Pharmacol. 64:719–725.
2013.PubMed/NCBI
|
|
14
|
Bowers CY, Momany FA, Reynolds GA and Hong
A: On the in vitro and in vivo activity of a new synthetic
hexapeptide that acts on the pituitary to specifically release
growth hormone. Endocrinology. 114:1537–1545. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Korbonits M and Grossman AB: Growth
hormone-releasing peptide and its analogues Novel stimuli to growth
hormone release. Trends Endocrinol Metab. 6:43–49. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith RG, Van der Ploeg LH, Howard AD, et
al: Peptidomimetic regulation of growth hormone secretion. Endocr
Rev. 18:621–645. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rotondo F, Cusimano M, Scheithauer BW,
Rotondo A, Syro LV and Kovacs K: Ghrelin immunoexpression in
pituitary adenomas. Pituitary. 14:318–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rak-Mardyla A: Ghrelin role in
hypothalamus-pituitary-ovarian axis. J Physiol Pharmacol.
64:695–704. 2013.PubMed/NCBI
|
|
19
|
Nanzer AM, Khalaf S, Mozid AM, et al:
Ghrelin exerts a proliferative effect on a rat pituitary
somatotroph cell line via the mitogen-activated protein kinase
pathway. Eur J Endocrinol. 151:233–240. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Avau B, De Smet B, Thijs T, et al: Ghrelin
is involved in the paracrine communication between neurons and
glial cells. Neurogastroenterol Motil. 25:e599–608. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu H, Xu G and Fan X: The effect of
ghrelin on cell proliferation in small intestinal IEC-6 cells.
Biomed Pharmacother. 67:235–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tian C, Zhang L, Hu D and Ji J: Ghrelin
induces gastric cancer cell proliferation, migration, and invasion
through GHS-R/NF-κB signaling pathway. Mol Cell Biochem.
382:163–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tian C, Ye F, Wang L, et al: Nitric oxide
inhibits ghrelin-induced cell proliferation and ERK1/2 activation
in GH3 cells. Endocrine. 38:412–416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cassoni P, Papotti M, Ghè C, et al:
Identification, characterization, and biological activity of
specific receptors for natural (ghrelin) and synthetic growth
hormone secretagogues and analogs in human breast carcinomas and
cell lines. J Clin Endocrinol Metab. 86:1738–1745. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu Y, Pang X, Dong M, Wen F and Zhang Y:
Ghrelin inhibits ovarian epithelial carcinoma cell proliferation in
vitro. Oncol Rep. 30:2063–2070. 2013.PubMed/NCBI
|
|
26
|
Jeffery PL, Murray RE, Yeh AH, et al:
Expression and function of the ghrelin axis, including a novel
preproghrelin isoform, in human breast cancer tissues and cell
lines. Endocr Relat Cancer. 12:839–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grönberg M, Fjällskog ML, Jirström K and
Janson ET: Expression of ghrelin is correlated to a favorable
outcome in invasive breast cancer. Acta Oncol. 51:386–393. 2012.
View Article : Google Scholar : PubMed/NCBI
|