Introduction
Plantar fasciitis is the most common cause of heel
pain (1). It has been estimated to
affect overweight individuals who spend long periods standing
(1) and ∼10% of runners (2). The patients feel initial pain on the
first step out of bed in the morning. The pain is relieved with
gradually increased activity and worsens during long periods of
standing (3). There are various
treatments for plantar fasciitis, including physical therapy,
orthotic devices, splinting and walking casts (4). Corticosteroid injection is usually one
of the first-line treatments for plantar fasciitis. Owing to its
anti-inflammatory effect, this treatment may relieve both acute and
chronic pain of the heel (4);
however, histological examination has shown that plantar fasciitis
is not an inflammatory response but a degenerative process with
microtears of the fascia and collagen necrosis, and a number of
investigators have recommended that plantar fasciitis should be
more exactly termed plantar fibrosis (2,5). As a
result, the efficacy of corticosteroid injection has been brought
into question. Corticosteroid injection is believed to provide only
short-term pain relief or even no benefit (3,4).
Furthermore, the patient may suffer from an increased risk of
plantar fascia rupture following the treatment (6).
At present, several published randomized controlled
trials (RCTs) (7–10) have compared the efficacy of
corticosteroid injection with that of placebo injection for the
management of plantar fasciitis; however, the efficacy of
corticosteroid injection remains controversial. The present
meta-analysis was performed to determine the efficacy of
corticosteroid versus placebo injection for the treatment of
plantar fasciitis.
Materials and methods
Search strategy
Databases, including Medline, the Cochrane Library,
Google Scholar and Embase, were systematically searched for RCTs
comparing the efficacy of corticosteroid and placebo injection for
plantar fasciitis. The time limitation was set from the
establishment of the databases to January 1, 2014. The free terms
‘plantar fasciitis’, ‘plantar fibrosis’, ‘heel pain’, ‘painful
heel’, ‘corticosteroid’ and ‘steroid’ and corresponding Medical
Subject Headings in different combinations were used for the
literature search. The citations and reference lists of relevant
articles were checked in a series for additional studies.
Inclusion criteria
The full texts of potentially relevant studies were
retrieved and reviewed. Studies that met the inclusion criteria
were eligible. The inclusion criteria were developed based on the
PICOS framework (patient, intervention, comparison, outcome and
study design) as follows: i) P, patients who felt pain in the heel
and had a point of tenderness over the calcaneal medial tubercle
and who were definitely diagnosed with plantar fasciitis; ii) I and
C, corticosteroid injection and placebo injection were compared;
iii) O, outcomes, including visual analogue score (VAS) and plantar
fascial thickness (PFT), were described; iv) S, studies of an RCT
design were included. Eligible studies were accepted for inclusion
without language restriction.
Data extraction
Two reviewers independently extracted the
demographic characteristics and outcomes. The demographic
characteristics included the first author, year of publication,
location, sample size, average age, male/female ratio,
intervention, comparison, study design and follow-up duration.
Quality assessment
The risk of bias tool (11) was used to assess the methodological
quality. All included studies were assessed in seven aspects:
Random sequence generation, allocation concealment, blinding of
patients, blinding of therapists, incomplete outcome data,
selective reporting and other bias.
Evidence grading
The Grading of Recommendations Assessment,
Development and Evaluation (GRADE) system (12) was used to grade the evidence quality
for all outcomes. Outcomes based on RCTs were of high quality. Five
factors (risk of bias, inconsistency, indirectness, imprecision and
publication bias) had the potential to downgrade the evidence
level. Finally, four evidence levels (high, moderate, low and very
low) were determined.
Statistical analysis
The outcomes were VAS and PFT. The relative risk
with 95% confidence interval (CI) was calculated for dichotomous
data, while the standardized mean difference (SMD) or MD with 95%
CI was adopted to analyze continuous variables. The statistical
heterogeneity across trials was estimated using the I2
value. A fixed-effects model was adopted when heterogeneity could
be ignored (I2<50%); otherwise the randomized-effects
model was used. Statistical analysis was performed with RevMan
software, version 5.2 (The Nordic Cochrane Center, The Cochrane
Collaboration, Copenhagen, Denmark), and P<0.05 was considered
to be statistically significant.
Results
Characteristics of the included
studies
The flow diagram of the literature search is shown
in Fig. 1. Four RCTs (7–10) with a
total of 289 patients were ultimately considered to be eligible
according to the inclusion criteria. The characteristics of the
included studies are presented in Table
I. All of the included studies compared corticosteroid
injection and placebo injection in the treatment of plantar
fasciitis. Two studies were performed in UK (7,10), one
in Australia (8) and one in Kenya
(9). The patients in the included
studies were middle-aged and elderly, with an average age of
between 43.1 and 58.2 years. The follow up duration was between two
and six months.
 | Table I.General characteristics of the
included studies. |
Table I.
General characteristics of the
included studies.
| First author, year
(ref.) | Location | Sample size (n) | Average age
(years) | Gender (M/F) | BMI in
kg/m2 (C/P) | Intervention | Comparison | Study design | Outcomes | Follow-up |
|---|
| Crawford, 1999
(7) | UK | 54 | 58.2 | - | - | 1 ml 2% lignocaine
with 25 mg prednisolone acetate | 2 ml 1% lignocaine
hydrochloride | RCT | VAS | 6 months |
| McMillan, 2012
(8) | Australia | 82 | 52.7 | 43/39 | 31.4/30.9 | 1 ml 4 mg/ml
dexamethasone sodium phosphate | 1 ml normal
saline | RCT | VAS, PFT | 12 weeks |
| Abdihakin, 2012
(9) | Kenya | 88 | 43.1 | 42/46 | 31.7 | Methylprednisolone
acetate 40 mg plus conservative treatment | Saline and lidocaine
plus conservative treatment | RCT | VAS | 2 months |
| Ball, 2013 (10) | UK | 65 | 49.4 | 29/36 | 31.3/32.4 | 0.5 ml (20 mg)
methylprednisolone acetate and 0.5 ml saline | 1 ml 0.9% saline | RCT | VAS, PFT | 12 weeks |
Quality assessment
The results of the quality assessment are shown in
Table II. Randomization was
mentioned in all the studies but one study did not report the
randomized sequence generation. Allocation concealment was reported
in two studies. Blinding of patients was used in all studies and
blinding of therapists was used in all studies. Incomplete outcome
data and selective reporting were generally of low risk. The
remaining items were of unclear risk.
 | Table II.Risk of bias of the included
studies. |
Table II.
Risk of bias of the included
studies.
| First author, year
(ref.) | Random sequence
generation | Allocation
concealment | Blinding of
patients | Blinding of
therapists | Incomplete outcome
data | Selective
reporting | Other bias |
|---|
| Crawford, 1999
(7) | Low | Low | Low | Low | High | Low | Unclear |
| McMillan, 2012
(8) | Low | Low | Low | Low | Low | Low | Unclear |
| Abdihakin, 2012
(9) | Low | Unclear | Low | Low | Low | Low | Unclear |
| Ball, 2013 (10) | Low | Unclear | Low | Low | Low | Low | Unclear |
Outcome measurements
The follow-up duration varied among the included
studies, and the results were pooled based on different follow-up
durations. The VAS scores after one, two and three month(s) were
reported in three (7–9), two (8,9) and
three (7,8,10)
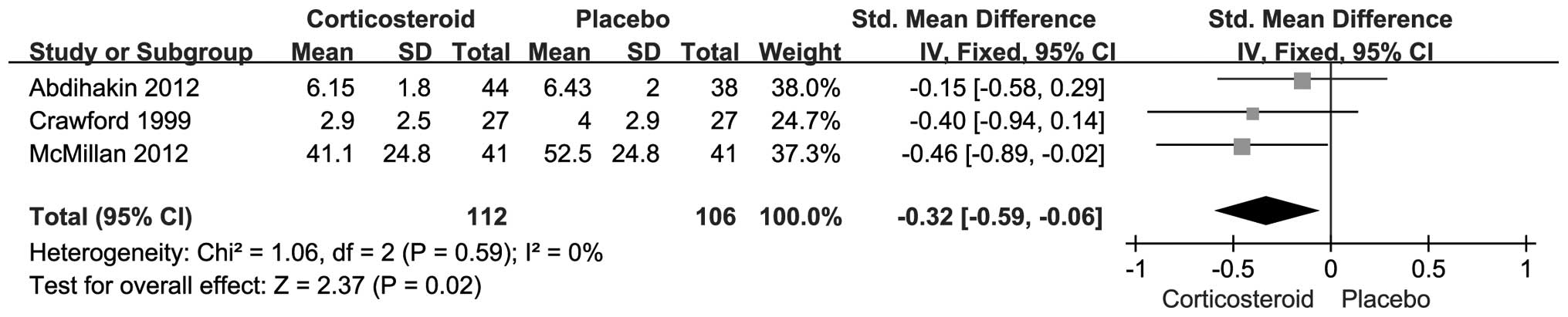
studies, respectively. The results showed that pain relief was
achieved after one month by corticosteroid injection (SMD, −0.32;
95% CI, −0.59--0.06; P=0.02) (Fig.
2); however, no difference was detected with respect to VAS
score after two months (SMD, −0.04; 95% CI, −0.35–0.27; P=0.79)
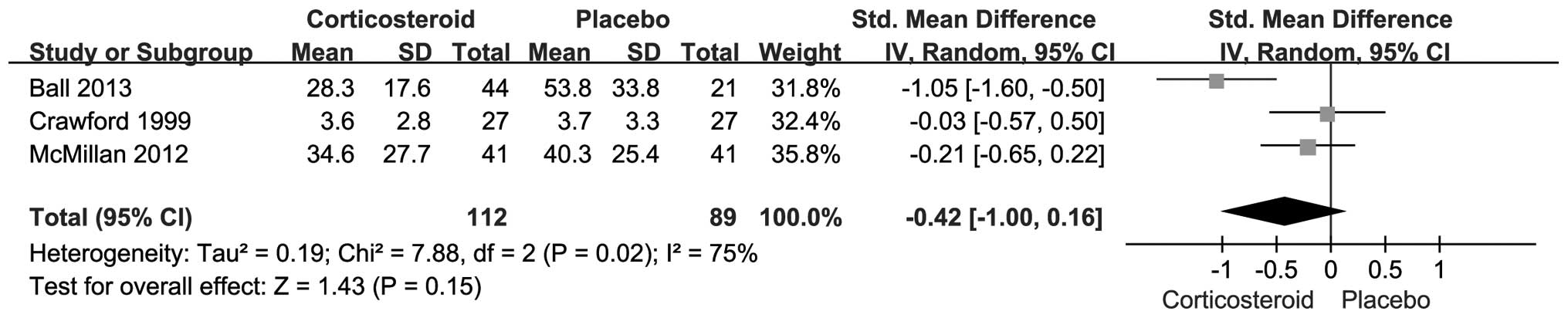
(Fig. 3) or three months (SMD,
−0.42; 95% CI, −1.00–0.16; P=0.15) (Fig.
4), although the corticosteroid injection group tended to have
a lower VAS score. The PFT was recorded in two studies (8,10) with a
total of 147 patients. The patients treated with corticosteroid
injection showed a tendency of a thinner PFT, although no
significant difference was found (MD, −0.70; 95% CI, −1.77–0.38;
P=0.20) (Fig. 5).
Evidence grading
Evidence grading according to the GRADE system is
shown in Table III. Four outcomes
in this meta-analysis were analyzed. The quality of evidence was
high for the VAS after one and two months, and moderate for the VAS
after three months and the PFT (Table
III).
 | Table III.GRADE assessment of outcomes. |
Table III.
GRADE assessment of outcomes.
| Outcome | Quality
assessment | |
|---|
|
|---|
| No. of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication
bias | Quality | Importance |
|---|
| VAS-1 | 3 | RCT | Not serious | No serious
inconsistenc | No serious
indirectness | No serious
imprecision | Undetected | High | Critical |
| VAS-2 | 2 | RCT | Not serious | No serious
inconsistency | No serious
indirectness | No serious
imprecision | Undetected | High | Critical |
| VAS-3 | 3 | RCT | Not serious |
Seriousa | No serious
indirectness | No serious
imprecision | Undetected | Moderate | Critical |
| PFT | 2 | RCT | Not serious |
Seriousa | No serious
indirectness | No serious
imprecision | Undetected | Moderate | Important |
Discussion
Local corticosteroid injection is a commonly used
treatment for patients with plantar fasciitis. At present, several
RCTs have compared corticosteroid injection with placebo injection;
however, the conclusions remain controversial. This meta-analysis
was therefore conducted to determine whether corticosteroid
injection is superior to placebo injection. The results showed that
patients benefit from corticosteroid injection in terms of the VAS
after one month. No notable improvement was observed with respect
to the VAS after two or months or the PFT.
The etiology of plantar fasciitis is not fully
understood and may be multifactorial (2,4,13). Evidence has suggested that gender,
age and weight contribute to the disease process (13). The association between plantar
fasciitis and gender is controversial. No difference was detected
with respect to the male/female ratio in this study. Despite a
study finding the incidence rates for men and women to be 9.2 and
18.0 per 1,000 person-years, respectively (13), another retrospective analysis
suggested that men are more likely to suffer plantar fasciitis than
women (14). The average age of
patients in the present study ranged between 43.1 and 58.2 years.
It has previously been demonstrated that plantar fasciitis is a
chronic degenerative process caused by repetitive microtrauma of
the plantar fascia (15). The
incidence rate of the condition increases with age (13). In the present study, the average body
mass index was mentioned in three studies (8–10) and
ranged from 30.9 to 32.4 kg/m2. The incidence of plantar
fasciitis in obese individuals has been found to be 5.6-fold higher
than that in the non-obese (16).
Other potential risk factors, including limited ankle dorsiflexion
and prolonged weight bearing, may also contribute to the disease
development (13); however, these
risk factors were not reported in the included studies.
In the present study, pain relief was gained with
corticosteroid injection after one month but not after two or three
months. Furthermore, no difference was detected with respect to the
PFT. The short-term efficacy of corticosteroid injection has been
previously confirmed, and was in accordance with the present
results; however, this modality was primarily used for shoulder and
elbow tendinitis (17). The plantar
fascia is a different structure and the results may not be exactly
the same. Considering the observation that corticosteroid injection
tended to produce more favorable results than placebo, without
significant difference, we speculated that it was due to the
illusion of small sample size. Thus, the power for each outcome was
calculated. The results showed that the power for PFT was 0.91,
while that for the remaining outcomes was <0.8, which confirmed
our speculation.
Plantar fasciitis causes heel pain and disables
activity. In the present analysis, foot function was meaningful
during the follow-up. Abdihakin et al (9) treated patients with plantar fasciitis
with steroid injection and the Foot Function Index scores were
recorded at study entry and at one and two months. The results
showed that steroid injection did not improve function compared
with the control group. Another RCT (18) compared corticosteroid and placebo
therapy for plantar fasciitis, and foot function was assessed using
the Maryland Foot Score. Compared with the placebo, corticosteroid
injection improved the function score at the end of treatment but
not at the one-month time-point.
Corticosteroid injection for the treatment of
plantar fasciitis can significantly improve the heel pain,
particularly in the short term; however, it can also cause serious
complications, including plantar fascia rupture, fat pad atrophy,
plantar fascia calcification, nerve injury, sterile abscess,
calcaneal osteomyelitis and even impaired vision (19–22). In
the present analysis, two studies (8,10)
reported that no adverse event occurred. This was not mentioned in
two other studies (7,9).
Plantar fasciitis is generally a self-limiting
disease, and the majority of patients report spontaneous heel pain
relief within one year, even without treatment (2,23);
however, ∼10% of patients seek care from the physician due to
unrelieved pain and disabled daily activities (2). Corticosteroid injection is considered
to be one of the first-line treatments and has been found to
produce satisfactory short-term results by blockading the
inflammatory response and improving local edema, swelling, pain and
foot function. Despite this, it has been revealed that inflammatory
cells are rarely present within the lesion and that plantar
fasciitis is therefore essentially a degenerative disease (5). This finding has led to the efficacy of
corticosteroid injection being questioned and to the suggestion
that there could be an increased risk of certain adverse events
with this method. The treatment should therefore not be used until
it has been confirmed to be effective.
The present meta-analysis was a secondary research
based on published studies and was certainly flawed. Firstly, only
four studies with a total of 289 patients were finally included;
thus, the power for all outcomes may be limited by the small sample
size. Secondly, the heterogeneity between the included studies
cannot be ignored. This heterogeneity may have been caused by a
number of factors, including different treatment algorithms,
clinical skills and physician experience. A physician with more
experience and skills would be considerably more likely to
administer an accurate injection with few adverse events.
Furthermore, the outcomes were mainly VAS, which is somewhat
subjective. Other objective changes included tenderness threshold,
heel tenderness index and heel pad thickness, which were not
reported in all the included studies. In addition, measurements on
safety, economy and quality of life were seldom provided. Despite
the weaknesses, the present meta-analysis did reveal that patients
with plantar fasciitis may benefit from local corticosteroid
injection with respect to the pain symptoms, at least in the short
term.
In conclusion, the present meta-analysis has found
that corticosteroid injection may provide short-term pain relief;
however, the effect may vanish with the progression of time.
Further studies are required to confirm the present findings.
References
|
1
|
Schwartz EN and Su J: Plantar fasciitis: A
concise review. Perm J. 18:e105–e107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martinelli N, Bonifacini C and Romeo G:
Current therapeutic approaches for plantar fasciitis. Orthop Res
Rev. 6:332014. View Article : Google Scholar
|
|
3
|
Buchbinder R: Clinical practice. Plantar
fasciitis. N Engl J Med. 350:2159–2166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kirkland P and Beeson P: Use of primary
corticosteroid injection in the management of plantar fasciopathy:
Is it time to challenge existing practice? J Am Podiatr Med Assoc.
103:418–429. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lemont H, Ammirati KM and Usen N: Plantar
fasciitis: A degenerative process (fasciosis) without inflammation.
J Am Podiatr Med Assoc. 93:234–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tatli YZ and Kapasi S: The real risks of
steroid injection for plantar fasciitis, with a review of
conservative therapies. Curr Rev Musculoskelet Med. 2:3–9. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crawford F, Atkins D, Young P and Edwards
J: Steroid injection for heel pain: Evidence of short-term
effectiveness. A randomized controlled trial. Rheumatology
(Oxford). 38:974–977. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McMillan AM, Landorf KB, Gilheany MF, Bird
AR, Morrow AD and Menz HB: Ultrasound guided corticosteroid
injection for plantar fasciitis: Randomised controlled trial. BMJ.
344:e32602012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abdihakin M, Wafula K, Hasan S and MacLeod
J: A randomised controlled trial of steroid injection in the
management of plantar fasciitis. SA Orthop J. 11:33–38. 2012.
|
|
10
|
Ball EM, McKeeman HM, Patterson C, et al:
Steroid injection for inferior heel pain: A randomised controlled
trial. Ann Rheum Dis. 72:996–1002. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Higgins JP, Altman DG, Gøtzsche PC, et al:
Cochrane Bias Methods Group; Cochrane Statistical Methods Group:
The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Atkins D, Best D, Briss PA, et al: GRADE
Working Group: Grading quality of evidence and strength of
recommendations. BMJ. 328:14902004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beeson P: Plantar fasciopathy: Revisiting
the risk factors. Foot Ankle Surg. 20:160–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taunton JE, Ryan MB, Clement DB, McKenzie
DC, Lloyd-Smith DR and Zumbo BD: A retrospective case-control
analysis of 2002 running injuries. Br J Sports Med. 36:95–101.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rosenbaum AJ, DiPreta JA and Misener D:
Plantar heel pain. Med Clin North Am. 98:339–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Riddle DL, Pulisic M, Pidcoe P and Johnson
RE: Risk factors for Plantar fasciitis: A matched case-control
study. J Bone Joint Surg Am. 85-A:872–877. 2003.PubMed/NCBI
|
|
17
|
Gaujoux-Viala C, Dougados M and Gossec L:
Efficacy and safety of steroid injections for shoulder and elbow
tendonitis: A meta-analysis of randomised controlled trials. Ann
Rheum Dis. 68:1843–1849. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gudeman SD, Eisele SA, Heidt RJ, Colosimo
AJ and Stroupe AL: Treatment of plantar fasciitis by iontophoresis
of 0.4% dexamethasone. A randomized, double-blind,
placebo-controlled study. Am J Sports Med. 25:312–316. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Molloy LA: Managing chronic plantar
fasciitis: when conservative strategies fail. JAAPA. 25:52–53.
2012.
|
|
20
|
Gidumal R and Evanski P: Calcaneal
osteomyelitis following steroid injection: a case report. Foot
Ankle. 6:44–46. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Buccilli Ta Jr, Hall HR and Solmen JD:
Sterile abscess formation following a corticosteroid injection for
the treatment of plantar fasciitis. J Foot Ankle Surg. 44:466–468.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anupama B, Puthran N, Hegde V and Andrews
S: Plantar fasciitis and impaired vision: A case report. Foot
(Edinb). 20:151–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goff JD and Crawford R: Diagnosis and
treatment of plantar fasciitis. Am Fam Physician. 84:676–682.
2011.PubMed/NCBI
|