Introduction
Stroke is a life-threatening disease that is
considered the most common cause of disability in adults. According
to the World Health Organization, 15 million individuals suffer
from stroke worldwide each year. Of these, 5 million succumb and a
further 5 million are permanently disabled (1). Ischemic stroke is by far the most
common type of stroke, accounting for ∼88% of all stroke cases.
Ischemic stroke results from a loss of blood supply to part of the
brain, initiating the ischemic cascade (2).
The mechanisms involved in ischemic stroke have been
suggested to involve a complex interplay of excitotoxicity,
oxidative stress, inflammation and apoptosis (3–6);
however, oxidative stress is considered to have a key role in the
pathogenesis of ischemia/reperfusion injury (4). Reactive oxygen species are found to be
over-produced during ischemia/reperfusion, accompanied by elevated
levels of free radicals. It is known that reactive oxygen species
are generated following N-methyl-D-aspartate receptor activation
(7), which enhances glutamate (Glu)
release (8) and leads to
excitotoxicity. This initiates a chain reaction or signaling
pathways and leads to the process of cell death. Furthermore, it
has been reported that oxidative stress and excitotoxicity may be
interdependent mechanisms involved in neuronal cell injury and
death (9).
To date, numerous antioxidants, such as vitamin E
and edaravone have shown neuroprotective effects in
ischemia/reperfusion-induced cerebral injury (10–12);
however, these agents do not exhibit satisfactory clinical outcomes
due to a variety of patient- and drug-associated factors. There has
recently been increased interest in the application of natural
products, particularly Traditional Chinese Medicine, for the
treatment of stroke. Gualou Guizhi decoction (GLGZD), a well-known
traditional Chinese formula, was first recorded in ‘Essentials from
the Golden Cabinet’, which was written during the Eastern Han
Dynasty, in ∼210 A.D (13).
According to the theory of Traditional Chinese Medicine, GLGZD is
formulated of six herbs, which collectively exert therapeutic and
modulatory effects. The formula has been used to treat muscular
spasticity following stroke, epilepsy or spinal cord injury in
China (14–16). The present authors' clinical study
revealed the promising effects of GLGZD in stroke patients (Zhang
et al, unpublished data); however, although reports from a
few clinical studies are available (17), little investigation has been
performed into the mechanism underlying the action of GLGZD against
cerebral ischemia/reperfusion injury. The present study was
therefore designed to confirm the potential effects of GLGZD on
focal cerebral ischemia/reperfusion injury and elucidate the
underlying therapeutic mechanism by using a middle cerebral artery
occlusion (MCAO) model of cerebral ischemia.
Materials and methods
Animals and materials
Male Sprague Dawley (specific pathogen-free) rats,
weighing 300±20 g, were provided by the Laboratory Animal Center of
Fujian University of Traditional Chinese Medicine (Fuzhou, China).
All experiments were conducted in accordance with the Institutional
Animal Care and Use Committee of Fujian University of Traditional
Chinese Medicine.
The six herbal medicines of GLGZD were purchased
from Tongchun Drugstore (Fuzhou, China) and identified by Professor
Chengzhi Yang (College of Pharmacy, Fujian University of
Traditional Chinese Medicine). Acetonitrile was high-performance
liquid chromatography grade and purchased from Merck Co.
(Darmstadt, Germany). Deionized water used throughout the
experiments was generated by a Millipore water purification system
(Milli-Q® Direct-Q 3; Millipore, Milford, MA, USA). All other
chemicals were obtained from commercial sources unless otherwise
stated.
Preparation of GLGZD
GLGZD was prepared from the six herbs
(Trichosanthes kirilowii Maxim., Paeonia lactiflora
Pall., Cinnamomum cassia Presl., Glycyrrhiza
uralensis Fisch., Zingiber officinale Rosc. and
Ziziphus jujuba Mill) with the ratio of 10:3:3:3:2:3 (dry
weight), respectively, and extracted with 80% ethanol twice, 1 h
per time. Filtrate was recovered from the ethanol and concentrated
to extraction with a relative density of 1.2 (50°C). The decoction
was obtained for further use.
Focal cerebral ischemia/reperfusion
model
The focal cerebral ischemia/reperfusion model was
generated using MCAO methodology, as described previously (18). Briefly, rats were anesthetized with
chloral hydrate (350 mg/kg) intraperitoneally, and the common left
carotid artery, external carotid artery (ECA) and internal carotid
artery (ICA) were exposed. A 3-0 surgical monofilament nylon suture
with a rounded tip was carefully inserted from the ECA into the ICA
and was advanced to occlude the origin of the left MCA, until a
light resistance was felt (18–22 mm). After 2 h of occlusion, the
nylon suture was withdrawn for blood reperfusion. During the
surgical procedures, the body temperature of the rats was
maintained at 37±0.5°C. Following surgery, the rats were allowed to
recover in pre-warmed cages. Sham-operated rats underwent the same
procedure, but arteries were not occluded. Successfully established
rat models of focal cerebral ischemia/reperfusion were divided into
two experimental groups to give three groups (n=15 per group) in
total: Sham surgery, model and GLGZD.
Drug administration protocol
GLGZD was administered at doses of 7.2 g/kg once a
day, starting 2 h after reperfusion, between days 1 and 7. Saline
solution (0.9%) was administered to the sham surgery and model
groups.
Evaluation of neurological deficit
score
Neurological deficit was scored using the criteria
of a five-point scale (19), as
follows: Score 0, no neurological symptoms; score 1, inability to
completely extend the front jaw on the contralateral side; score 2,
rotation while crawling and falling to the contralateral side;
score 3, inability to walk without assistance and score 4,
unconsciousness. Rat behavioral tests were performed after 60 min
of ischemia followed by 7 days of exercise. The evaluation was
blindly performed by a single observer.
Evaluation of cerebral infarct
volume
Six rats were sacrificed by decapitation and the
intact brain was obtained on the seventh day after MCAO in each
group. The frozen brains were sliced into uniform coronal sections,
and then immediately stained with 2% 2,3,5-triphenyltetrazolium
chloride (TTC) solution in phosphate buffer (pH 7.4). The sections
were kept at 37°C for 1 h and turned over several times. Following
staining, the infarct volume was measured using image analyzer
software (Motic Med 6.0; Xiamen Motic Software Engineering Co.,
Ltd., Shenzhen, China) and calculated as a percentage fraction of
non-viable cerebral tissue of the global brain. Color images of
these slices were captured using a digital camera (Canon 550D;
Canon Inc., Tokyo, Japan).
Biochemical assays
The activity of superoxide dismutase (SOD), as well
as the concentrations of glutathione (GSH) and malondialdehyde
(MDA), in the blood samples were measured using total superoxide
dismutase (T-SOD), cell MDA and reduced GSH assay kits from Nanjing
Jiancheng Institute of Bioengineering (Nanjing, China) in
accordance with the manufacturer's instructions.
Determination of EAA level in
cerebrospinal fluid
Cerebrospinal fluid was collected for the
determination of Glu and aspartate (Asp) levels by a Hitachi
automatic L-8900 amino acid analyzer (Hitachi, Tokyo, Japan)
(20).
Immunohistochemical analysis of Glu
receptor 1 (GluR1)
Six rats in each group were anesthetized and were
fixed with a buffered 4% paraformaldehyde solution by transcardial
perfusion. The intact brain was then embedded with paraffin, and
paraffin-embedded sections were used for the GluR1
immunohistochemistry assay. In brief, paraffin sections were
dewaxed and incubated in boiling citrate buffer for antigen
retrieval. The sections were subsequently incubated with polyclonal
rabbit anti-GluR1 primary antibodies (1:500; #bs-10042R; Beijing
Biosynthesis Biotechnology Co., Ltd., Beijing China) at room
temperature for 2 h, following incubation with 3%
H2O2 and blocking by normal goat serum.
Following a rinse in phosphate-buffered saline (PBS), the sections
were incubated with a biotinylated polyclonal anti-rabbit
IgG/streptavidin secondary antibody (1:1,000; #bs-0295G-SA; Beijing
Biosynthesis Biotechnology Co., Ltd.), washed and incubated with
horseradish peroxidase-labeled streptavidin (Beijing Biosynthesis
Biotechnology Co., Ltd.). The sections were then developed with
diaminobenzidine and counterstained with hematoxylin. PBS replaced
the primary antibody to determine specific binding for the negative
control. Microscopic images were acquired using a Leica microscope
(DM4000B; Leica Microsystems GmbH, Wetzlar, Germany) and five
high-power fields (magnification, ×400) were randomly selected in
each slide. The average proportion of positive cells in each field
was calculated by the true color multi-functional cell image
analysis management system (Image-Pro Plus; Media Cybernetics,
Inc.).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) staining
TUNEL staining was performed in accordance with the
manufacturer's instructions using an apoptosis detection kit
(Promega Corp., Madison, WI, USA). The apoptotic index was
calculated by multiplying the quantity and staining intensity
scores (21).
Statistical analysis
Data are presented as the mean ± standard deviation.
The statistical significance of differences was analyzed by one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of GLGZD on neurological
deficits and cerebral infract volume
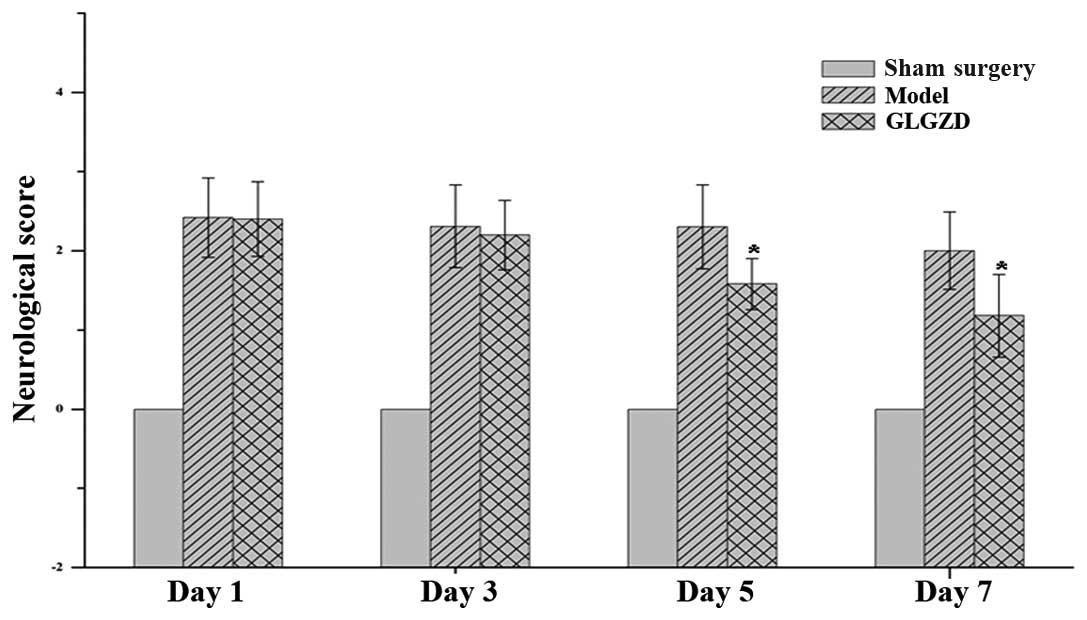
According to the criteria of the five-point scale,
neurological symptoms were not observed in the sham surgery group,
but were evident in the two cerebral ischemia/reperfusion groups
(Fig. 1). GLGZD treatment for the
first 3 days did not significantly reduce the neurological deficit
scores compared with the model group; thereafter, however, the
scores were significantly reduced by GLGZD (P<0.01).
Following TTC staining, the living cells were
stained deep red, while the infarcted cells remained pale due to
dehydrogenase loss at 7 days after ischemia (Fig. 2A). GLGZD significantly decreased the
infarct volume to 0.20±0.06 (P<0.05), as compared with the
cerebral ischemia/reperfusion group (0.32±0.05) (Fig. 2B).
Effect of GLGZD on SOD activity and
MDA and GSH levels in the blood
As shown in Table I,
the levels of MDA and GSH were 2.120±0.79 nmol/ml and 18.231±0.90
µmol/ml, respectively, and the SOD activity was 20.213±0.02 U/ml in
the sham surgery group; however, the SOD activity and GSH levels
were markedly decreased and the MDA level was significantly
increased in the model group compared with those in the sham
surgery group. In the GLGZD group, it was observed that the MDA and
GSH levels and SOD activity were significantly altered when
compared with those in the model group (P<0.05).
 | Table I.Effect of GLGZD on SOD activity and
MDA and GSH levels in the blood of rats subjected to cerebral
ischemia/reperfusion injury. |
Table I.
Effect of GLGZD on SOD activity and
MDA and GSH levels in the blood of rats subjected to cerebral
ischemia/reperfusion injury.
| Groups | SOD (U/ml) | MDA (nmol/ml) | GSH (µmol/l) |
|---|
| Sham surgery |
20.213±0.02 |
2.120±0.79 |
18.231±0.90 |
| Model |
3.689±0.03a |
5.680±1.25a |
3.001±0.29a |
| GLGZD |
19.686±0.02b |
2.190±0.29b |
16.539±1.30b |
Effect of GLGZD on EAAs in
cerebrospinal fluid
In the model group, the levels of EAAs, such as Glu
and Asp, were increased by 150 and 146%, respectively (P<0.05),
compared with those in the sham surgery group. Following treatment
with GLGZD, the EAA levels were decreased by 36 and 47%,
respectively, compared with those in the model group (P<0.05)
(Fig. 3).
Effect of GLGZD on apoptosis in the
brain
Apoptosis (programmed cell death) was detected by
TUNEL assay. Data in Fig. 4A show
that GLGZD treatment decreased the proportion of TUNEL-positive
cells compared with the model group, thus showing the
anti-apoptotic activity of GLGZD in vivo.
Effect of GLGZD on the expression of
GluR1 in brain
The protein expression of GluR1 was evaluated via
immunohistochemical analysis. Protein expression was significantly
elevated in the model group compared with that in the sham surgery
group, while decreased expression was observed in the GLGZD group
compared with that in the model group (Fig. 4B).
Discussion
Ischemic stroke is the most commonly encountered
type of stroke in humans. In experimental stroke, the two models
used are the permanent MCAO model and the focal cerebral ischemia
model. The focal cerebral ischemia model more closely replicates
the condition in human beings; therefore, a reversible model of
focal ischemia (i.e. the temporary MCAO model) is more relevant
than the permanent occlusion model (22). The major advantages of this model are
that the method is relatively simple and that the MCA can be
occluded and reperfused without craniotomy (23,24).
In the present study, the neuroprotective effect of
GLGZD was evaluated in vivo using an MCAO rat model. The
results showed that GLGZD significantly decreased the focal infarct
volume, neurological deficit score and level of apoptosis compared
with the model rats. These findings were consistent with those of a
previous report (18).
The neuroprotective effect of GLGZD may involve a
number of different mechanisms, including reduced inflammatory
cytokine levels (25), an
antioxidation effect (26) and the
modulation of Glu levels and
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
(AMPAR) expression (18). In the
MCAO rats, a significant increase was observed in the level of MDA
and a decrease in the levels of endogenous antioxidants, i.e. SOD
and GSH. This was indicative of marked oxidative stress. Excessive
free radical generation occurs at the time of reperfusion and
accounts for free radical-induced injury (27). In the GLGZD-treated rats, a
significant attenuation in the level of MDA and increases in the
activity of SOD and level of GSH were noted.
Oxidative stress can cause cellular damage and
enhance Glu release, thus leading to excitotoxicity. Glu is the
most common excitatory neurotransmitter in the central nervous
system and is involved in numerous aspects of normal brain
function; however, abnormally elevated levels of Glu may induce
excitatory neural toxicity (28).
Excess Glu-induced excitotoxicity is involved in the pathogenesis
of several human neurological diseases, such as stroke. It has been
reported that cerebral ischemia or brain injury markedly elevates
Glu concentrations (29), with the
resulting excitotoxicity leading to neuronal injury or death
(30). The present study showed that
Glu and Asp concentrations were significantly decreased in
GLGZD-treated rats compared with those in the model rats; similar
results were observed for GluR1 expression. GluR1 is one of the
AMPAR subunits. AMPAR is a member of the ionotropic glutamate
receptor family and mediates excitatory synaptic transmission in
the brain. It additionally has an important role in motor function
following cerebral ischemia. Gottlieb and Matute (31) found that AMPAR subunits were
upregulated in immunoreactive hypertrophic astrocytes in the CA1
hippocampal region following transient forebrain ischemia (31); therefore, a normal Glu concentration
is important to prevent glutamate-induced neurotoxicity.
In this study, initial evidence was provided that
GLGZD exerts a neuroprotective effect in vivo by promoting
endogenous antioxidant enzymatic activities, decreasing neuronal
cell death and Glu concentration and inhibiting GluR1 expression.
The findings lead to the speculation that GLGZD may be a potential
therapeutic agent for cerebral ischemia.
Acknowledgements
This study was carried out in the State Key
Laboratory of Chinese Pharmacies of the Fujian Provincial
Department of Science and Technology, the Collaborative Innovation
Center for Rehabilitation Technology and the TCM Rehabilitation
Research Center of SATCM. It was funded by the Important Subject of
Fujian Province Science and Technology Hall of China (no.
2012Y0041) and the Important Subject of Fujian Province Education
Hall of China (no. JA12176).
References
|
1
|
World Health Organization: The World
Health Report 2002. World Health Organization; France: 2002
|
|
2
|
Deb P, Sharma S and Hassan KM:
Pathophysiologic mechanisms of acute ischemic stroke: An overview
with emphasis on therapeutic significance beyond thrombolysis.
Pathophysiology. 17:197–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Candelario-Jalil E: Injury and repair
mechanisms in ischemic stroke: considerations for the development
of novel neurotherapeutics. Curr Opin Investig Drugs. 10:644–654.
2009.PubMed/NCBI
|
|
4
|
Janardhan V and Qureshi AI: Mechanisms of
ischemic brain injury. Curr Cardiol Rep. 6:117–123. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amantea D, Nappi G, Bernardi G, et al:
Post-ischemic brain damage: pathophysiology and role of
inflammatory mediators. FEBS J. 276:13–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doyle KP, Simon RP and Stenzel-Poore MP:
Mechanisms of ischemic brain damage. Neuropharmacology. 55:310–318.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lafon-Cazal M, Pietri S, Culcasi M, et al:
NMDA-dependent superoxide production and neurotoxicity. Nature.
364:535–537. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gilman SC, Bonner MJ and Pellmar TC:
Peroxide effects on [3H] L-glutamate release by synaptosomes
isolated from the cerebral cortex. Neurosci Lett. 140:157–160.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Won MH, Kang T, Park S, et al: The
alterations of N-Methyl-D-aspartate receptor expressions and
oxidative DNA damage in the CA1 area at the early time after
ischemia-reperfusion insult. Neurosci Lett. 301:139–142. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
El-Abhar HS: Possible neuroprotective
effects of melatonin against ischemia/reperfusion insult in rat
brain. Med Sci Res. 27:605–608. 1999.
|
|
11
|
Bora KS and Sharma A: Neuroprotective
effect of Artemisia absinthium L. on focal ischemia and
reperfusion-induced cerebral injury. J Ethnopharmacol. 129:403–409.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoneda Y, Uehara T, Yamasaki H, et al:
Hospital-based study of the care and cost of acute ischemic stroke
in Japan. Stroke. 34:718–724. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Z, Lin Y, Yang P, Hou X and Yang Y:
Synopsis of Golden Chamber. Macmillan Press; Beijing: pp. 203–204.
2008
|
|
14
|
Yang C, Chen L and Tao J: New usage of a
classical formula-Gua Lou Gui Zhi decotion. Liaoning Zhong Yi Za
Zhi. 8:166–167. 2010.
|
|
15
|
Sun X: Research on formula treating
paralysis and spasticity from ‘treatise on febrile and
miscellaneous diseases’. Zhongguo Zhong Yi Ji Chu Yi Xue Za Zhi.
8:644–645. 2010.[(In Chinese)].
|
|
16
|
Zhang L and Ai H: Effects of Gua Lou Gui
Zhi decoction on c-fos and c-jun in epileptic rats. Shi Yong Zhong
Yi Yao Za Zhi. 23:21–22. 2005.[(In Chinese)].
|
|
17
|
Yang C, Chen L and Tao J: New usage of a
classical formula-Gua Lou Gui Zhi decoction. Liaoning Zhong Yi Za
Zhi. 8:166–167. 2010.[(In Chinese)].
|
|
18
|
Huang J, Tao J, Xue X, et al: Gua Lou Gui
Zhi decoction exerts neuroprotective effects on post-stroke
spasticity via the modulation of glutamate levels and AMPA receptor
expression. Int J Mol Med. 31:841–848. 2013.PubMed/NCBI
|
|
19
|
Camerlingo M, Salvi P, Belloni G, et al:
Intravenous heparin started within the first 3 h after onset of
symptoms as a treatment for acute nonlacunar hemispheric cerebral
infarctions. Stroke. 36:2415–2420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo C, Tong L, Xi M, et al:
Neuroprotective effect of calycosin on cerebral ischemia and
reperfusion injury in rats. J Ethnopharmacol. 144:768–774. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Xu W, Li H, et al: Therapeutic
effects of total alkaloids of Tripterygium wilfordii Hook f. on
collagen-induced arthritis in rats. J Ethnopharmacol l. 45:699–705.
2013. View Article : Google Scholar
|
|
22
|
Hossmann KA: Cerebral ischemia: models,
methods and outcomes. Neuropharmacology. 55:257–270. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Belayev L, Alonso OF, Busto R, et al:
Middle cerebral artery occlusion in the rat by intraluminal suture.
Neurological and pathological evaluation of an improved model.
Stroke. 27:1616–1622. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu XY, Lin SG, Zhou ZW, et al: Tanshinone
IIB, a primary active constituent from Salvia miltiorrhiza,
exhibits neuro-protective activity in experimentally stroked rats.
Neurosci Lett. 417:261–265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu H, Li Z, Zhu X, et al: Gua Lou Gui Zhi
decoction suppresses LPS-induced activation of the TLR4/NF-κB
pathway in BV-2 murine microglial cells. Int J Mol Med.
31:1327–1332. 2013.PubMed/NCBI
|
|
26
|
Mao JJ, Li ZF and Guang J: Impacts of
Gualou Guizhi decoction extract on the expression of NrF2 and HO-1
mRNA in oxidative stress PC12 cells. Shijie Zhongxiyi Jiehe Zazhi.
8:563–566. 2013.[(In Chinese)].
|
|
27
|
Yu XQ, Xue CC, Zhou ZW, et al: In vitro
and in vivo neuroprotective effect and mechanisms of glabridin, a
major active isoflavan from Glycyrrhiza glabra (licorice). Life
Sci. 82:68–78. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Danbolt NC: Glutamate uptake. Prog
Neurobiol. 65:1–105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Parelkar NK and Wang JQ: Upregulation of
metabotropic glutamate receptor8 mRNA expression in the rat
forebrain after repeated amphetamine administration. Neurosci Lett.
433:250–254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bonde C, Noraberg J, Noer H and Zimmer J:
Ionotropic glutamate receptors and glutamate transporters are
involved in necrotic neuronal cell death induced by oxygen-glucose
deprivation of hippocampal slice cultures. Neuroscience.
136:779–794. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gottlieb M and Matute C: Expression of
ionotropic glutamate receptor subunits in glial cells of the
hippocampal CA1 area following transient forebrain ischemia. J
Cereb Blood Flow Metab. 17:290–300. 1997. View Article : Google Scholar : PubMed/NCBI
|