Introduction
Renal cell carcinoma (RCC) is one of the most
progressive urological cancers among men and women (1,2), and is
considered as one of the most difficult cancers to diagnose and to
treat (3). Advanced RCC is highly
resistant to radiotherapy and chemotherapy. Approximately 25–30% of
RCC patients suffer metastatic or advanced disease, with a 5-year
survival rate <10% (4). Prior to
recent advancements in understanding of the molecular mechanism of
RCC and the development of angiogenesis inhibitors, cytokine-based
therapy such as interleukin-2 (IL-2) and interferon (IFN) was the
main therapy used to treat RCC (5).
However, the therapeutic effect of these treatments is quite
limited, as there is only a 4–6% combined response rate for most
single agent or combination regimens (6).
Therapeutic options for patients have significantly
expanded due to the successful development and adaptation of
several targeting agents in the first-line treatment of advanced
RCC, including multikinase inhibitors sorafenib (7), sunitinib (8), pazopanib (9) and axitinib (10); the combination of the anti-vascular
endothelial growth factor (VEGF) agent bevacizumab with IFN-α
(11); and mammalian target of
rapamycin (mTOR) inhibitors temsirolimus (12) and everolimus (13). However, considering the poor physical
condition, such as cachexia, that is common among patients with
advanced RCC (14), selection of
treatment should not only consider the therapeutic effect, but also
adverse events. In order to make a more rational choice of
treatment for individual patients with advanced RCC, it is
necessary to identify the level of adverse effects of these
options. In this scenario, a meta-analysis of randomized trials was
performed to evaluate the therapeutic and adverse effects of the
multikinase inhibitors sorafenib, sunitinib, pazopanib and
axitinib.
Materials and methods
Study design
This study was a meta-analysis based on data
collected from previous randomized controlled trials (RCTs) of
first-line chemotherapies for patients with advanced RCC. Two
reviewers (QT and YL) selected and reviewed the evidence
independently. Disagreements were handled through group
discussion.
Search strategy
Studies were search among PubMed/Medline, Embase and
Cochrane Central Register of Controlled Trials (CENTRAL) databases
using the following terms and strategy: (‘sorafenib’ or ‘sunitinib’
or ‘pazopanib’ or ‘axitinib’) and (‘renal cell carcinoma’ or ‘RCC’
or ‘metastatic renal cell carcinoma’ or ‘advanced RCC’) and
(‘randomized trials’ or ‘random*’ or ‘RCT’) in the abstract. The
databases were searched for studies published up to February 2014.
Only trials published in English were considered. Reference lists
of related articles were manually checked to search for additional
eligible publications. All references of relevant articles were
scanned and all additional studies of potential interest were
retrieved for further analysis.
Selection criteria
Eligible trials were required to meet the following
criteria: Patients involved were diagnosed with advanced RCC
through cytologic diagnosis or pathological diagnosis; RCTs
evaluated multikinase inhibitors individually with a control
intervention as the sole treatment; patients did not undergo
surgery or other non-antiangiogenic treatment; and ≥100 patients
were enrolled. Animal studies, non-randomized trials and
pharmacokinetic studies were excluded.
The bias risk of included publications was evaluated
based on the Cochrane Handbook for Systematic Reviews of
Interventions, version 5.0.0 (15).
The major quality components include: i) Sequence generation of the
allocation; ii) allocation concealment; iii) blinding of
participants, personnel and outcome assessors; iv) incomplete
outcome data; v) selective outcome reporting; and vi) other sources
of bias (15). Trials were
classified into three levels according to the bias risk. Trials
with appropriate and sufficient support of index of outcome
assessment that have minimal risk of bias were classified into
level A; trials with one or more high or unclear risks for bias
among the quality components and with a moderate level risk of bias
were in level B; trials with three or more high or unclear risks
for bias among the quality components and with the highest level of
bias were in level C.
Data extraction
Two reviewers (QT and YL) separately and
independently extracted data from the trials. Disagreements were
handled by consensus. All data were checked for internal
consistency. The trials were identified with the first author and
the year of publication. For the trials that did not report the
required data to determine the outcomes, the reviewers contacted
the authors to obtain required information. The baseline
information of patients and details of intervention of each trial
were extracted to assess the heterogeneity. The outcomes assessed
included tumor progression, objective response rate (ORR) and
progressive disease rate (PDR). Toxicity data reported commonly in
the trials involved were retrieved, extracted and assessed
respectively.
Statistic analysis
Meta-analysis was conducted with Review Manager 5.2
(RevMan 5.2; The Nordic Cochrane Centre, The Cochrane
Collaboration, Copenhagen, Denmark). Risk ratio (RR) was used for
evaluation and the 95% confidence interval (CI) was calculated for
each estimate. P≤0.05 was used to denote statistical significance.
Heterogeneity of the results of the trials was assessed with the
I2 statistic using the χ2 test at α=0.1
(15). Primary assessment was
conducted with a fixed model. When P≥0.05 and I2≤50%, it
was considered that the trials were without heterogeneity and a
fixed-effect model was used to perform the meta-analysis. When
P<0.05 and I2>50%, it was considered that the
trials had significant heterogeneity (16). The source of the heterogeneity was
further analyzed. If there was no significant clinical
heterogeneity, a secondary confirmatory analysis was performed with
a random-effect model. Otherwise, descriptive analysis was
performed. Where necessary, sensitivity analyses were performed to
test the stability of identified outcomes.
Results
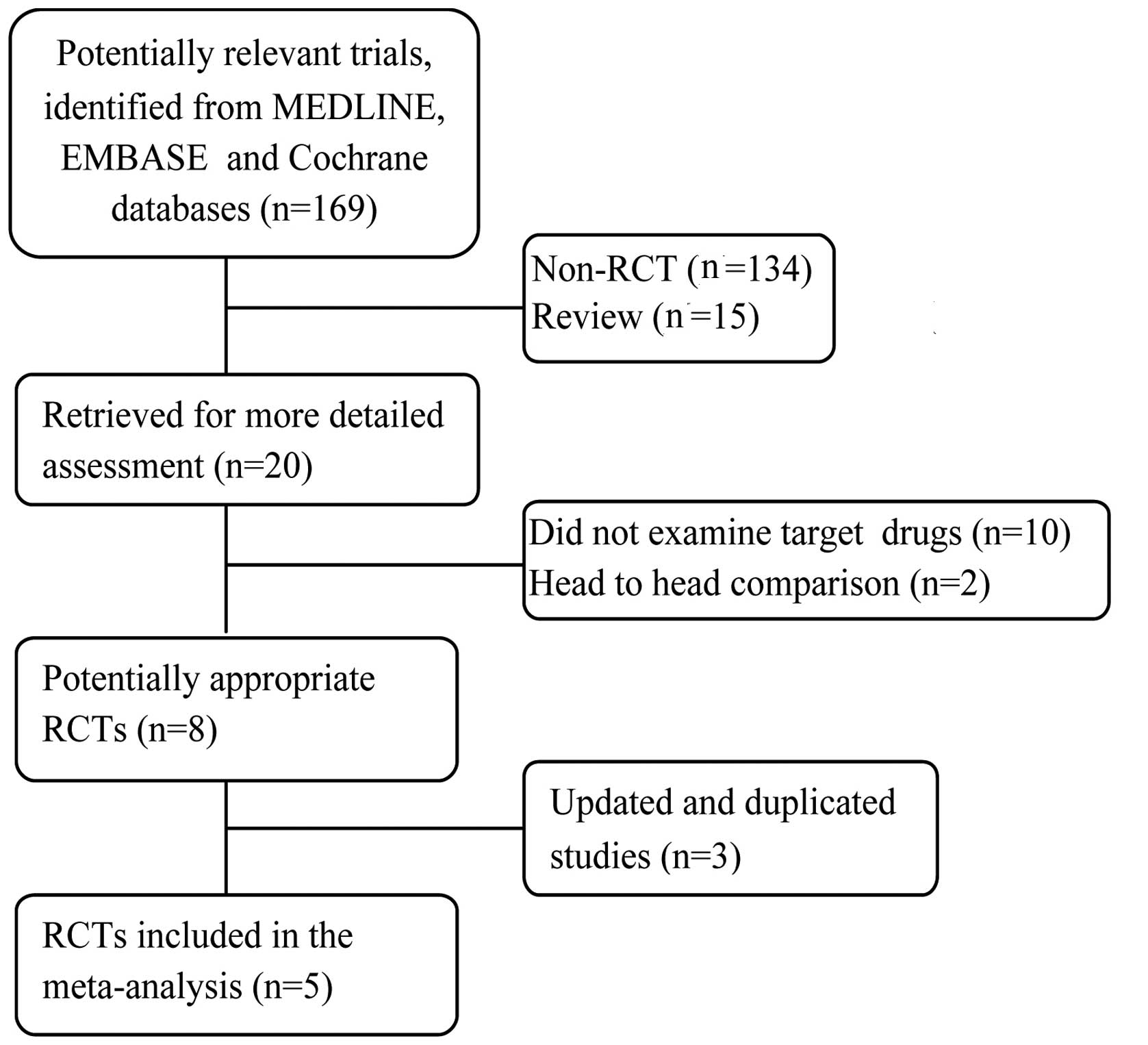
Literature search
The whole search process was as described in the
flowchart in Fig. 1. The primary
literature search identified 169 studies. Of these, 149 were
excluded since they were not RCTs or were review studies. The
remaining 20 studies were reviewed in full-length. Among them, 10
were excluded because they did not examine the target drugs, 2 were
excluded since they were head-to-head comparisons, and 3 were
excluded since the studies were based on the same patients and the
same trial. Finally, 5 RCTs remained for inclusion in this
meta-analysis. No patients in the involved studies had received
previous systemic therapy. The 5 trials are randomized,
multicenter, controlled and phase II or III trials. Among them, two
assessed sorafenib (17,18), one evaluated sunitinib (19), one evaluated pazopanib (20) and one evaluated axitinib (21). The methodological details relevant to
bias and the treatment arms of the selected studies are presented
in Table I.
 | Table I.Summary of the included trials. |
Table I.
Summary of the included trials.
| Trial (ref.) | N | Intervention | Control | Quality
components | Quality level |
|---|
| Escudier 2007
(17) | 903 | Sorafenib (400 mg,
twice daily, 6 week cycles) | Placebo | R; S and RPB; C; BR;
F; ITT | B |
| Escudier 2009
(18) | 189 | Sorafenib (twice
daily; 400 mg, period 1; 600 mg, period 2) | IFN-α | R; S; C; BR; F;
ITT | B |
| Motzer 2009 (22) | 750 | Sunitinib (50 mg once
daily, with a 4 weeks on and 2 weeks off schedule) | IFN-α | R; S and RPB; C; NB;
F; ITT | B |
| Sternberg 2010
(20) | 435 | Pazopanib (800 mg,
once daily) | Placebo | R; S and RPB; C; DB;
F; ITT | A |
| Rini 2013 (21) | 112 | Axitinib (5 mg + 2
mg, twice daily) | Placebo | R; S and RPB; C; B R;
F; ITT | B |
Meta-analysis of the therapeutic
efficiency of multikinase inhibitors
Progression free survival (PFS) and overall
survival (OS)
All studies reported PFS data and two of the studies
reported OS data. Detailed information about the included trials is
presented in Table II. The pooled
HR of PFS is presented in Fig. 2.
This shows that, compared with controls, multikinase inhibitors
contributed to significantly longer PFS [hazard ratio (HR)=0.58;
95% confidence interval (CI): 0.45–0.74; P<0.0001]. Of the two
studies that reported OS data, one study (17) reported a significantly longer OS for
the multikinase inhibitor compared with the control (19.3 vs. 15.9
months, P=0.02), while the other (22) did not find a significant difference
(26.4 vs. 21.8 months, not significant).
 | Table II.Progression-free survival (PFS) and
overall survival (OS) in the included trials. |
Table II.
Progression-free survival (PFS) and
overall survival (OS) in the included trials.
| Trials (ref.) | N | Median
PFSa | HR (95% CI) | P-value | Median OS
(months) | HR (95% CI) | P-value |
|---|
| Escudier 2007
(17) | 903 | 5.5 vs. 2.8 | 0.44 (0.35–0.55) | 0.01 | 19.3 vs. 15.9 | 0.72 (0.54–0.94) | 0.02 |
| Escudier 2009
(18) | 189 | 5.7 vs. 5.6 | 0.88 (0.61–1.27) | 0.50 | N/A | – | – |
| Motzer 2009
(22) | 750 | 11.0 vs. 5.0 | 0.54
(0.45–0.64) | 0.01 | 26.4 vs. 21.8 | 0.82
(0.64–1.00) | NS |
| Sternberg 2010
(20) | 435 | 9.2 vs. 4.2 | 0.46
(0.34–0.62) | 0.001 | N/A | – | – |
| Rini 2013 (21) | 112 | 14.5 vs. 15.7 | 0·85
(0.54–1.35) | 0.24 | N/A | – | – |
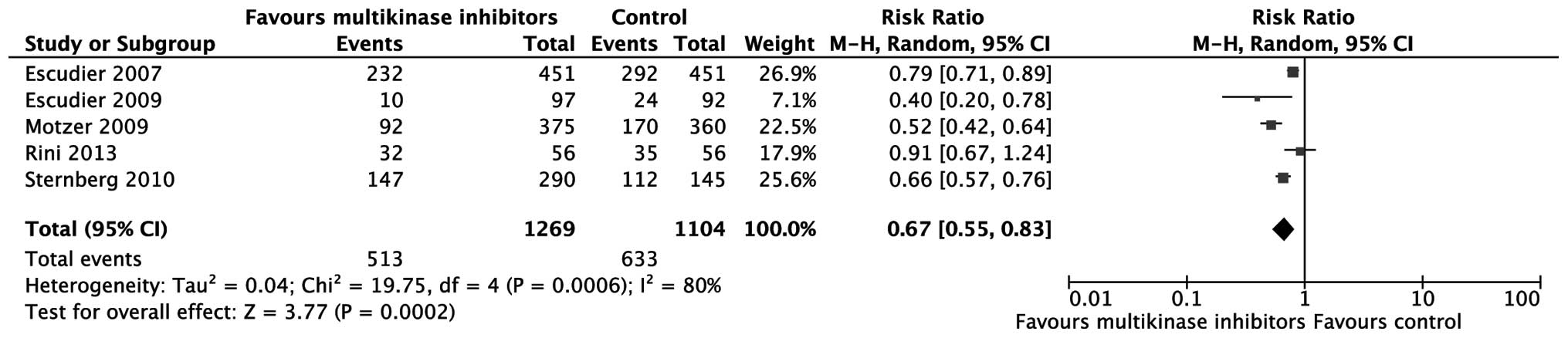
PDR and ORR
The number of patients with progressive disease was
extracted to assess tumor progression. According to the
meta-analysis, multikinase inhibitors are more effective in
controlling tumor progression compared with placebo or IFN-α
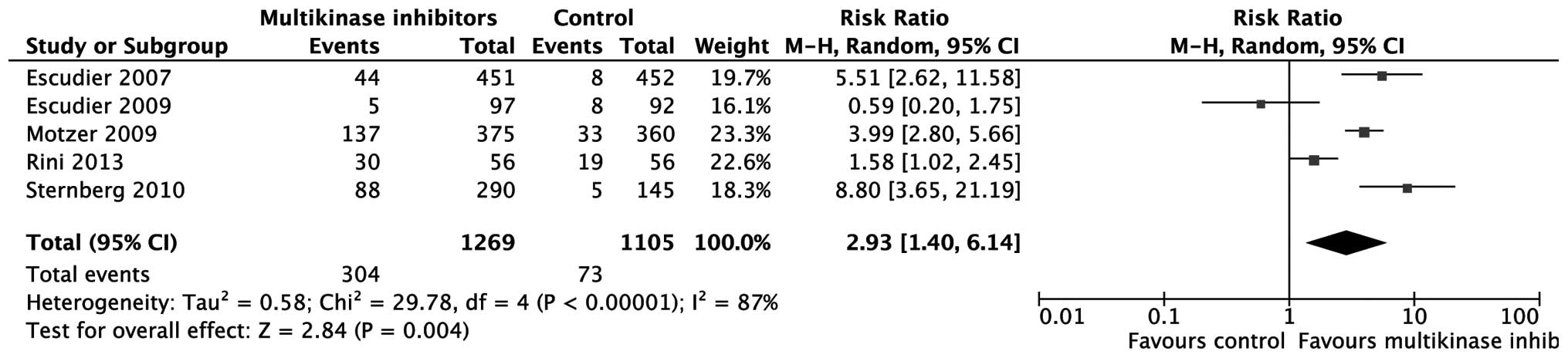
[relative risk (RR)=0.67; 95% CI: 0.55–0.83; P=0.0002; Fig. 3). As to ORR, the effect of
multikinase inhibitors was also superior to that of placebo or
IFN-α (RR=2.93; 95% CI: 1.40–6.14; P=0.004; Fig. 4).
Adverse effects
The reported adverse effects associated with drug
administration in the 5 trials were extracted and are summarized in
Table III. Six types of adverse
effect, including cardiac (hypertension), constitutional (fatigue)
and gastrointestinal (diarrhea, anorexia and vomiting) effects and
pain (abdominal) were reported. Only adverse effects reported in ≥4
studies were pooled for RR evaluation. According to the
meta-analysis, compared with IFN or placebo, multikinase inhibitors
were associated with significantly higher rates of grade 3 or 4
hypertension (RR=6.00; 95% CI: 3.36–10.69; P<0.00001), diarrhea
(RR=5.84; 95% CI: 3.06–11.16; P<0.00001), nausea (RR=2.30; 95%
CI: 1.16–4.54; P=0.02), vomiting (RR=1.84; 95% CI: 1.00–3.41;
P=0.05) and hand-foot skin reaction (RR=11.78; 95% CI: 5.16–26.93;
P<0.00001; Table III). However,
the difference in fatigue between the multikinase inhibitor group
and the IFN or placebo group was not significant (RR=0.93; 95% CI:
0.69–1.25; P=0.61; Table III).
 | Table III.Meta-analysis of adverse effects. |
Table III.
Meta-analysis of adverse effects.
|
| Escudier 2007 | Escudier 2009 | Motzer 2009 | Rini 2013 | Sternberg 2010 |
|
|
|
|
|---|
|
|
|
|
|
|---|
| Variable | I | C | I | C | I | C | I | C | I | C | Pooled RR (95%
CI) | I2
(%) | P-H | P-value |
|---|
| No. of
patients | 451 | 451 | 97 | 90 | 375 | 360 | 56 | 56 | 290 | 145 |
|
|
|
|
| Cardiac,
hypertension | 16 | 2 | 2 | 1 | 45 | 4 | 10 | 5 | 13 | 1 | 6.00 (3.36,
10.69) | 42 | 0.14 | <0.00001 |
| Hematologic,
decreased Hb | 12 | 20 | – | – | – | – | – | – | – | – |
|
|
|
|
| Constitutional |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fatigue | 22 | 16 | 5 | 9 | 41 | 47 | 3 | 2 | 7 | 4 | 0.93 (0.69,
1.25) | 0 | 0.52 | 0.61 |
| Weight
loss | 3 | 0 | 2 | 1 | – | – | 4 | 3 | – | – |
|
|
|
|
|
Asthenia | – | – | – | – | 26 | 14 | – | – | 8 | 0 |
|
|
|
|
| Other
symptoms | 6 | 6 | 0 | 0 | – | – |
|
| – | – |
|
|
|
|
|
Gastrointestinal |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diarrhea | 11 | 3 | 6 | 0 | 34 | 4 | 6 | 2 | 11 | 1 | 5.84 (3.06,
11.16) | 0 | 0.76 | <0.00001 |
|
Nausea | 3 | 3 | 0 | 3 | 19 | 4 | 3 | 0 | 2 | 0 | 2.30 (1.16,
4.54) | 41 | 0.15 | 0.02 |
|
Anorexia | 3 | 5 | 0 | 2 | 8 | 7 | – | – | 6 | 1 | 0.95 (0.47,
1.93) | 0 | 0.43 | 0.89 |
|
Vomiting | 4 | 6 | 2 | 1 | 15 | 4 | 3 | 0 | 7 | 3 | 1.84 (1.00,
3.41) | 23 | 0.27 | 0.05 |
|
Constipation | 3 | 3 | – | – | – | – | 0 | 0 | – | – |
|
|
|
|
|
Neurologic-sensory |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Neuropathy | 2 | 3 | – | – | – | – | 0 | 0 | – | – |
|
|
|
|
| Pain |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Abdominal | 7 | 9 | 3 | 1 | 8 | 0 | 0 | 1 | 6 | 0 | 1.81 (0.91,
3.63) | 44 | 0.13 | 0.09 |
|
Muscular | – | – | 1 | 0 | – | – | 0 | 0 | – | – |
|
|
|
|
|
Stomach | – | – | 0 | 0 | – | – |
|
| – | – |
|
|
|
|
|
Headache | 1 | 2 | – | – | 4 | 0 | 0 | 0 | 0 | 0 | 1.84 (0.11,
31.90) | 56 | 0.13 | 0.67 |
|
Joint | 7 | 1 | – | – | – | – | 1 | 2 | – | – |
|
|
|
|
|
Bone | 3 | 15 | – | – | – | – | 0 | 0 | – | – |
|
|
|
|
|
Tumor | 13 | 8 | – | – | – | – | – | – | – | – |
|
|
|
|
| Pulmonary |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cough | 1 | 1 | 0 | 0 | – | – | 0 | 0 | – | – |
|
|
|
|
|
Dyspnea | 16 | 11 | 0 | 0 | 8 | 4 | 1 | 2 | – | – | 1.45 (0.79,
2.67) | 0 | 0.61 | 0.23 |
| Voice
changes | – | – | 0 | 0 | – | – | – | – | – | – |
|
|
|
|
| Dermatologic |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Rash or
desquamation | 4 | 1 | 6 | 0 | – | – | 0 | 0 | – | – |
|
|
|
|
|
Hand-foot skin reaction | 25 | 0 | 11 | 0 | 34 | 4 | 2 | 1 | – | – | 11.78 (5.16,
26.93) | 22 | 0.28 | <0.00001 |
|
Alopecia | 1 | 0 | 0 | 0 | 0 | 0 | – | – | – | – |
|
|
|
|
|
Pruritus | 1 | 0 | 0 | 0 | – | – | – | – | – | – |
|
|
|
|
Discussion
This meta-analysis aimed to identify the therapeutic
effect and associated adverse events of the currently used
multikinase inhibitors sorafenib, sunitinib, pazopanib and axitinib
in advanced RCC. The results demonstrate that individual use of
sorafenib, sunitinib, pazopanib and axitinib is more effective in
terms of PDR and ORR. However, the results also show that
multikinase inhibitors are associated with a higher risk of grade 3
or 4 adverse events for hypertension and gastrointestinal effects,
including diarrhea, nausea and vomiting. Although much progress has
been achieved in understanding the molecular mechanism of advanced
RCC and in the development of targeting drugs, the overall
efficiency of these therapies is far from satisfactory. Considering
the poor physical condition of patients with advanced RCC, the
appropriate selection of treatments and the proper management of
adverse events are required to improve the quality of life of such
patients.
Although the combination of bevacizumab and IFN may
provide a higher ORR compared with either agent alone, the higher
frequency or grade of adverse events may be unbearable for some
patients (23). However, although
the individual use of sorafenib, sunitinib or temsirolimus is less
toxic, the efficiency of these drugs in improving the ORR is
limited (23). In clinical practice,
the balance between therapeutic effect and associated adverse
events should be carefully evaluated prior to the final selection
of a treatment. Previous studies observed that patients suffered
hypertension as early as the first day of taking sorafenib
(24) and axitinib (25). Rini et al (26) observed that the median time of
all-grade hypertension onset was independent of baseline
antihypertensive use. However, the onset of grade 3 or 4
hypertension occurred significantly earlier in patients that
received antihypertensive medications than in those that did not at
baseline. Thus, patients receiving antihypertensive medication
might be more biologically susceptible to earlier axitinib-induced
hypertension (26). Therefore, it is
recommended that clinicians consider collaborating with patients
and other practitioners to manage treatment-induced hypertension
actively. Prior to treatment, it is necessary for practitioners to
assess the blood pressure status, cardiovascular risk factors and
previous antihypertensive medication use of the patients. Based on
this assessment, patients may be divided into normotensive,
uncontrolled hypertensive, sub-optimally medication-controlled
hypertensive or medication-controlled hypertensive groups (25). For patients with uncontrolled
hypertension, short-acting antihypertensive agents could be
provided. Following the initiation of multikinase treatment,
short-acting antihypertensive agents may be switched to long-acting
agents. For patients with sub-optimally controlled hypertension,
treatment-induced hypertension could be controlled through
increasing the dose of current medication or adding new medication.
Pretreatment evaluation of risk factors of cardiovascular diseases,
such as peripheral vascular disease, diabetes mellitus, cardiac
conditions and renal disease is essential since patients with these
risk factors may require close monitoring and the aggressive
management of cardiovascular events during treatment (27). In addition, clinicians should to
check whether patients have received hypertension-inducing agents
such as hormones or steroids, which may complicate the management
of axitinib-induced hypertension (28). It is recommended that during
treatment with multikinase inhibitors, blood pressure should be
monitored regularly in-clinic and at home. In order to gain a
higher level of compliance of the patient to treatment, blood
pressure assessment prior to treatment, including an assessment of
the likelihood of hypertension developing during therapy and home
monitoring could be provided. The clinician could support patients
by training them and their family members in the proper use of the
blood pressure-monitoring device, calibration of the device and
recording and the reporting of measurements.
Gastrointestinal adverse events, including diarrhea,
nausea and vomiting were commonly reported for these multikinase
inhibitors. Although these events might not lead to treatment
discontinuation and can be properly managed by pharmacological
intervention and dietary modifications, in elderly patients, these
events might lead to serious dehydration if they are not well
controlled (29). Previous studies
also reported that treatment-related diarrhea can be persistent for
the duration of multikinase therapy and mild-to-moderate diarrhea
can reduce the mobility and independence of patients, impairing
quality of life (30–32). Therefore, it is recommended that
clinical guidelines for the management of cancer treatment-related
gastrointestinal adverse events should be followed (33). The major limitation of this study is
the small number of studies involved. This small selection of
studies was not adequate to perform patient subgroup analyses.
In conclusion, multikinase inhibitors can
significantly control disease progress and improve the ORR.
However, they are also associated with a higher risk of grade 3 and
4 hypertension and gastrointestinal events. Proper management of
these events is necessary to improve quality of life.
References
|
1
|
Decastro GJ and McKiernan JM:
Epidemiology, clinical staging and presentation of renal cell
carcinoma. Urol Clin North Am. 35:581–592. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garcia JA, Cowey CL and Godley PA: Renal
cell carcinoma. Curr Opin Oncol. 21:266–271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Motzer RJ, Bander NH and Nanus DM:
Renal-cell carcinoma. N Engl J Med. 335:865–875. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Spronsen DJ, de Weijer KJ, Mulders PF
and De Mulder PH: Novel treatment strategies in clear-cell
metastatic renal cell carcinoma. Anticancer Drugs. 16:709–717.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Veldhuizen PJ, Hussey M, Lara PN Jr,
et al: A phase II study of gemcitabine and capecitabine in patients
with advanced renal cell cancer: Southwest Oncology Group Study
S0312. Am J Clin Oncol. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Escudier B, Eisen T, Stadler WM, et al:
TARGET Study Group: Sorafenib in advanced clear-cell renal-cell
carcinoma. N Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Motzer RJ, Michaelson MD, Rosenberg J, et
al: Sunitinib efficacy against advanced renal cell carcinoma. J
Urol. 178:1883–1887. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu CF, Bing NX, Ball HA, et al: Pazopanib
efficacy in renal cell carcinoma: evidence for predictive genetic
markers in angiogenesis-related and exposure-related genes. J Clin
Oncol. 29:2557–2564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cella D, Escudier B, Rini B, et al:
Patient-reported outcomes for axitinib vs sorafenib in metastatic
renal cell carcinoma: phase III (AXIS) trial. Br J Cancer.
108:1571–1578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Escudier B, Pluzanska A, Koralewski P, et
al: AVOREN Trial Investigators: Bevacizumab plus interferon alfa-2a
for treatment of metastatic renal cell carcinoma: a randomised,
double-blind phase III trial. Lancet. 370:2103–2111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hudes G, Carducci M, Tomczak P, et al:
Temsirolimus, interferon alfa, or both for advanced renal-cell
carcinoma. N Engl J Med. 356:2271–2281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Motzer RJ, Escudier B, Oudard S, et al:
Efficacy of everolimus in advanced renal cell carcinoma: a
double-blind, randomised, placebo-controlled phase III trial.
Lancet. 372:449–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim HL, Belldegrun AS, Freitas DG, et al:
Paraneoplastic signs and symptoms of renal cell carcinoma:
implications for prognosis. J Urol. 170:1742–1746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions. John Wiley … Sons, Inc;
Chichester, UK: 2008, View Article : Google Scholar
|
|
16
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Escudier B, Eisen T, Stadler WM, et al:
TARGET Study Group: Sorafenib in advanced clear-cell renal-cell
carcinoma. N Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Escudier B, Szczylik C, Hutson TE, et al:
Randomized phase II trial of first-line treatment with sorafenib
versus interferon Alfa-2a in patients with metastatic renal cell
carcinoma. J Clin Oncol. 27:1280–1289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Motzer RJ, Hutson TE, Tomczak P, et al:
Sunitinib versus interferon alfa in metastatic renal-cell
carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sternberg CN, Davis ID, Mardiak J, et al:
Pazopanib in locally advanced or metastatic renal cell carcinoma:
results of a randomized phase III trial. J Clin Oncol.
28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rini BI, Melichar B, Ueda T, et al:
Axitinib with or without dose titration for first-line metastatic
renal-cell carcinoma: a randomised double-blind phase 2 trial.
Lancet Oncol. 14:1233–1242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Motzer RJ, Hutson TE, Tomczak P, et al:
Overall survival and updated results for sunitinib compared with
interferon alfa in patients with metastatic renal cell carcinoma. J
Clin Oncol. 27:3584–3590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Escudier B, Albiges L and Sonpavde G:
Optimal management of metastatic renal cell carcinoma: Current
status. Drugs. 73:427–438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maitland ML, Kasza KE, Karrison T, et al:
Ambulatory monitoring detects sorafenib-induced blood pressure
elevations on the first day of treatment. Clin Cancer Res.
15:6250–6257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rixe O, Bukowski RM, Michaelson MD, et al:
Axitinib treatment in patients with cytokine-bractory metastatic
renal-cell cancer: a phase II study. Lancet Oncol. 8:975–984. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rini BI, Quinn DI, Baum M, et al:
Hypertension among patients with renal cell carcinoma receiving
axitinib or sorafenib: analysis from the randomized phase III AXIS
trial. Target Oncol. 2014.PubMed/NCBI
|
|
27
|
Edmonds K, Hull D, Spencer-Shaw A, et al:
Strategies for assessing and managing the adverse events of
sorafenib and other targeted therapies in the treatment of renal
cell and hepatocellular carcinoma: recommendations from a European
nursing task group. Eur J Oncol Nurs. 16:172–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hutson TE, Figlin RA, Kuhn JG and Motzer
RJ: Targeted therapies for metastatic renal cell carcinoma: an
overview of toxicity and dosing strategies. Oncologist.
13:1084–1096. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Brien BE, Kaklamani VG and Benson AB
III: The assessment and management of cancer treatment-related
diarrhea. Clin Colorectal Cancer. 4:375–381; discussion 382–373.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bellmunt J, Eisen T, Fishman M and Quinn
D: Experience with sorafenib and adverse event management. Crit Rev
Oncol Hematol. 78:24–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
La Vine DB, Coleman TA, Davis CH,
Carbonell CE and Davis WB: Frequent dose interruptions are required
for patients receiving oral kinase inhibitor therapy for advanced
renal cell carcinoma. Am J Clin Oncol. 33:217–220. 2010.PubMed/NCBI
|
|
32
|
Dutcher JP, Tannir N, Bellmunt J and
Escudier B: Experience with sorafenib and the elderly patient. Med
Oncol. 27:1359–1370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Benson AB III, Ajani JA, Catalano RB, et
al: Recommended guidelines for the treatment of cancer
treatment-induced diarrhea. J Clin Oncol. 22:2918–2926. 2004.
View Article : Google Scholar : PubMed/NCBI
|