Introduction
Parkinson's disease (PD) is a common degenerative
disease in the nervous system. The neuropathological
characteristics of the disease include the progressive pallidal
degeneration of dopaminergic neurons in the pars compacta of the
substantia nigra, and the formation of intracellular inclusion
bodies known as Lewy bodies (LBs) (1). It is believed that the nerve cell death
in PD is induced by the change in the protein conformation of
α-synuclein to form amyloid filaments, resulting in abnormal
aggregation (2–5). Furthermore, studies of transgenic mice
and Drosophila models have demonstrated that the formation
of α-synuclein inclusion bodies is associated with the degeneration
of the nervous system (6,7), and that proteasomal dysfunction may
cause the formation of the α-synuclein protein aggregates and
inclusion bodies. The proteasomal inhibitor lactacystin has been
shown to be able to induce the death of PC12 cells and the
formation of cell inclusion bodies (8). Previous studies have demonstrated that
systematic drug administration can result in behavioral changes
similar to those of PD in mice; furthermore, the damage to the
central nervous system is strikingly similar to that in patients
with PD (9,10).
Protein degradation in the cell can also occur
through the lysosomal pathway; however, few studies have
investigated the association between the lysosomal pathway and PD,
the effect of the lysosomal and ubiquitin-proteasome pathways on
α-synuclein protein degradation or the correlation between the
pathways. The aim of the present study, therefore, was to observe
the effect of the lysosomal and proteasomal inhibitors
trans-epoxysuccinyl-L-leucylamido-(4-guanidino) butane (E64) and
lactacystin, respectively, on α-synuclein protein degradation, and
to explore the effect of lysosomal pathway degradation on
proteasomal pathway degradation. Furthermore, the apoptotic status
of the inclusion body-positive cells was evaluated in order to
elucidate the association between the inhibition of the lysosomal
and proteasomal pathways and the death of dopaminergic neurons, and
to provide an experimental and theoretical foundation for the
pathogenesis of PD.
Materials and methods
Cell culture
In this study, a rat pheochromocytoma cell line
(PC12) was provided by the China Center for Type Culture Collection
(Wuhan University, Wuhan, China). The cells were placed into
Dulbecco's modified Eagle's medium (Gibco-BRL, Grand Island, NY,
USA) containing 10% inactivated calf serum (Gibco-BRL), 5% horse
serum (Gibco-BRL), penicillin (100 U/ml) and streptomycin (100
U/ml), and cultured in a Forma™ CO2 cell incubator
(3195/N; Thermo Fisher Scientific Inc., Waltham, MA, USA) with 5%
CO2 and at 37°C. The culture medium was renewed every
two days. Nerve growth factor (NGF; BeiDaZhiLu Biological
Engineering Co., Ltd., Xiamen, China) at a final concentration of
50 ng/ml was used to induce the neuronal differentiation of the
PC12 cells one week prior to the drug treatment, and the
morphological changes prior and subsequent to the PC12 cell
induction were observed using an inverted phase contrast microscope
(Olympus Corp., Tokyo, Japan). On the day of medication,
lactacystin and E64 (Sigma-Aldrich) were added to 1 and 10 mmol/l
culture medium, respectively. The final concentrations of
lactacystin were 5, 10 and 20 µmol/l, while those of E64 were and
2, 20 and 200 µmol/l.
MTT assay
The PC12 cell density was regulated to
2×105/ml, and the cells were inoculated into a 96-well
tissue culture plate with 100 µl in each well. After 24 h,
different concentrations of lactacystin (5, 10 and 20 µmol/l), E64
(2, 20 and 200 µmol/l) and lactacystin plus E64 (5+2, 10+20 and
20+200 µmol/l) were added, respectively, and left to react for 24
h. Six wells were assigned to each treatment, and parallel control
wells without treatment reagent were also established. A total of
10 µl MTT (5 mg/l) (Sigma-Aldrich, St. Louis, MO, USA) was added to
each well after 24 h, and the cells were cultured for an additional
4 h. The culture medium was subsequently removed and 100 µl
dimethyl sulfoxide (Sigma-Aldrich) was added to each well and mixed
through oscillation. A microplate reader (Bio-Rad, Hercules, CA,
USA) was used to test the optical density (OD) value of each well
at a wavelength of 570 nm. The A570 OD value was
considered to represent the viability of the PC12 cells. All
experiments were repeated at least three times.
Phospholipid binding protein
(Annexin-V)-propidium iodide (PI) double-staining method
The PC12 cells were treated with lactacystin (20
µmol/l), E64 (200 µmol/l) and lactacystin plus E64 (20+200 µmol/l),
respectively, for 24 h, prior to being collected and digested with
0.14 g/l EDTA. The cells were then washed with precooled
phosphate-buffered saline (PBS) at 4°C to generate a single cell
suspension. The cell density was adjusted to 5×105/ml,
and then 100 µl cell suspension was removed and supplemented with 5
µl Annexin-V/fluorescein isothiocyanate (FITC) and 10 µl PI at 20
µg/ml. PBS (400 µl) was added after 15 min incubation in the dark
at room temperature, and the fluorescence intensity and rates of
early cell apoptosis were detected via flow cytometry using the
FACSCalibur™ system (BD Biosciences, Franklin Lakes, NJ, USA).
Immunofluorescence method
The PC12 cells were inoculated into a 24-well tissue
culture plate with cover glass (poly-L-lysine pretreatment). When
the cells reached the logarithmic growth phase, lactacystin (20
µmol/l), E64 (200 µmol/l) and lactacystin plus E64 (20+200 µmol/l)
were added, respectively, and a control group was additionally
established. After 24 h of incubation, the PC12 cells were
immobilized by ice-cold acetone/absolute ethyl alcohol (1:1) for 10
min; 2% Triton X-100-PBS was then added at room temperature for 20
min and the cells were incubated with 0.1% thioflavin S for a
further 10 min. Following incubation, the cells were transferred to
80% alcohol for differentiation for 5 min, oscillated and washed.
Bovine serum albumin (3%) was added for an additional 30 min of
incubation at room temperature, excess liquid was extracted and
goat polyclonal anti-rat α-synuclein protein antibody (1:100;
sc-7012; Santa Cruz Biotechnology, Inc., Santa-Cruz, CA, USA) was
added for incubation at 4°C for 24 h. In the negative control
group, the goat anti-rat α-synuclein protein antibody was replaced
by PBS. Following incubation with the primary antibody,
tetramethylrhodamineisothiocyanate (TRITC; Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China)-labeled rabbit
anti-goat fluorescent secondary antibody (1:100; Santa Cruz
Biotechnology, Inc.) was added and incubated for 90 min at room
temperature. A total of 500 µl Hoechst 33258 stain (2 µg/ml) was
added to each well, and the cells were incubated in the dark for 15
min at room temperature to facilitate the development of the
double-staining of the cell nucleus. The PC12 cells were then
washed three times with PBS for 5 min each time. A fluorescence
microscope and Image-J (version 1.43 h) image processing system
(Olympus Corp., Tokyo, Japan) were utilized to observe the
formation of α-synuclein protein- and thioflavin S-positive
inclusion bodies within the cytoplasm and the morphological
variations in the nuclear chromatin during apoptosis. Each
treatment had four wells and each experiment was repeated three
times.
Statistical analysis
SPSS software, version 16.0 (SPSS Inc., Chicago, IL,
USA) was used to perform the statistical analysis. All data are
expressed as the mean ± standard deviation. Comparisons of the data
between each treatment group and the control group were conducted
through the t-test or rank sum test. A homogeneity of variance test
was implemented prior to the comparison of different groups;
analysis of variance was applied when homogeneity was observed,
while the rank sum test was applied when there was no homogeneity.
The Student-Newman-Keuls method was applied to compare the
difference between each group, and P<005 was considered to
indicate a statistically significant difference.
Results
Morphological changes prior and
subsequent to the NGF induction
As shown in Fig. 1,
the PC12 cells had a regular shape (circular or oval) prior to the
induction, with each cell exhibiting few bulges and a short length.
Intercellular connections were rare. Following the induction, the
cells became irregularly shaped, with a polygonal or spindle
outline. In addition, more bulges appeared, the cells became longer
and intercellular connections became more common.
Effect of lactacystin and E64 on the
activity and metabolic status of the cells
The A570 value of the control group was
0.618±0.055. As the lactacystin was administered in increasing
concentrations, the viability of the cells decreased steadily,
exhibiting a dose-response association (Fig. 2). An evident dose-response
association was also observed for E64, with the viability of the
cells decreasing markedly following the administration of
increasing concentrations of E64. Notably, the OD values of the
lactacystin plus E64 treatment groups showed more marked reductions
than those in the lactacystin or E64 groups, indicating that the
viability of the cells in the lactacystin plus E64 treatment groups
had decreased considerably.
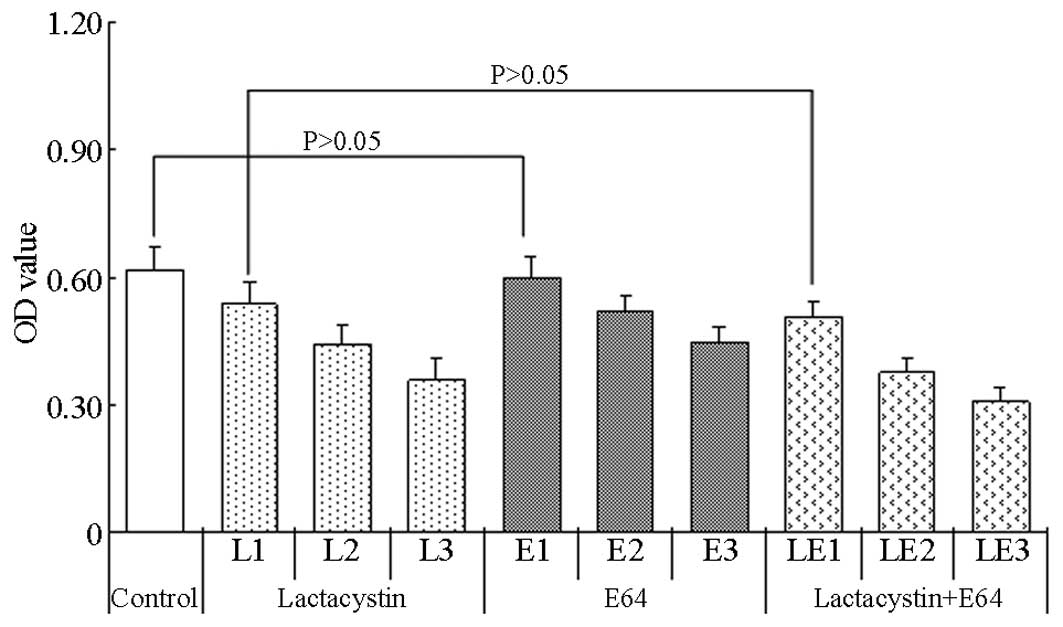 | Figure 2.Viability of PC12 cells after 4 h of
MTT treatment and the administration of pathway inhibitors at
different concentrations. The cells were divided into the
lactacystin (concentrations for L1, L2 and L3 were 5, 10 and 20
µmol/l, respectively), E64 (concentrations for E1, E2 and E3 were
2, 20 and 200 µmol/l, respectively) and lactacystin plus E64
(concentrations for LE1, LE2 and LE3 were 5+2, 10+20, 20+200
µmol/l, respectively) groups. Results are presented as the mean ±
standard deviation. OD, optical density; E64,
trans-epoxysuccinyl-L-leucylamido-(4-guanidino) butane. |
Statistical analysis showed that the differences in
the OD values at each concentration between the lactacystin and
lactacystin plus E64 groups, as well as between the E64 and
lactacystin plus E64 groups, were significant (P<0.05), with the
exception of the comparison between the 5 µmol/l lactacystin and
the 5 µmol/l lactacystin plus 2 µmol/l E64 groups (P>0.05). In
comparison with the OD values of the control group, significant
differences were found in the values of the lactacystin, E64 and
lactacystin plus E64 groups at all concentrations (P<0.05), with
the exception of the comparison between the OD values of the 2
µmol/l E64 and control groups (P>0.05).
Effect of lactacystin, E64 and
lactacystin plus E64 on apoptosis and necrosis in the PC12
cells
Live cells exhibited no FITC and PI staining
(FITC−, PI−), as shown by the lower-left cell
cluster in the analysis charts. Apoptotic cells were negative for
PI and highly stained by FITC (FITC+, PI−),
as shown by the lower-right cell cluster in the analysis charts.
Necrotic cells were highly stained by PI and FITC
(FITC+, PI+), as shown by the upper-right
cell cluster in the analysis charts (Table I and Fig.
3).
 | Table I.Rates of apoptosis and necrosis
following the treatment of PC12 cells with lactacystin, E64 and
lactacystin plus E64, respectively, for 24 h. |
Table I.
Rates of apoptosis and necrosis
following the treatment of PC12 cells with lactacystin, E64 and
lactacystin plus E64, respectively, for 24 h.
| Group | Apoptotic cell death
(%) | t-value | P-value | Necrotic cell death
(%) | t-value | P-value |
|---|
| Lactacystin |
38.09±1.71 | 10.34 | <0.01 |
1.78±0.46 | 0.97 | >0.05 |
| E64 |
29.05±0.77 | 8.72 | <0.01 |
6.76±0.51 | 6.39 | <0.01 |
| Lactacystin +
E64 |
44.36±1.19 | 12.03 | <0.01 |
7.15±0.87 | 7.03 | <0.01 |
| Control |
0.79±0.55 | – | – |
1.01±0.36 | – | – |
Rates of α-synuclein protein and
thioflavin S double-stained cells and apoptosis in the
double-stained cells after 24 h of lactacystin, E64 and lactacystin
plus E64 treatment in PC12 cells
In the control group, few PC12 cells exhibited
α-synuclein protein and thioflavin S double-positive inclusion
bodies (3.78±0.40%). After 24 h of 20 µmol/l lactacystin treatment,
however, the number of cells that were positive for inclusion
bodies was increased to 20.33±2.4%; after 24 h of 200 µmol/l E64
treatment, the number of inclusion body-positive cells was also
increased (7.94±0.97). The greatest increase in the number of
inclusion body-positive cells was observed in the lactacystin plus
E64 treatment group (36.77±3.5%). Comparisons with the control
group showed that the differences were all significant (P<0.05)
(Table II).
 | Table II.Rates of α-synuclein protein and
thioflavin S double-stained cells and apoptosis in the
double-stained cells after 24 h of lactacystin, E64 and lactacystin
plus E64 treatment, respectively, in PC12 cells. |
Table II.
Rates of α-synuclein protein and
thioflavin S double-stained cells and apoptosis in the
double-stained cells after 24 h of lactacystin, E64 and lactacystin
plus E64 treatment, respectively, in PC12 cells.
| Group | Inclusion-harboring
cells (%) | Apoptotic
inclusion-harboring cells (%) |
|---|
| Lactacystin |
20.33±2.40a |
16.42±4.46 |
| E64 |
7.94±0.97a |
18.75±4.42 |
| Lactacystin +
E64 |
36.77±3.50a, b |
18.33±6.04 |
| Control |
3.78±0.40 |
16.67±5.56 |
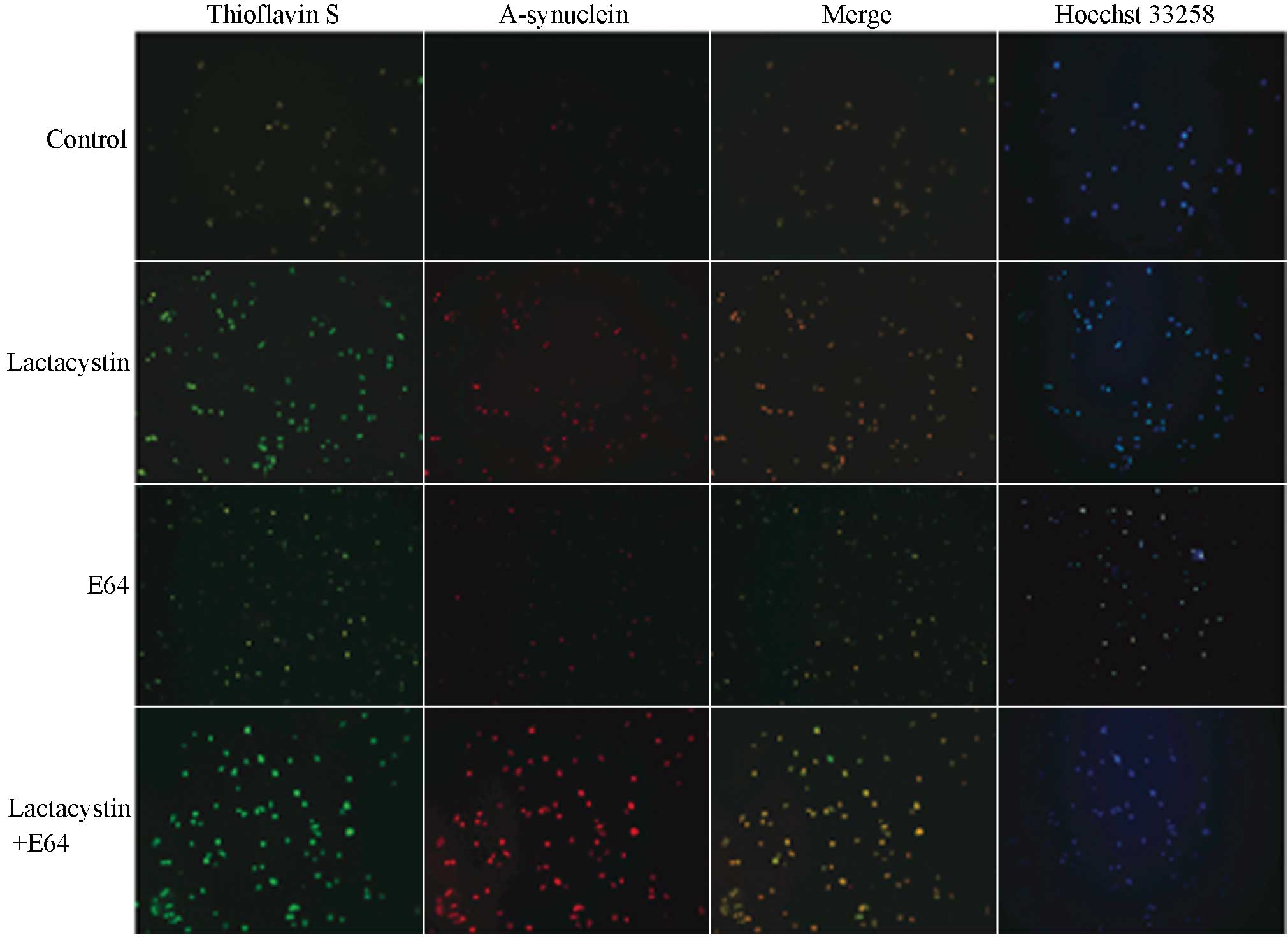
Thioflavin S staining is used to indicate the
presence of amyloids; the protein in its normal state or small
protein aggregates cannot undergo combination with thioflavin S
(11). When the protein conformation
changes, large clumps can combine with thioflavin S, and the
combined complex can be stimulated to emit green fluorescence under
the blue light of the fluorescence microscope. By comparison, the
α-synuclein that has combined with the TRITC can emit red
fluorescence under the fluorescence microscope. Fig. 4 shows the staining as observed under
the fluorescence microscope. Where double-staining of α-synuclein
protein and thioflavin S occurred, the PC12 cells appeared yellow
or orange. The nuclei of normal cells stained by Hoechst 33258
appeared even and hazy blue, while in the apoptotic cells, the
chromatin appeared in clusters, the size of particles was different
and the blue was brighter; these cells therefore exhibited that the
morphological characteristics of chromosome condensation. The
microscopic observations indicated that the double-positive cells
showed an apoptotic tendency (17.29±1.54%); however, the
differences in the apoptotic states between the control and
treatment groups were not significant (P>0.05).
Discussion
In 1988, Maroteaux et al (12) isolated a 143-amino acid protein from
Torpedo electric organ cholinergic nerve terminals and named it
synuclein. Following the discovery of synuclein came the
identification of its protein homologs, and synuclein was renamed
α-synuclein (13). At the end of the
20th century, it was found that certain patients with familial PD
exhibited an α-synuclein gene mutation, which incited interest into
the study of α-synuclein (5). It has
subsequently been found that, whether in familial or sporadic PD,
the aggregation of abnormal α-synuclein forms LBs and that this
abnormal α-synuclein aggregation in the neurons of the substantia
nigra constitutes a pathological morphological marker for PD
(14).
In normal conditions, α-synuclein has an unordered
and unfolded structure; however, under high concentrations or
conditions conducive to abnormal modifications the protein
undergoes a conformational change into fibers rich in β-sheets,
which form oligomers and exhibit cytotoxicity (15,16).
Since it is believed that α-synuclein aggregation could play an
important role in the occurrence and development of PD, it is
crucial to elucidate the mechanism underling the α-synuclein
aggregation in cells in order to facilitate the understanding of PD
pathogenesis.
There are two principal pathways of cellular protein
degradation: The ubiquitin-proteasome pathway and the lysosomal
pathway (17). The
ubiquitin-proteasome pathway involves substrate ubiquitination and
is an orderly process mediated by a series of enzymes. Ubiquitin is
first activated by the ubiquitin-activating enzyme E1, and then
transferred directly to the ubiquitin-carrier enzyme E2, which acts
to conjugate ubiquitin to the ε-amino group of the lysine residues
in the substrate protein. E2 can ubiquitinate the substrate
directly or in conjunction with a ubiquitin ligase. In this way,
through a cascade of enzymatic reactions, ubiquitin C-terminal
glycine residues covalently bond with the ε-amino group of the
lysine residues in the target protein. The ubiquitin molecules can
then form a polyubiquitin chain via the connection of lysine 48
residues, and this polyubiquitin chain is the activated signal of
protein degradation, which can be identified and degraded by the
26S proteasome. The ubiquitin and proteasome-mediated degradation
of specific proteins in cells underlies cell adjustment in
processes such as the cell cycle, cell apoptosis, pinocytosis,
immunization and the inflammatory reaction. At present, it is
believed that in certain harmful environments, such as under
conditions of oxidative or endoplasmic reticulum stress and in
protein misfolding in the ageing process, damaging abnormal protein
aggregation can be found in the cell. This abnormal protein
aggregation is initiated as a result of modifications in protein
synthesis, abnormal protein cleavage and the decreased capacity of
the cell to deal with the abnormal protein expression, and
ultimately leads to cell dysfunction or death (18).
Lysosomes are dynamic organelles that are surrounded
by a single membrane, often present as a cycle and contain
electron-dense material (19).
Lysosomes contain >50 species of hydrolase in an acid
environment, and acid phosphatase is the marker enzyme, which
controls the degradation of intracellular macromolecules, including
protein, lipid and cytoplasmic organelles. Lysosomes are known as
the cellular alimentary organs. According to the different stages
of physiological function, lysosomes can be divided into primary
and secondary lysosomes and residual bodies. It is generally
believed that proteins with a long half-life can be degraded by
lysosomes. In addition to acting as the alimentary organs in the
cell, lysosomes are associated with autocytolysis, cytophylaxis and
the usage of certain substances, such as antibodies and hormones.
Lysosomes degrade intracellular protein via the autophagy
mechanism. There are at least three different types of autophagy:
Macroautophagy, microautophagy and chaperone-mediated autophagy
(CMA). In macroautophagy, the soluble proteins and damaged
organelles within the cytoplasm are bundled into a double-membrane
structure of non-lysosomal origin, which is known as an
autophagosome; the autophagosome then brings the proteins to the
lysosome for degradation. Microautophagy differs from
macroautophagy in that the cytoplasmic substrates are directly
engulfed by the lysosome via the deformation of the lysosomal
membrane. By contrast, the process of CMA is selective for certain
proteins; these are transferred to the lysosomal membrane and then
translocated into the lysosomal lumen for hydrolase-mediated
degradation.
Webb et al (20) found that α-synuclein protein could
undergo degradation via two pathways: Proteasomal and lysosomal. A
previous study demonstrated that there is a molecular switch in the
body called E3 ubiquitin-protein ligase CHIP, which controls the
proteasomal and lysosomal pathways (21). CHIP is a component of LBs in the
human brain and co-localizes with α-synuclein and heat shock 70 kDa
protein. The structural domains of CHIP facilitate the mediation of
α-synuclein degradation through the proteasomal pathway (via its
tetratricopeptide repeat structural domain) and through the
lysosomal pathway (via its U-box domain). Numerous studies have
demonstrated that proteasome inhibitors are able to induce
dopaminergic neuronal cell death and the formation of intracellular
inclusion bodies (22,23). It has also been found that the
soluble oligomeric formation of α-synuclein protein can be degraded
by the lysosomal pathway (24), and
may be associated with CMA (25,26).
Lactacystin is a metabolite of Streptomyces
and is hydrolyzed in the body to produce an activated intermediate
product, β-lactone. The activity of the 20S subunit of the
proteasomes can be exclusively inhibited by binding between the
proteasome proteins and the 20S β-subunit (27). E64, a specific inhibitor of
lysosomes, inhibits the activity of cysteine proteinases in the
lysosome and affects the degradation of the substrate (28).
PC12 cells are positive in the tyrosine hydroxylase
(TH) immune response, have the ability to compose and excrete
dopamine and can undergo differentiation into a neuronal phenotype
following induction by NGF. In the present study, NGF was utilized
to induce the PC12 cells. As a result, the cell body of the PC12
cells became enlarged, the cells became polygonal, the adherent
ability of the cells was enhanced and cell growth was attenuated.
Consequently, these NGF-induced PC12 cells were used as the ideal
neuronal dopaminergic cell model to study the pathogenesis of
PD.
The present study demonstrated that, when the
proteasomal and lysosomal pathway inhibitors lactacystin and E64
were incubated with the PC12 cells independently or in combination,
the activity and metabolism of the PC12 cells were notably
decreased, while the rate of apoptosis was increased. This
phenomenon was more evident when lactacystin and E64 were incubated
with PC12 cells in combination. According to the study results, the
dysfunction of the proteasomal or lysosomal pathway damages the
cell, and the effect of the pathway damage is synergistic.
In this study, it was observed that a low level of
α-synuclein protein aggregation occurred following the
administration of the lysosomal inhibitor, while marked aggregation
occurred with the proteasomal inhibitor. The highest level of
aggregation occurred when the lysosomal and proteasomal inhibitors
were applied in combination. A recent study has shown that the half
life of the α-synuclein protein in the monomeric form is shorter
than that of the protein in the aggregated form. In mammalian
cells, the short half-life protein is degraded by the proteasomal
pathway and the long half-life protein is degraded by the lysosomal
pathway (29).
According to the results of the present study, we
speculated that the selectivity of the α-synuclein protein
degradation pathways could be associated with the half-life and
form of the protein; under normal circumstance, those proteins with
a short half-life existing in a soluble monomeric form would be
degraded by the ubiquitin-proteasome system, while those proteins
with a long half-life existing in an aggregated form would be
degraded by a lysosomal pathway, in interdependent and mutual
processes. Following damage to one or both of these pathways, or
when the levels of α-synuclein protein exceed the degradation
ability of the pathways, α-synuclein protein would not be able to
undergo timely degradation, and the accumulation of the α-synuclein
protein would cause toxicity and lead to the degeneration and death
of dopaminergic neurons. The mechanism of cell death could be
associated with apoptosis, since the study results indicated that
17.29% of the α-synuclein protein and thioflavin S double-positive
cells were apoptotic. This indicated that the α-synuclein inclusion
bodies had a toxic effect on the cells, which led to cell apoptosis
(30). Due to the apoptotic cell
death evidenced in the substantia nigra of patients with PD
(31,32), it is reasonable to speculate that the
inclusion bodies formed from accumulated α-synuclein may lead to
the death of dopaminergic neurons through the apoptotic pathway. In
addition, it was suggested in the present study that dysfunction of
the proteasomal and lysosomal pathways could play an important role
in dopaminergic neuron degeneration and the process of protein
accumulation and inclusion body formation. α-synuclein linked the
heredity and the environmental factors of PD.
The association between α-synuclein degradation and
the formation of LBs, as well as the understanding that α-synuclein
accumulation may be involved in the development of PD, may provide
fresh insights to further understand the pathogenesis of PD. In
future studies, we aim to continually focus on exploring the
mechanism of α-synuclein degradation and accumulation, in order to
seek more effective methods to prevent the pathological
deposition.
References
|
1
|
Ghosh A, Roy A, Liu X, et al: Selective
inhibition of NF-kappaB activation prevents dopaminergic neuronal
loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci
USA. 104:18754–18759. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bennett MC: The role of alpha-synuclein in
neurodegenerative diseases. Pharmaco1 Ther. 105:311–331. 2005.
View Article : Google Scholar
|
|
3
|
Bisaglia M, Mammi S and Bubacco L:
Structural insights on physiological functions and pathological
effects and alpha-synuclein. FASEB J. 23:329–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nemani VM, Lu W, Berge V, et al: Increased
expression of alpha-synuclein reduces neurotransmitter release by
inhibiting synaptic vesicle reclustering after endocytosis. Neuron.
65:66–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu J, Kao SY, Lee FJ, Song W, Jin LW and
Yankner BA: Dopamine-dependent neurotoxicity of α-synuclein: A
mechanism for selective neurodegeneration in Parkinson disease. Nat
Med. 8:600–606. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamanka K, Saito Y, Yamamori T, Urano Y
and Noguchi N: 24(S)-hydroxycholesterol induces neuronal cell death
through necroptosis, a form of programmed necrosis. J Biol Chem.
286:24666–34673. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beyer K and Ariza A: Protein aggregation
mechanism in synucleinopathies: Commonalities and differences. J
Neuropathol Exp Neurol. 66:965–974. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu X, Zhang H, Zhang Y, et al:
Differential protein profile of PC12 cells exposed to proteasomal
inhibitor lactacystin. Neurosci Lett. 575:25–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ryu EJ, Angelastro JM and Greene LA:
Analysis of gene expression changes in a cellular model of
Parkinson disease. Neurobiol Dis. 18:54–74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McNaught KS, Shashidharan P, Perl DP,
Jenner P and Olanow CW: Aggresome-related biogenesis of Lewy
bodies. Eur J Neurosci. 16:2136–2148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pouplana S, Espargaro A, Galdeano C, et
al: Thioflavin-S staining of bacterial inclusion bodies for the
fast, simple, and inexpensive screening of amyloid aggregation
inhibitors. Curr Med Chem. 21:1152–1159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maroteaux L, Campanelli JT and Scheller
RH: Synuclein: A neuron-specific protein localized to the nucleus
and presynaptic nerve terminal. J Neurosci. 8:2804–2815.
1988.PubMed/NCBI
|
|
13
|
Jo E, McLaurin J, Yip CM, St George-Hyslop
P and Fraser PE: alpha-Synuclein membrane interactions and lipid
specificity. J Biol Chem. 275:34328–34334. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McNaught KS, Björklund LM, Belizaire R,
Isacson O, Jenner P and Olanow CW: Proteasome inhibition causes
nigral degeneration with inclusion bodies in rats. Neuroreport.
13:1437–1441. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Banerjee K, Munshi S, Sen O, Pramanik V,
Roy Mukherjee T and Chakrabarti S: Dopamine cytotoxicity involves
both oxidative and nonoxidative pathways in SH-SY5Y cells:
Potential role of alpha-synuclein overexpression and proteasomal
inhibition in the etiopathogenesis of Parkinson's disease.
Parkinsons Dis. 2014:8789352014.PubMed/NCBI
|
|
16
|
Bucciantini M, Giannoni E, Chiti F, et al:
Inherent toxicity of aggregates implies a common mechanism for
protein misfolding diseases. Nature. 416:507–511. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ciechanover A: The ubiquitin-proteasome
pathway: On protein death and cell life. EMBO J. 17:7151–7160.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Česen MH, Pegan K, Spes A and Turk B:
Lysosomal pathways to cell death and their therapeutic
applications. Exp Cell Res. 318:1245–1251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boya P and Kroemer G: Lysosomal membrane
permeabilization in cell death. Oncogene. 27:6434–6451. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Webb JL, Ravikumar B, Atkins J, Skepper JN
and Rubinsztein DC: Alpha-Synuclein is degraded by both autophagy
and the proteasome. J Biol Chem. 278:25009–25013. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shin Y, Klucken J, Patterson C, Hyman BT
and McLean PJ: The co-chaperone carboxyl terminus of
Hsp70-interacting protein (CHIP) mediates alpha-synuclein
degradation decisions between proteasomal and lysosomal pathways. J
Biol Chem. 280:23727–23734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lazzeri G, Lenzi P, Busceti CL, et al:
Mechanisms involved in the formation of dopamine-induced
intracellular bodies within striatal neurons. J Neurochem.
101:1414–1427. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeon SM, Cheon SM, Bae HR, Kim JW and Kim
SU: Selective susceptibility of human dopaminergic neural stem
cells to dopamine-induced apoptosis. Exp Neurobiol. 19:155–164.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee HJ, Khoshaghideh F, Patel S and Lee
SJ: Clearance of alpha-synuclein oligomeric intermediates via the
lysosomal degradation pathway. J Neurosci. 24:1888–1896. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cuervo AM, Stefanis L, Fredenburg R,
Lansbury PT and Sulzer D: Impaired degradation of mutant
alpha-synuclein by chaperone-mediated autophagy. Science.
305:1292–1295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eskelinen EL, Illert AL, Tanaka Y,
Schwarzmann G, Blanz J, Von Figura K and Saftig P: Role of LAMP-2
in lysosome biogenesis and autophagy. Mol Biol Cell. 13:3355–3368.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu W and Silverman RB: Stereospecific
total syntheses of proteasome inhibitor omuralide and lactacystin.
J Org Chem. 76:8287–8293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wijayanti MA, Sholikhah EN, Hadanu R,
Jumina J, Supargiyono S and Mustofa M: Additive in vitro
antiplasmodial effect of N-alkyl and N-benzyl-1,10-phenanthroline
derivatives and cysteine protease inhibitor e64. Malar Res Treat.
2010:5407862010.PubMed/NCBI
|
|
29
|
Mo JS, Yoon JH, Hong JA, et al:
Phosphorylation of nicastrin by SGK1 leads to its degradation
through lysosomal and proteasomal pathways. PLoS One. 7:e371112012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rideout HJ and Stefanis L: Proteasomal
inhibition-induced inclusion formation and death in cortical
neurons require transcription and ubiquitination. Mol Cell
Neurosci. 21:223–238. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anglade P, Vyas S, Javoy-Agid F, et al:
Apoptosis and autophagy in nigral neurons of patients with
Parkinson's disease. Histol Histopathol. 12:25–31. 1997.PubMed/NCBI
|
|
32
|
Tain LS, Chowdhury RB, Tao RN, et al:
Drosophila HtrA2 is dispensable for apoptosis but acts downstream
of PINK 1independently from Parkin. Cell Death Differ.
16:1118–1125. 2009. View Article : Google Scholar : PubMed/NCBI
|