Introduction
Drug-induced liver injury (DILI) has been associated
with ~1,000 types of drug (1) and is
the most common reason for regulatory actions concerning drugs.
DILI accounts for over half of the cases of acute liver failure,
with acetaminophen (APAP, 4-hydroxyacetanilide) being the principal
offending drug (2).
APAP is a widely used over-the-counter agent that
exhibits antipyretic and analgesic activity. The hospitalization
rate due to accidental and intentional APAP overdose has been
estimated to be >26,000 cases per year in the USA (3). APAP-induced hepatic damage has been
extensively investigated in mice as hepatic centrilobular necrosis
occurs within hours following APAP administration (4,5). In
addition to hepatotoxicity, APAP exerts a nephrotoxic effect that
may be mechanistically independent of the liver damage it induces.
In cases of nephrotoxicity, tubular cell loss is the characteristic
feature of acute and chronic renal failure (6). Phase I metabolism of APAP, which is
predominantly mediated by CYP2E1, produces toxic metabolites
(7).
Protein binding is the critical initiating event
underlying the cell death observed during APAP-induced liver
injury. The subsequent results of this binding process include
mitochondrial dysfunction, and oxidative stress and injury
(8). Hence, the detection of reduced
glutathione (GSH) serves as a useful marker of the injury cycle as
it indicates the degree of oxidative stress 4induced. Although
previous studies have suggested that binding to mitochondrial
proteins is a key process in liver injury, an improved mechanistic
understanding is required as other factors may also be involved
(9). These include immunomodulators,
for example, the toll-like receptors (TLRs).
TLRs are a family of transmembrane proteins that
represent the major pattern recognition receptors (PRRs). TLR-2 and
TLR-4 are extracellular TLRs with a wide range of potential
endogenous ligands, including heat shock proteins, high mobility
group box 1 and breakdown products of fibronectin, heparin sulfate
and hyaluronic acid (10). Binding
of these endoligands to TLR-2 and TLR-4 leads to the stimulation of
adaptor proteins, such as MYD88, TRIF, TRAF and NF-κB, followed by
increased cytokine and chemokine production (11). The portal vein drains blood from the
gastrointestinal tract and supplies 75–80% of the blood supply to
the liver. Although a constant inflow of gut-derived microbes to the
liver occurs, hepatic TLRs are not constantly activated. This high
threshold for the activation of liver TLRs is known as ʻtoleranceʼ.
Tolerance is associated with the low-level expression of TLRs and
signaling molecules, such as MD2 and MYD88 (12), in addition to the upregulation of
interleukin-1 receptor-associated kinase-M (13). Therapeutic manipulation of the
hepatic TLR system may be key to the development of novel
treatments for the management and treatment of chronic inflammatory
liver diseases. Various animal models (14) and phase III clinical trials (15) involving the use of small molecules,
such as lipid A and TAK-242 (a TLR-4 blocker/antagonist) have
indicated the beneficial effects of these agents in the management
of septic patients. Agents such as these may be of benefit in the
management of various liver diseases. Furthermore, Xu et al
(16) demonstrated that TLR-2 and
TLR-4 are major receptors for extracellular histone-mediated
sterile inflammation, tissue injury and death in a mouse model of
APAP. These results support the use of TLRs as therapeutic targets.
Therefore, the present study aimed to evaluate the role of
anti-TLR-4 agents as a potential therapy for APAP-induced organ
injury, which may act through immunomodulation and the mitigation
of APAP-induced inflammatory processes. Furthermore, the TLR-4
antagonist treatment was compared with the conventional APAP
therapy (N-acetyl cysteine, NAC) that reduces the oxidative stress
induced by APAP toxicity.
Materials and methods
Study design
The efficiency of TAK-242 (Invitrogen Life
Technologies, Carlsbad, CA, USA), a TLR-4 blocker, in the treatment
of APAP-induced injury was evaluated by histological and
biochemical analysis in a mouse model. Mice treated with TAK-242
exhibit downregulation of TLR-4 in liver and kidney tissue. The
model of APAP toxicity used in the present study has been verified
in previous studies (4,5), which indicated that centrilobular liver
damage was induced within a 4-h period post-treatment with APAP.
This was verified again in the Medical Experimental Research Center
(MERC) of Mansoura University (Mansoura, Egypt) prior to proceeding
with this study. Reagents were purchased from Sigma-Aldrich, St.
Louis, MO, USA, unless otherwise stated.
Animals
The present study was approved by the ethics
committee of Mansoura University. A total of 40 male, 5-week-old
C57BL/6 mice (Animal House, MERC, Mansoura University), weighing
16–20 g, were maintained at 21–23°C, with a humidity of 40–55% and
12 h light cycle (lights on 06:00–18:00). Mice were acclimatized
for a 7-day period prior to the initiation of any procedures. Mice
were fasted overnight, but had free access to water prior to the
experiments.
Mice were allocated at random into the following
groups (n=10 per group): Vehicle-treated/control (VEH);
APAP-treated (APAP); NAC-pretreated plus APAP (APAP + NAC); and
TAK-242-pretreated plus APAP (APAP + TAK) groups. Mice were fasted
as described above. Mice were injected intraperitoneally (ip) with
400 mg/kg APAP (26 mg/ml in water) or water (VEH) at 10:00 a.m. At
1 h prior to APAP or VEH injection (09:00 a.m.), mice were injected
ip with 1.25 mmol/kg NAC (204 mg/kg, 40 mg/ml pH 7 in water) or 1
mg/kg TAK-242. Plasma, and liver and kidney tissues were collected
4 h after APAP administration.
Animal assessment
Mice were examined for clinical manifestations of
liver failure, such as disturbed sensorium, by an experienced
researcher. Furthermore, following sacrifice by transcardial
perfusion, the sizes and weights of the mouse livers were compared
among groups.
Serum enzyme assays
Plasma samples were collected and stored at 4°C
until required for the measurement of the levels of alanine
transaminase (ALT), alanine aspartate (AST) and creatinine (ALT/AST
activity kit, Sigma; creatinine assay kit, Erba Mannheim, Mannheim,
Germany). The serum enzyme assay (Slim+ spectrophotometer, SEAC,
Florence, Italy) was conducted to ensure the development of liver
and kidney damage in all study groups.
Reduced glutathione (GSH)
determination
Tissue samples (200 mg) were homogenized in 500 µl
sulfosalicylic acid (0.5%) and adjusted to a volume of 1 ml. Total
GSH was determined using GSH reductase and NADPH-coupled reaction,
with 5,5′-dithiobis(2-nitrobenzoic acid), as previously described
(17). Values are expressed as
nmol/g tissue.
Histology
Segments of liver and kidney tissue were fixed in 15
ml neutral buffered formalin solution. Tissue samples were embedded
in Paraplast and processed as described previously (5). The tissue samples were sectioned to
~4-µm thickness and stained with hematoxylin and eosin (H&E).
The tissues were subsequently examined under a light microscope
(CX31RTSF; Olympus, Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± standard deviation.
Two groups of data were analyzed by Student's t-test. Three groups
of data were analyzed by analysis of variance with a Tukey post hoc
test. For all tests, P<0.05 was considered to indicate a
statistically significant difference.
Results
Animal assessment
Animals treated with APAP exhibited disturbed
sensorium and hepatomegaly. By contrast, mice treated with NAC or
TAK-242 exhibited improvements of these symptoms, which were more
marked in the APAP + TAK group. The APAP + TAK group mice displayed
improved normalization of liver size and weight compared with the
APAP and APAP + NAC groups. Although NAC treatment resulted in a
reduction in liver size, the APAP + NAC group mice exhibited
significant hepatomegaly compared with the APAP + TAK and VEH group
mice.
Serum enzyme assays
The results of ALT, AST and creatinine assays for
all groups are presented in Table I.
Animals treated with APAP exhibited a significant increase in the
serum levels of all three enzymes compared with those in the
control group. By contrast, the APAP + NAC and APAP + TAK groups
displayed normalized serum levels of creatinine, which were
comparable to the level in the VEH group and significantly reduced
compared with that in the APAP group. Furthermore, the APAP + NAC
and APAP + TAK groups presented with reduced levels of ALT and AST
compared with those in the APAP group. However, these levels
remained increased in comparison with those in the VEH group.
 | Table I.Serum enzymes assay in different
treatment groups. |
Table I.
Serum enzymes assay in different
treatment groups.
| Group | ALT (IU/l) | AST (IU/l) | Creatinine
(µmol/l) |
|---|
| VEH |
83.02±12.01 |
91.11±11.20 |
0.73±0.07 |
| APAP |
800.10±27.11a |
1320.41±32.02a |
3.21±0.20a |
| APAP + NAC |
452.23±15.03a,b |
789.72±22.10a,b |
0.42±0.03b |
| APAP + TAK |
588.33±23.02a,b |
757.32±33.13a,b |
0.42±0.04a,b |
GSH levels
Tables II and
III display the levels of GSH in
the liver and kidney tissues, respectively, of all study groups.
GSH levels were higher in the VEH group mice than in the other
groups. APAP treatment led to a significant reduction in GSH levels
in the liver and kidney tissues. The APAP + NAC and APAP + TAK
groups exhibited significant elevations in GSH levels in liver and
kidneys compared with those in the APAP group; however, their
levels remained reduced in comparison with those in the VEH group
mice.
 | Table II.Levels of the oxidative stress marker
GSH in the liver tissues of different treatment groups. |
Table II.
Levels of the oxidative stress marker
GSH in the liver tissues of different treatment groups.
| Group | GSH (nmol/g) |
|---|
| VEH |
38.91±5.02 |
| APAP |
1.25±0.04a |
| APAP + NAC |
12.26±0.27a,b |
| APAP + TAK |
12.85±0.31a,b |
 | Table III.Levels of the oxidative stress marker
GSH in the kidney tissues of different treatment groups. |
Table III.
Levels of the oxidative stress marker
GSH in the kidney tissues of different treatment groups.
| Group | GSH (nmol/g) |
|---|
| VEH |
15.92±0.81 |
| APAP |
5.91±0.34a |
| APAP + NAC |
11.30±0.66a,b |
| APAP + TAK |
13.89±0.71b |
Histology
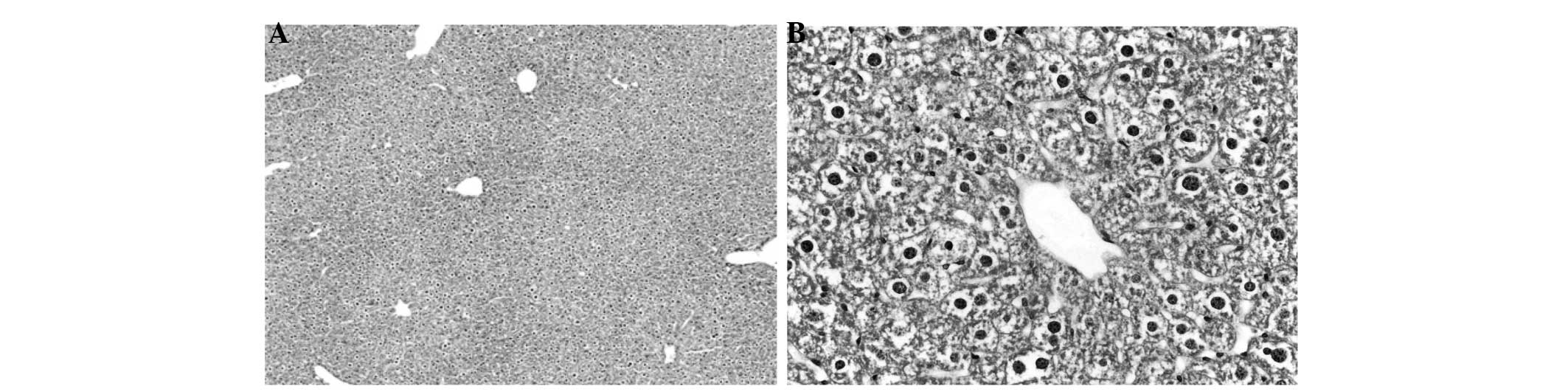
Figs. 1–4 display the results of H&E staining of
liver and kidney tissues. The mice in the APAP group exhibited
liver centrilobular hemorrhage, degeneration and necroinflammation,
with multiple foci of focal lytic necrosis with replacement by
inflammatory cells (Fig. 1). The
livers of the mice in the APAP + NAC and APAP + TAK groups
exhibited mild degenerative changes, with no hemorrhage or
necroinflammation (Figs. 2 and
3). Kidneys from the mice in the
APAP group presented with focal tubular cell detachment, necrosis
and apoptosis (Fig. 4A). However,
the kidneys of the mice in the APAP + NAC and APAP + TAK groups
displayed no evidence of tubular cell necrosis or apoptosis
(Fig. 4B and C).
Discussion
In the present study, the role of TLR-4 in
APAP-induced organ failure was investigated. This was conducted by
blocking TLR-4 using TAK-242 prior to treating the mice with a
toxic dose of APAP. Liver and kidney tissues were subsequently
harvested and analyzed using H&E histopathology. The results
indicated that blocking TLR-4 significantly protected the liver and
kidney tissues against APAP toxicity. This approach may provide an
alternative to the conventional NAC therapy for APAP, which
exhibits a number of limitations. First, NAC produces certain
side-effects (intractable vomiting, allergic reaction) in clinical
practice (18). Furthermore, NAC
therapy requires the administration of high doses of NAC over an
extended treatment period (5).
Blocking TLR-4 with the administration of a single dose may provide
a more reliable alternative to the prolonged NAC protocol. The
successful mitigation of APAP-induced hepato-renal toxicity by the
administration of a TLR-4 antagonist in the present study supports
the results of the study by Xu et al (16), which indicated a potential pathogenic
role of TLR-4 in APAP toxicity. The results of the present study
are consistent with the previous observations of Shah et al
(19), which demonstrated the
beneficial effects of TLR-4 blockade in an APAP toxicity model.
It is notable that the protective effects of
blocking TLR-4 were observed in both liver and kidney tissues. This
result underlines the crucial function of TLRs in various organs,
and their role in systemic conditions, such as septicemia and
toxicity (16). Furthermore, TLR-4
has been previously demonstrated to serve a key function in organ
crosstalk (20). Thus, TLR-4 may
protect tissues against APAP-induced nephrotoxicity that occurs
directly, for example via toxic metabolites attacking renal
targets, or indirectly through hepato-renal crosstalk.
The effect of TLR-4 blockade on oxidative stress was
also evaluated in the present study, as it is well established that
oxidative stress is involved in the pathogenesis of APAP-induced
toxicity (21). Notably, blocking
TLR-4 mitigated the effects of APAP-induced oxidative stress. The
association between oxidative stress and inflammation is readily
understood. Conditions that promote significant oxidative stress
may precipitate cellular death and extracellular matrix (ECM)
breakdown. Necrotic cells and damaged ECM in turn release various
intracellular and extracellular molecules, which function as
‘alarmins’, triggering inflammatory cascades following recognition
by PRRs (22). Furthermore,
oxidative stress conditions may induce various modifications within
lipids and proteins, generating so-called oxidation-specific
epitopes. These function as potent damage-associated molecular
patterns, and are able to trigger innate immune responses by
binding to multiple PRRs (23).
Hence, previous studies support the hypothesis that antioxidant
therapy may inhibit inflammatory processes by preventing the
initiation of this cycle. However, in the present study, the
reverse was observed; as the blocking of a proinflammatory agent,
i.e. TLR-4, led to a reduction in oxidative stress. Similar results
were obtained in a study of intestinal epithelial cells conducted
by Latorre et al (24).
Latorre et al observed that TLR-2, −3 and −4 activation may
induce pro-oxidant effects. As a result, it was hypothesized that
TLRs may possess pro-oxidant properties in addition to their
inherent innate immunity properties. Another study of the
association between TLR-4 and oxidative stress (25) indicated that the pro-oxidative
properties of these molecules are not associated with their innate
immunity modulatory effects. However, the mechanisms underlying
these properties remain unspecified and require further
evaluation.
In conclusion, the present study demonstrated the
protective effects of the TLR-4 blocker TAK-242 against acute APAP
toxicity in liver and kidney tissues. These effects were
demonstrated clinically, histologically and biochemically.
Furthermore, TAK-242 appeared to exert antioxidative effects in
addition to its anticipated anti-inflammatory effects.
Acknowledgements
The present study was funded by a grant from the
Medical Experimental Research Center of Mansoura University (no.
014-1).
Glossary
Abbreviations
Abbreviations:
|
APAP
|
acetaminophen
|
|
NAC
|
N-acetyl cysteine
|
|
TLR-4
|
toll-like receptor 4
|
|
GSH
|
reduced glutathione
|
References
|
1
|
Abboud G and Kaplowitz N: Drug-induced
liver injury. Drug Saf. 30:277–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tujios S and Fontana RJ: Mechanisms of
drug-induced liver injury: From bedside to bench. Nat Rev
Gastroenterol Hepatol. 8:202–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nourjah P, Ahmad SR, Karwoski C and Willy
M: Estimates of acetaminophen (Paracetomal)-associated overdoses in
the United States. Pharmacoepidemiol Drug Saf. 15:398–405. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitchell JR, Jollow DJ, Potter WZ,
Gillette JR and Brodie BB: Acetaminophen-induced hepatic necrosis.
IV. Protective role of glutathione. J Pharmacol Exp Ther.
187:211–217. 1973.PubMed/NCBI
|
|
5
|
Terneus MV, Kiningham KK, Carpenter AB,
Sullivan SB and Valentovic MA: Comparison of
S-adenosyl-L-methionine and N-acetylcysteine protective effects on
acetaminophen hepatic toxicity. J Pharmacol Exp Ther. 320:99–107.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cermik H, Taslipinar MY, Aydin I, et al:
The relationship between N-acetylcysteine, hyperbaric oxygen and
inflammation in a rat model of acetaminophen-induced
nephrotoxicity. Inflammation. 36:1145–1152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McGill MR and Jaeschke H: Metabolism and
disposition of acetaminophen: Recent advances in relation to
hepatotoxicity and diagnosis. Pharm Res. 30:2174–2187. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jaeschke H, McGill MR and Ramachandran A:
Oxidant stress, mitochondria and cell death mechanisms in
drug-induced liver injury: Lessons learned from acetaminophen
hepatotoxicity. Drug Metab Rev. 44:88–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jaeschke H and Bajt ML: Intracellular
signaling mechanisms of acetaminophen-induced liver cell death.
Toxicol Sci. 89:31–41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arslan F, Keogh B, McGuirk P and Parker
AE: TLR2 and TLR4 in ischemia reperfusion injury. Mediators
Inflamm. 2010:7042022010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zager RA, Johnson AC, Lund S and
Randolph-Habecker J: Toll like receptor (TLR4) shedding and
depletion: acute proximal tubular cell responses to hypoxic and
toxic injury. Am J Physiol. Renal Physiol. 292:F304–F312. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hayashi F, Smith KD, Ozinsky A, et al: The
innate immune response to bacterial flagellin is mediated by
Toll-like receptor 5. Nature. 410:1099–1103. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi K, Hernandez LD, Galán JE,
Janeway CA Jr, Medzhitov R and Flavell RA: IRAK-M is a negative
regulator of Toll-like receptor signaling. Cell. 110:191–202. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Daubeuf B, Mathison J, Spiller S, et al:
TLR4/MD-2 monoclonal antibody therapy affords protection in
experimental models of septic shock. J Immunol. 179:6107–6114.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanzler H, Barrat FJ, Hessel EM and
Coffman RL: Therapeutic targeting of innate immunity with Toll-like
receptor agonists and antagonists. Nat Med. 13:552–559. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu J, Zhang X, Monestier M, Esmon NL and
Esmon CT: Extracellular histones are mediators of death through
TLR2 and TLR4 in mouse fatal liver injury. J Immunol.
187:2626–2631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Valentovic M, Terneus M, Harmon RC and
Carpenter AB: S-Adenosylmethionine (SAMe) attenuates acetaminophen
hepatotoxicity in C57BL/6 mice. Toxicol Lett. 154:165–174. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kao LW, Kirk MA, Furbee RB, Mehta NH,
Skinner JR and Brizendine EJ: What is the rate of adverse events
after oral N-acetylcysteine administered by the intravenous route
to patients with suspected acetaminophen poisoning? Ann. Emerg Med.
42:741–750. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shah N, Montes de Oca M, Jover-Cobos M, et
al: Role of toll-like receptor 4 in mediating multiorgan
dysfunction in mice with acetaminophen induced acute liver failure.
Liver Transpl. 19:751–761. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salama M, Farrag SM, Abulasrar SA, et al:
Up-regulation of TLR-4 in the brain after ischemic kidney-induced
encephalopathy in the rat. CNS Neurol Disord Drug Targets.
12:583–586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hartley DP, Kolaja KL, Reichard J and
Petersen DR: 4-Hydroxynonenal and malondialdehyde hepatic protein
adducts in rats treated with carbon tetrachloride: Immunochemical
detection and lobular localization. Toxicol Appl Pharmacol.
161:23–33. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan JK, Roth J, Oppenheim JJ, et al:
Alarmins: Awaiting a clinical response. J Clin Invest.
122:2711–2719. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lugrin J, Rosenblatt-Velin N, Parapanov R
and Liaudet L: The role of oxidative stress during inflammatory
processes. Biol Chem. 395:203–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Latorre E, Mendoza C, Layunta E, Alcalde
AI and Mesonero JE: TLR2, TLR3 and TLR4 activation specifically
alters the oxidative status of intestinal epithelial cells. Cell
Stress Chaperones. 19:289–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pierre N, Deldicque L, Barbé C, Naslain D,
Cani PD and Francaux M: Toll-like receptor 4 knockout mice are
protected against endoplasmic reticulum stress induced by a
high-fat diet. PLoS One. 8:e650612013. View Article : Google Scholar : PubMed/NCBI
|