Introduction
Acute kidney injury (AKI) is a common complication
in trauma due to hemorrhage. Despite advances in the provision of
hospital emergency procedures, the incidence of AKI is increasing
and remains an independent predictor of morbidity and mortality
(1–3). An impediment toward improving outcomes
has been the continued reliance on outdated and unreliable markers,
and there has long been a requirement for a marker that accurately
responds to changes in glomerular function
Cystatin C (CysC) is a 13-kDa endogenous cysteine
proteinase inhibitor that plays a major role in the intracellular
catabolism of various peptides and proteins (4). CysC is synthesized at a relatively
constant rate by all nucleated cells in the human body, and is then
released into the plasma. In excess of 99% of CysC undergoes
filtration by the glomeruli, prior to being reabsorbed by proximal
renal tubular epithelial cells and catabolized (5). Levels of CysC can therefore be used to
measure glomerular function. Furthermore, since an increased
presence of CysC in the urine may indicate damage to the tubular
epithelium, the protein has been proposed as an additional urine
biomarker for AKI (6).
Estimates for the glomerular filtration rate (GFR)
based on measurements of CysC have been suggested to be superior to
those based on serum creatinine (SCr) in selected patient
populations, such as the elderly and children, as well as
transplant, cirrhotic and hypertensive patients (7–10);
however, the results in cardiac surgery and critically ill patients
are inconclusive (11–15). Several studies have also shown that
serum CysC has a similar, and not necessarily superior, value to
SCr for the detection of AKI in the early stage of the disease.
Patients with traumatic hemorrhagic shock are highly susceptible to
the development of AKI, but little data are available regarding the
changes in CysC in patients with traumatic hemorrhagic shock. The
aim of the present study, therefore, was to investigate whether
CysC has a higher value than SCr and urea for use in monitoring
glomerular function in traumatic hemorrhagic shock.
Patients and methods
Subject population
For the experimental group, diagnostic serum samples
were obtained from 57 patients who were diagnosed with traumatic
hemorrhagic shock in the Emergency Center of the First Affiliated
Hospital of Xinjiang Medical University (Ürümqi, China). All
patients admitted to the Emergency Center between July 1, 2012 and
July 1, 2013 were included in the cohort, which comprised 41 men
and 16 women, aged 34.49±17.28 years. A total of 57 normal serum
samples were randomly collected from individuals during the same
period to form a control group. The control group also consisted of
41 men and 16 women, aged 36.24±15.22 years. All of the patients
and volunteers provided written informed consent, and the study was
approved by the University and Institutional Review Board (First
Affiliated Hospital of Xinjiang Medical University). No significant
differences in gender and age were found between the groups. The
initial values of CysC, SCr and urea in the patients with trauma
and normal subjects were measured.
Definitions
For inclusion in the experimental group, patients
had to exhibit at least one of the following injuries: Fracture to
the pelvis, two or more long bones or multiple ribs; pulmonary
contusion; or major blunt or penetrating trauma to the extremities
or torso (16). In addition,
patients had to have two systolic blood pressure measurements of
<90 mmHg or a base deficit of ≥6 mEq/l within 60 min of arrival
at the Emergency Center. All of the samples were collected within
the first 8 h after trauma. Patients who succumbed prior to
reaching the Emergency Center were excluded from the study.
Normal subjects were defined as those without the
following conditions: High blood pressure; diabetes; liver, kidney,
heart and infectious diseases; acute infection within the past
month; and any malignancy. AKI was defined as an SCr increase of
≥50% or ≥27 mmol/l within 48 h. SCr was measured using the isotope
dilution mass spectrometry traceable enzymatic method (Roche
Diagnostics, Mannheim, Germany), urea was measured using an
enzymatic kit (Roche) and CysC was measured by nephrometric assay
(Dade Behring, Pittsburgh, PA, USA) according to the manufacturer's
instructions.
Statistical analysis
Data are expressed as the mean ± standard deviation
and were analyzed using SPSS 17.0 statistical software (SPSS, Inc.,
Chicago, IL, USA). Comparisons between groups were made using the
t-test or one-way analysis of variance for continuous variables and
either the Fisher's exact or χ2 test for categorical
variables. Correlation analysis for quantitative data was performed
using Pearson's test. To investigate the diagnostic value of serum
CysC, SCr and urea as predictors of AKI, receiver operating
characteristic (ROC) curve analysis was performed; these data are
expressed as the area under the curve (AUC) and 95% confidence
interval (CI). Multivariate logistic regression analysis was used
to investigate the association between these values and mortality.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characteristics of the study
subjects
Table I shows the
characteristics of the experimental group subjects. The mean CysC
value of the female patients was lower compared with the male
patients (0.96±0.22 vs. 1.16±0.39 mg/l, respectively), but no
significant difference was found (P>0.05). The CysC level was
not significantly affected by age, mechanism of injury or time
between injury and arrival at the Emergency Center.
 | Table I.Characteristics of the study
subjects. |
Table I.
Characteristics of the study
subjects.
| Clinical
parameter | N | CysC (mg/l) | P-value |
|---|
| Gender |
|
| 0.066 |
| Male | 41 |
1.16±0.39 |
|
|
Female | 16 |
0.96±0.22 |
|
| Age (years) |
|
| 0.110 |
| 0 to
<20 | 10 |
1.04±0.23 |
|
| 20 to
<40 | 23 |
0.97±0.24 |
|
| 40 to
<60 | 20 |
1.26±0.48 |
|
| ≥60 | 4 |
1.27±0.33 |
|
| Mechanism of
injury |
|
| 0.865 |
|
Blunt | 41 |
1.09±0.38 |
|
|
Penetrating | 6 |
1.17±0.35 |
|
| Both | 10 |
1.12±0.34 |
|
| Time between injury
and arrival (h) |
|
| 0.623 |
| 0 to
<5 | 28 |
1.07±0.38 |
|
| 5–8 | 29 |
1.13±0.35 |
|
The CysC level of the patients with traumatic
hemorrhagic shock was significantly higher than that of the normal
subjects (1.10±0.36 vs. 0.91±0.34 mg/l). The SCr and urea levels in
the experimental group were also significantly increased compared
with those in the control group (Table
II).
 | Table II.Comparative analysis of levels of
CysC, SCr and urea between patients with traumatic hemorrhagic
shock and normal subjects. |
Table II.
Comparative analysis of levels of
CysC, SCr and urea between patients with traumatic hemorrhagic
shock and normal subjects.
| Parameter | Experimental
group | Control group | P-value |
|---|
| CysC (mg/l) |
1.10±0.36 |
0.91±0.34 | 0.004 |
| SCr (µmol/l) |
81.93±41.35 |
58.15±16.13 | <0.001 |
| Urea (mmol/l) |
5.06±1.95 |
3.89±1.07 | <0.001 |
Association between AKI and SCr, serum
CysC and urea
A total of 18 of the 57 patients with traumatic
hemorrhagic shock enrolled in the study had AKI (SCr increase of
≥50% or ≥27 mmol/l within 48 h). Fourteen of these 18 patients with
AKI had elevated SCr concentrations in the first 6 h of trauma,
whereas 13 had elevated serum CysC levels at that time. Only 10 of
the patients had elevated urea levels.
Nonparametric ROC plots of the sensitivity and
specificity of SCr, CysC and urea for the detection of AKI are
shown in Fig. 1. The AUCs for SCr,
CysC and urea were 0.901 (95% CI, 0.791–1.000), 0.728 (95% CI,
0.570–0.886) and 0.709 (95% CI, 0.552–0.865), respectively
(Table III and Fig. 1).
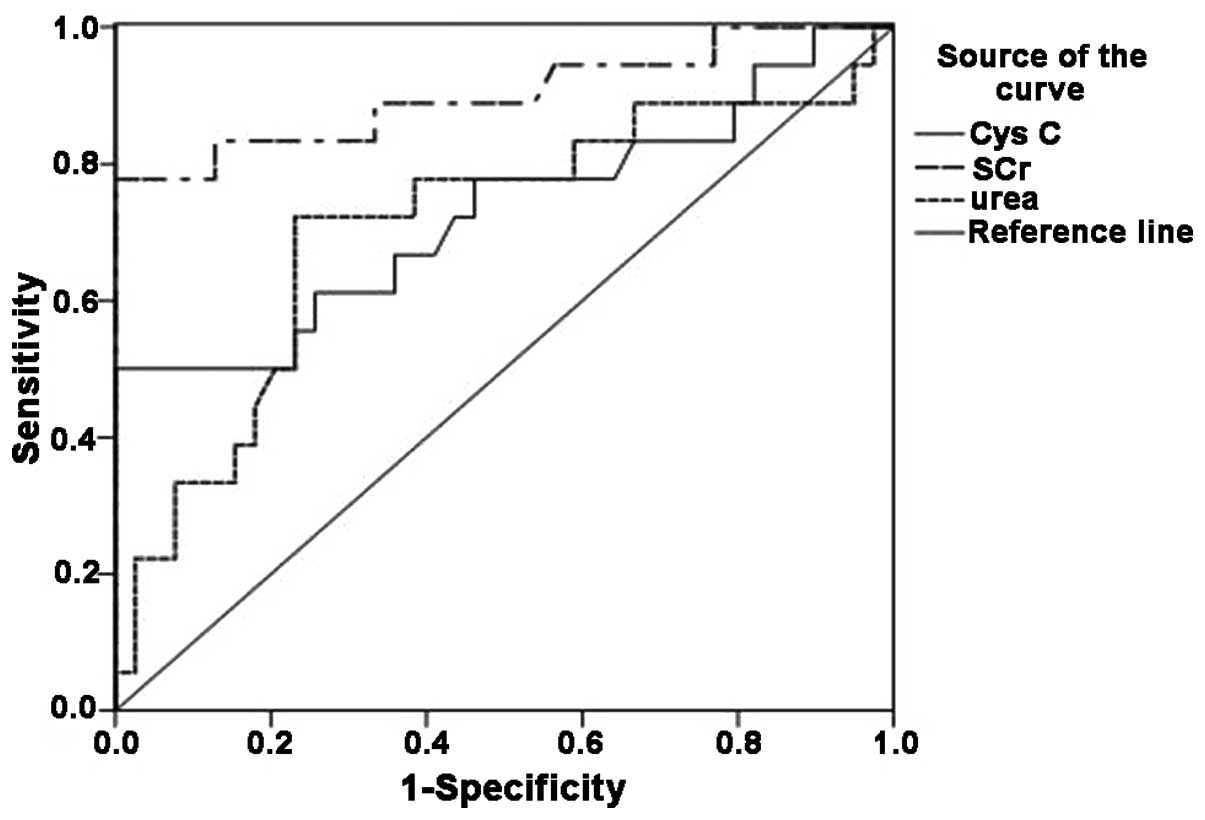 | Figure 1.Receiver operating characteristic
curve of CysC, SCr and urea. The area under the curve for the three
indicators was as follows: SCr, 0.901 (95% CI, 0.791–1.000); CysC,
0.728 (95% CI, 0.570–0.886); and urea, 0.709 (95% CI, 0.552–0.865).
CysC, cystatin C; SCr, serum creatinine; CI, confidence
interval. |
 | Table III.Area under the curve values for CysC,
SCr and urea. |
Table III.
Area under the curve values for CysC,
SCr and urea.
| Variable | Area | P-value | 95% CI |
|---|
| CysC | 0.728 | 0.006 | 0.570–0.886 |
| SCr | 0.901 | <0.001 | 0.791–1.000 |
| Urea | 0.709 | 0.012 | 0.552–0.865 |
Correlation analysis for serum
indicators
Pearson correlation analysis was used to evaluate
the correlations among the quantitative data. A significant
correlation was found between any two of CysC, SCr and urea
(P<0.01; Table IV).
 | Table IV.Pearson correlation analysis of CysC,
SCr and urea. |
Table IV.
Pearson correlation analysis of CysC,
SCr and urea.
| Parameter | CysC | SCr | Urea |
|---|
| CysC |
|
|
|
| Pearson
correlation | 1.000 | 0.457 | 0.369 |
|
P-value |
| 0.000a | 0.005a |
| SCr |
|
|
|
| Pearson
correlation | 0.457 | 1.000 | 0.439 |
|
P-value | 0.000a |
| 0.001a |
| Urea |
|
|
|
| Pearson
correlation | 0.369 | 0.439 | 1.000 |
|
P-value | 0.005a | 0.001a |
|
Association between serum indicators
and mortality
Six of the 57 patients enrolled in the study
succumbed. Using multivariate logistic regression analysis, the
association between mortality and the levels of CysC, SCr and urea
was determined in the study population. The three variables were
excluded from the multivariate model, and no significant
correlation was found between mortality and the levels of CysC, SCr
and urea.
Discussion
AKI is commonly encountered in cases of traumatic
hemorrhagic shock. The development of AKI is associated with poorer
clinical outcomes, including a prolonged period of hospitalization,
a dependence upon renal replacement therapy, the development of
chronic kidney disease and an increased risk of mortality (2,3,16).
Traditionally, the diagnosis of AKI has been
dependent upon the use of surrogate markers to detect abnormalities
in kidney function, such as elevations in SCr or urea. Although
considerable research has been performed in this area, the
diagnosis of AKI still relies on the same surrogates of kidney
function, which often are poor markers of early kidney injury;
therefore, the identification of novel kidney-specific biomarkers
for the early detection of AKI and kidney dysfunction is considered
to be a priority.
CysC is easy to measure and has been regarded as an
ideal marker of kidney function (17). Data suggest that CysC is modified by
gender, age, muscle mass, obesity, smoking status, thyroid
function, inflammation and malignancy (18). In the present study, the levels of
CysC detected were highest in male patients and patients aged
>40 years; however, the level of CysC was not significantly
associated with gender, age, mechanism of injury or time between
injury and arrival at the Emergency Center in the patients with
traumatic hemorrhagic shock.
Normal serum samples, collected during the same
period as that during which the patient samples were collected,
were used to form the control group; the two groups were matched in
terms of gender and age. The early stage of traumatic hemorrhagic
shock is associated with a decline in kidney function and, in the
present study, it was found that the levels of CysC, SCr and urea
in the patients with traumatic hemorrhagic shock were significantly
higher than those in the normal subjects.
The GFR in individuals suffering from critical
trauma can change rapidly, and changes in the SCr can be apparent
for several days until the stabilization phase is reached (19). In the present study, 18 out of the 57
(31.6%) enrolled subjects developed AKI and exhibited high SCr
levels within 48 h. This result was similar to findings in other
studies (20,21). The diagnostic utility of CysC (AUC,
0.728) observed in the present study was similar to that of urea
(AUC, 0.709); however, SCr (AUC, 0.901) remained the most suitable
marker for the prediction of AKI. Although previous reports have
suggested that CysC represents a better marker than SCr in several
fields, the present findings have shown the diagnostic utility of
CysC to be lower than that of SCr in the early stage of traumatic
hemorrhagic shock.
A strong correlation between any two of CysC, SCr
and urea was found in this study. This result was similar to
findings in other studies (22–24).
None of the serum indicators, however, showed a significant
correlation with mortality. Previous reports have found that
approximately one-third of patients with traumatic hemorrhagic
shock develop AKI (25–27). Hospital care should be adjusted
accordingly, but the incidence of AKI cannot be use to predict
mortality in cases of traumatic hemorrhagic shock.
In conclusion, the present data indicate that CysC
levels are increased significantly in the early stage of traumatic
hemorrhagic shock. CysC can be used as a marker to predict AKI, but
the diagnostic utility of CysC remains lower than that of SCr in
the early stage of traumatic hemorrhagic shock.
References
|
1
|
de Abreu KL, Silva Júnior GB, Barreto AG,
et al: Acute kidney injury after trauma: Prevalence, clinical
characteristics and RIFLE classification. Indian J Crit Care Med.
14:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bihorac A, Delano MJ, Schold JD, et al:
Incidence, clinical predictors, genomics and outcome of acute
kidney injury among trauma patients. Ann Surg. 252:158–165. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Costantini TW, Fraga G, Fortlage D, et al:
Redefining renal dysfunction in trauma: Implementation of the Acute
Kidney Injury Network staging system. J Trauma. 67:283–288. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldstein SL: A novel use for novel acute
kidney injury biomarkers: Fenoldopam's effect on neutrophil
gelatinase-associated lipocalin and cystatin C. Crit Care.
15:1772011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bagshaw SM and Bellomo R: Cystatin C in
acute kidney injury. Curr Opin Crit Care. 16:533–539. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soto K, Coelho S, Rodrigues B, et al:
Cystatin C as a marker of acute kidney injury in the emergency
department. Clin J Am Soc Nephrol. 5:1745–1754. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gerhardt T, Pöge U, Stoffel-Wagner B, et
al: Estimation of glomerular filtration rates after orthotopic
liver transplantation: Evaluation of cystatin C-based equations.
Liver Transpl. 12:1667–1672. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pöge U, Gerhardt T, Stoffel-Wagner B, et
al: Calculation of glomerular filtration rate based on cystatin C
in cirrhotic patients. Nephrol Dial Transplant. 21:660–664. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pöge U, Gerhardt T, Stoffel-Wagner B, et
al: Cystatin C-based calculation of glomerular filtration rate in
kidney transplant recipients. Kidney Int. 70:204–210. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodilla E, Costa JA, Pérez Lahiguera F,
González C, Miralles A and Pascual JM: Cystatin C and other
cardiovascular markers in hypertension. Med Clin (Barc). 130:1–5.
2008.(In Spanish). View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haase M, Bellomo R, Devarajan P, et al:
Novel biomarkers early predict the severity of acute kidney injury
after cardiac surgery in adults. Ann Thorac Surg. 88:124–130. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koyner JL, Bennett MR, Worcester EM, et
al: Urinary cystatin C as an early biomarker of acute kidney injury
following adult cardiothoracic surgery. Kidney Int. 74:1059–1069.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ristikankare A, Pöyhiä R, Kuitunen A, et
al: Serum cystatin C in elderly cardiac surgery patients. Ann
Thorac Surg. 89:689–694. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nejat M, Pickering JW, Walker RJ and Endre
ZH: Rapid detection of acute kidney injury by plasma cystatin C in
the intensive care unit. Nephrol Dial Transplant. 25:3283–3289.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perianayagam MC, Seabra VF, Tighiouart H,
Liangos O and Jaber BL: Serum cystatin C for prediction of dialysis
requirement or death in acute kidney injury: A comparative study.
Am J Kidney Dis. 54:1025–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morrison CA, Carrick MM, Norman MA, et al:
Hypotensive resuscitation strategy reduces transfusion requirements
and severe postoperative coagulopathy in trauma patients with
hemorrhagic shock: Preliminary results of a randomized controlled
trial. J Trauma. 70:652–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ortuno-Anderiz F, Cabello-Clotet N,
Vidart-Simon N, et al: Cystatin C as an early marker of acute
kidney injury in septic shock. Rev Clin Esp. 215:83–90.
2015.PubMed/NCBI
|
|
18
|
Bagshaw SM and Bellomo R: Cystatin C in
acute kidney injury. Curr Opin Crit Care. 16:533–539. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bihorac A, Baslanti TO, Cuenca AG, et al:
Acute kidney injury is associated with early cytokine changes after
trauma. J Trauma Acute Care Surg. 74:1005–1013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Abreu KL, Silva Junior GB, Barreto AG,
et al: Acute kidney injury after trauma: Prevalence, clinical
characteristics and RIFLE classification. Indian J Crit Care Med.
14:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Talving P, Karamanos E, Skiada D, Lam L,
Teixeira PG, Inaba K, Johnson J and Demetriades D: Relationship of
creatine kinase elevation and acute kidney injury in pediatric
trauma patients. J Trauma Acute Care Surg. 74:912–916. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hei ZQ, Li XY, Shen N, et al: Prognostic
values of serum cystatin C and beta2 microglobulin, urinary beta2
microglobulin and N-acetyl-beta-D-glucosaminidase in early acute
renal failure after liver transplantation. Chin Med J.
121:1251–1256. 2008.PubMed/NCBI
|
|
23
|
Liu J: Evaluation of serum cystatin C for
diagnosis of acute rejection after renal transplantation.
Transplant Proc. 44:1250–1253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lagos-Arevalo P, Palijan A, Vertullo L, et
al: Cystatin C in acute kidney injury diagnosis: early biomarker or
alternative to serum creatinine? Pediatr Nephrol. 30:665–676. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gomes E, Antunes R, Dias C, et al: Acute
kidney injury in severe trauma assessed by RIFLE criteria: A common
feature without implications on mortality? Scand J Trauma Resusc
Emerg Med. 18:12010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Podoll AS, Kozar R, Holcomb JB, et al:
Incidence and outcome of early acute kidney injury in
critically-ill trauma patients. PLoS One. 8:e773762013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Skinner DL, Hardcastle TC, Rodseth RN, et
al: The incidence and outcomes of acute kidney injury amongst
patients admitted to a level I trauma unit. Injury. 45:259–264.
2014. View Article : Google Scholar : PubMed/NCBI
|















