Introduction
Intracranial atherosclerosis (ICAS) is the most
common cause of ischemic stroke worldwide. To date, the incidence
of ICAS-induced stroke in China has exceeded those in other
countries and is the primary cause of mortality and disability in
adult patients (1). The risk of
stroke is associated with the degree of artery stenosis and the
stability of atherosclerotic plaques. However, only a small
proportion of stroke cases are a result of intracranial arterial
stenosis only. Magnetic resonance angiography (MRA) technology is
able to visualize cerebral arterial plaque composition, activity
characteristics and morphological alterations of blood vessels, in
addition to the corresponding changes of cerebral artery plaques in
cerebral infarction patients following clinical drug therapy.
Cerebral infarction may be caused by a number of factors, including
ICAS plaque rupture, hemorrhage, thrombogenesis and plaque
detachment (2,3). Thus, the early determination of ICAS,
particularly the early determination of plaques and the
vulnerability of plaques, is a key method for reducing the
incidence rate of cerebral infarction. In the present study,
qualitative analysis of ICAS plaques was conducted using 3.0 T
black-blood high-resolution magnetic resonance imaging (HRMRI),
with the aim to assess the clinical value of black-blood MRA and
the prevention of ICAS plaque formation.
Subjects and methods
Subjects
A total of 110 patients with cerebral infarction
were recruited from the outpatient and inpatient departments of the
Second Hospital of Hebei Medical University (Hebei, China) between
the 1st January 2012 and 1st December 2013.
The study population consisted of 63 male and 47 female patients,
with an average age of 58.6±5 years (range, 30–80 years).
Three-dimensional time-of-flight (3D-TOF) MRA, also known as
bright-blood technology, presented cerebral artery stenosis, local
vertebrobasilar stenosis or signal interruptions. Subsequently,
black-blood MRA was used to scan the abnormal blood vessels and to
observe whether plaques existed in the vessel walls. The MRI signal
characteristics of the plaques were observed and categorized. The
study was conducted in accordance with the Declaration of Helsinki
and with approval from the Ethics Committee of the Second Hospital
of Hebei Medical University. Written informed consent was obtained
from all the participants.
MRI examinations
A Signa Excite HD 3.0 T MRI scanner (GE Healthcare
Life Sciences, Bethesda, MD, USA) was employed in the study for all
imaging techniques, using the standard eight-channel orthogonal
array coil. For the black-blood MRA examinations, double inversion
recovery fast spin echo (DIR-FSE) T1-weighted imaging (T1WI;
fat-suppressed) was performed with a repetition time (TR) of 800
msec, an inversion time (TI) of 300 msec and an echo time (TE) set
at the minimum time at which a full echo was obtainable (min full).
DIR-FSE proton density-weighted imaging (PDWI) was performed with a
TR of 3,000 msec and a TE of min full. Furthermore, DIR-FSE
T2-weighted imaging (T2WI) was conducted with a TR of 3,000 msec
and a TE of 102 msec. The field of vision was 120×120 mm and the
array was 512×512, with a number of excitation (NEX) of three. For
the bright-blood imaging, the 3D-TOF scanning method was applied
with a TR of 29 msec and a TE of 2.1 msec.
Image analysis
In accordance with the revised American Heart
Association Pathological and Histological Classification (4), two senior neuroradiologists evaluated
the plaques in the lesion areas of the M1 section of the middle
cerebral artery and basilar artery using MRI classification
criteria, as described by Cai et al (2). The plaque characteristics, including
the type and vulnerability, were subsequently analyzed. The plaque
signal level was determined using the level in the adjacent brain
parenchyma as the reference, and was characterized as a high
signal, equisignal or low signal. The main components in the
analysis included the presence of a lipid core, calcification,
hemorrhage and fibrous caps.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 15.0 (SPSS Inc., Chicago, IL, USA). Categorical
variables were tested using the χ2 test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Detection of plaques
Bright-blood 3D-TOF magnetic resonance angiography
(MRA) identified 110 cases with abnormal cerebral vessels. However,
black-blood MRA identified 16 cases (14.5%) without an abnormality
or plaque in the cerebral artery lumen or walls, and 94 cases
(85.5%) with various types of cerebral artery plaques, as well as
lumen stenosis at varying degrees.
Classification of plaques
According to the MRI signal characteristics and
classification criteria of the plaques, an atherosclerotic plaque
was detected in 94 cases, which included four cases of types I and
II, 15 cases of type III, 26 cases of type IV and V, 23 cases of
type VI, 11 cases of type VII, 14 cases of type VIII and one case
of a mixed/complex plaque (Table
I).
 | Table I.Plaque classification, signal
characteristics of black-blood MRA and vascular wall condition. |
Table I.
Plaque classification, signal
characteristics of black-blood MRA and vascular wall condition.
|
|
|
|
| MRI signal
characteristics |
|---|
|
|
|
|
|
|
|---|
| Plaque type | Cases (n) | Ratio (%) | Wall
characteristics | T1WI (fat
saturation) | T2WI | PDW |
|---|
| I,II | 4 | 4.3 | No significant
thickening | Equisignal | Equisignal | Equisignal |
| III | 15 | 16.0 | Nonuniform thickening
and small plaque | Comparatively uniform
equisignal | Comparatively uniform
and high signal | Comparatively uniform
and high signal |
| IV,V | 26 | 27.7 | Nonuniform thickening
and eccentric plaque | Equisignal and
comparatively low signal | Equisignal and high
signal | Equisignal and high
signal |
| VI | 23 | 24.5 | Eccentric plaque | Equisignal and
comparatively high signal | Equisignal and
comparatively high signal | Equisignal and
comparatively high signal |
| VII | 11 | 11.7 | Eccentric plaque | Low signal | Low signal | Low signal |
| VIII | 14 | 14.9 | Nonuniform thickening
and eccentric plaque | Equisignal and low
signal | Equisignal and low
signal | Equisignal and low
signal |
| Mixedp laque | 1 | 1.1 | Irregular concentric
ring plaque | Equisignal and low
signal | Mixture of high,
equisignal and low signals | Mixture of high,
equisignal and low signals |
Incidence of plaque types
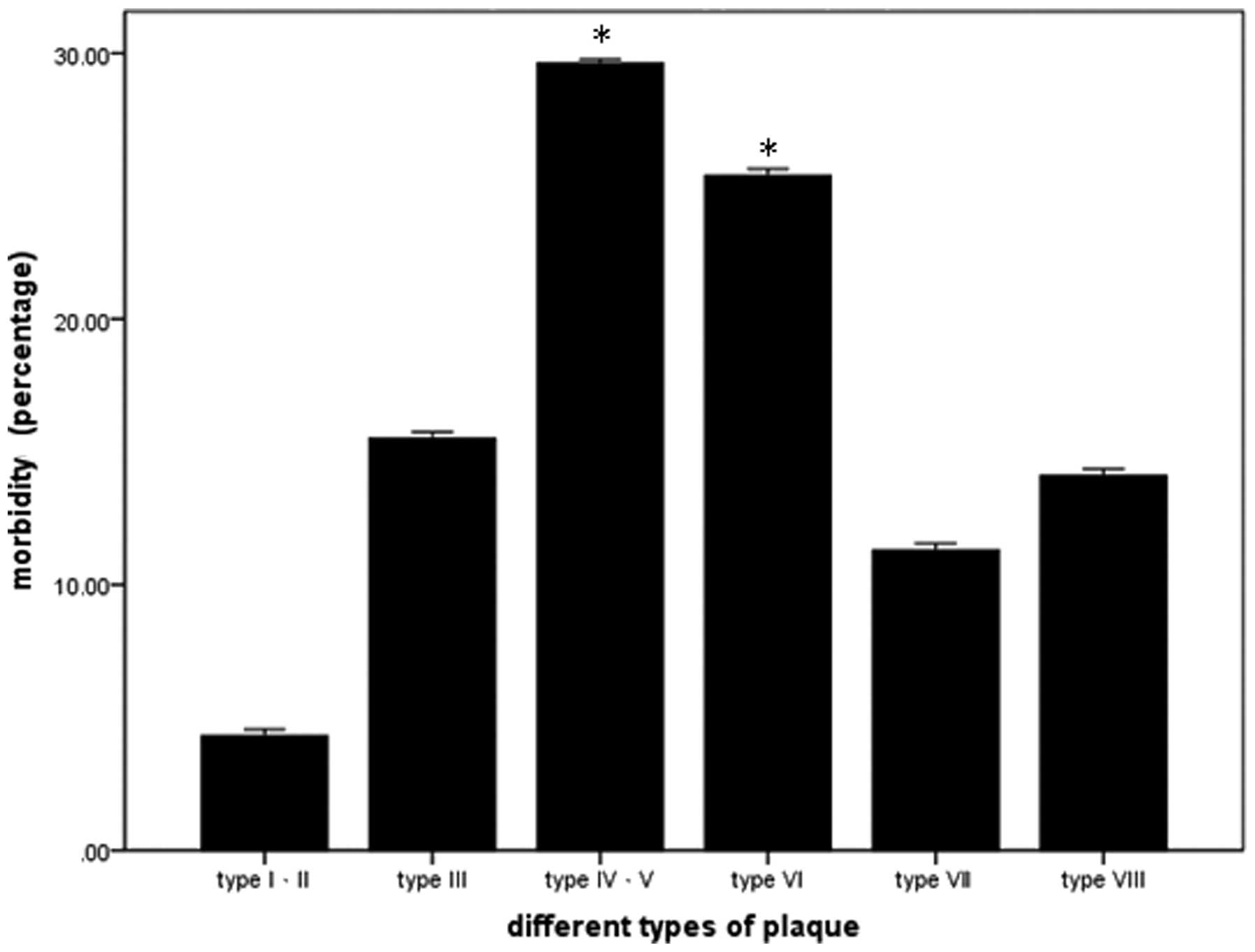
Statistical analysis indicated that the incidence of
type IV, V and VI atherosclerotic plaques was significantly higher
compared with other types of plaque (Fig. 1).
Discussion
As quality of life improves and the population
subsequently ages, ICAS-induced cerebrovascular diseases have
become increasingly prevalent in clinical practice. Various
mechanisms underlying cerebrovascular diseases caused by
intracranial artery lesions have been proposed, including a reduced
cerebral blood flow due to cerebral artery stenosis and an arterial
embolism caused by ICAS due to plaque rupture and detachment, thus
resulting in transient ischemic attack and stroke (5,6). ICAS
plaque vulnerability has presented increased risk of cerebral
infarction compared with hemadostenosis (7). Plaque stability is primarily determined
by the size of the lipid core, the thickness of the fibrous cap,
and the extent of hemorrhage, inflammatory reaction or new vessel
formation. Additionally, a histopathological study by Cappendijk
et al (8) demonstrated that a
large, necrotic lipid core is the most important factor affecting
plaque vulnerability. Thus, early determination of plaque
vulnerability is a key approach for the prevention of plaque
detachment and subsequent cerebral infarction.
Various traditional methods of intracranial artery
examination exist, including transcranial Doppler ultrasound (TCD),
bright-blood 3D-TOF MRA, computed tomography angiography (CTA) and
digital subtraction angiography (DSA). TCD has limited use in
cerebral artery examination due to the position of the cranium. A
number of studies have used TCD in cerebral artery examination;
however, these studies observed the extent of stenosis and blood
flow velocity in the cerebral artery, but did not conduct a
detailed examination of the plaques. Thus, the plaque type and
stability were unable to be determined. Cerebral artery CTA focuses
on identifying the existence and size of arterial aneurysms, in
addition to the degree of stenosis. CTA permits approximate
observation of calcified, soft and mixed plaques. DSA is the gold
standard for determining the degree of cerebrovascular stenosis and
is applicable to invasive examinations. However, DSA requires the
administration of a significant dose of radiation, and the DSA
method can result in embolus detachment, which may inadvertently
cause cerebral infarction. Therefore, the clinical applications of
DSA are limited (9).
MRA possesses a number of advantages, including
non-invasiveness, no requirement for radiation, a high
distinguishing capacity in soft tissues and the possibility of
multiparameter comparison and imaging (10). 3D-TOF MRA is the most common
angiography technology in clinical practice, and the primary
advantage of 3D-TOF MRA is the acquisition of a wide range of
cerebral artery MRA images in a relatively short period. The
negative results are highly accurate; however, 3D-TOF MRA possesses
the disadvantage of exaggerating the extent of cerebrovascular
stenosis. In certain cases, lumens with no hemadostenosis may
exhibit interrupted imaging or no image development with 3D-TOF
MRA.
Black-blood MRA, also known as spatial presaturation
MRI, is an alternative MRA technology. Black-blood MRA is able to
convert the lumen into a low signal and display the vessel walls
with increased image clarity. Therefore, black-blood MRA is able to
determine the presence and extent of stenosis, in addition to
accurately identifying plaques. Furthermore, by optimizing the TR,
TE and NEX parameters, different components of the plaque may be
examined. Using black-blood MRA, the type and vulnerability of the
plaque are identifiable and the potential risk of cerebral
arteriosclerosis may be preliminarily assessed. Thus, black-blood
MRA provides an effective approach to the early clinical detection
of cerebral infarction (11).
To date, numerous studies have been published on
carotid artery HRMRI (12–19), and comparisons between MRI
observations of the atherosclerotic plaques in living organisms and
pathological specimens have been conducted. Lipid produces a high
signal in T1W1, T2W1 and PDWI, while fat saturation is presented as
a low signal in T1WI. In addition, fibrous caps are equisignal or
produce relatively low signals, and a hemorrhage produces a high
signal in T1W1, T1W1 fat saturation, T2W1 and PDWI. For each
sequence that occurs, calcification produces a low signal. However,
there are limited number of studies that have used MRI to assess
the various components of ICAS plaques. ICAS is an integral factor
in systematic atherosclerosis. The histological and pathological
development of ICAS plaques is similar to that of cervical
atherosclerotic plaques. The primary features of the plaques
include a fibrous cap, lipid core, calcification and the exudation
of inflammatory cells (20).
Therefore, the identification of components in ICAS plaques is
feasible through comparison with living carotid artery plaque
MRI.
In the present study, 110 patients with cerebral
infarction were examined using bright-blood 3D-TOF MRA in order to
identify the existence of cerebral artery stenosis. Subsequently,
black-blood MRA was used for the multisequencing examination of
vessels in the abnormal sections. Since the blood vessels in the
brain are thinner and exhibit smaller plaques compared with the
carotid artery, the scanning vision was reduced and the NEX was
increased during MRI scanning. Furthermore, the matrix should be
enlarged and the scanning angle adjusted. Ultimately, the expected
images were obtained. Bright-blood MRA identified that 16/110
patients possessed an abnormal cerebral artery, with no lumen
stenosis, walls or plaques. This result may explain the findings of
the clinical bright-blood test. For patients with cerebral artery
stenosis or occlusion, no significant ischemia or infarction was
identified in the cerebral tissues in the area supplied by the
vessel. Therefore, these observations may prevent misdiagnosis and
excessive treatment. For the 94 patients with arteriosclerotic
plaques identified by black-blood MRA, the revised plaque standard
of atherosclerotic plaque classification by Cai et al
(2) was adopted in order to observe
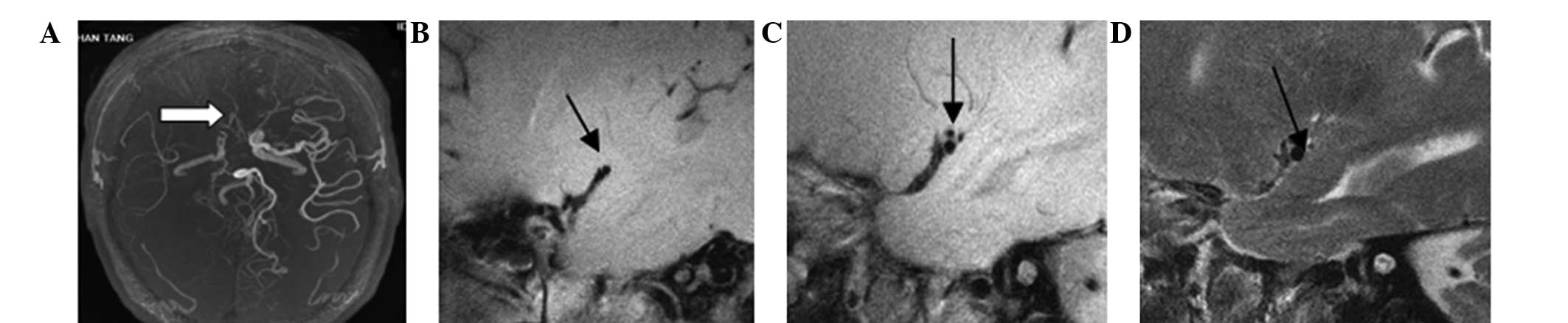
and analyze the ICAS plaques. Six plaques were categorized as types
I–II, in which the wall thickness was approximately normal and four
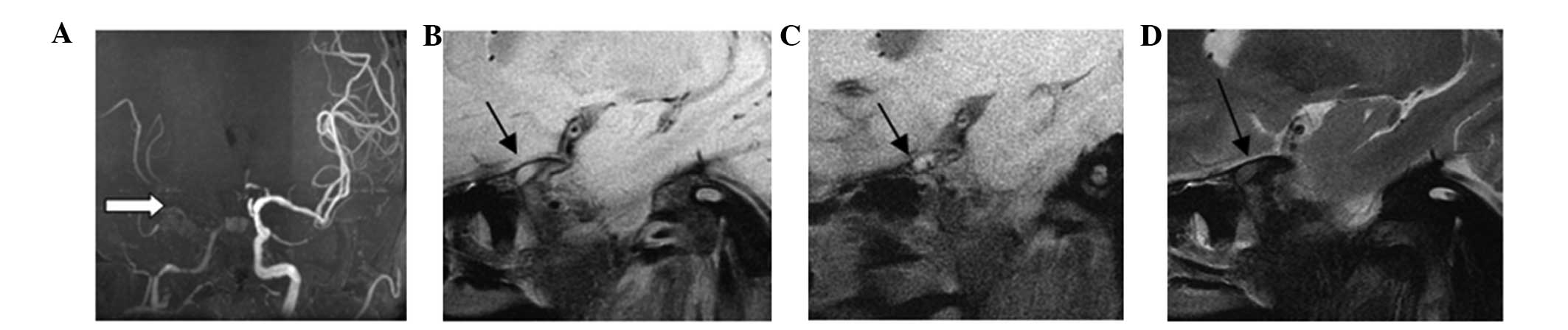
cases exhibited no calcification (Fig.
2). There were 15 plaques classified as type III, which
exhibited artery intima diffusion or eccentric thickening (Fig. 3). In total, 26 plaques were
classified as types IV–V, and these exhibited a wide range of
necrosis with lipid cores and the formation of a fibrous cap in the
plaques, which were combined with a small amount of calcification
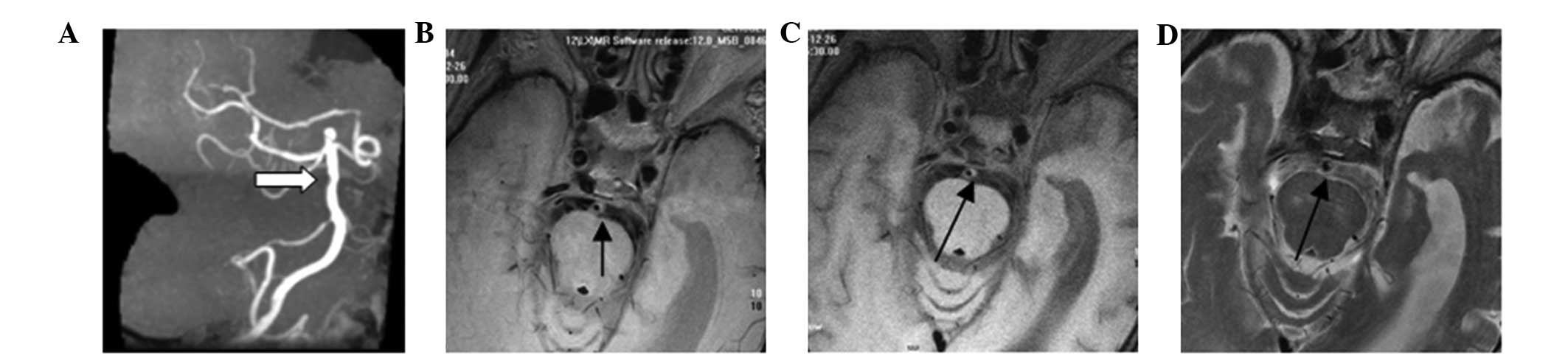
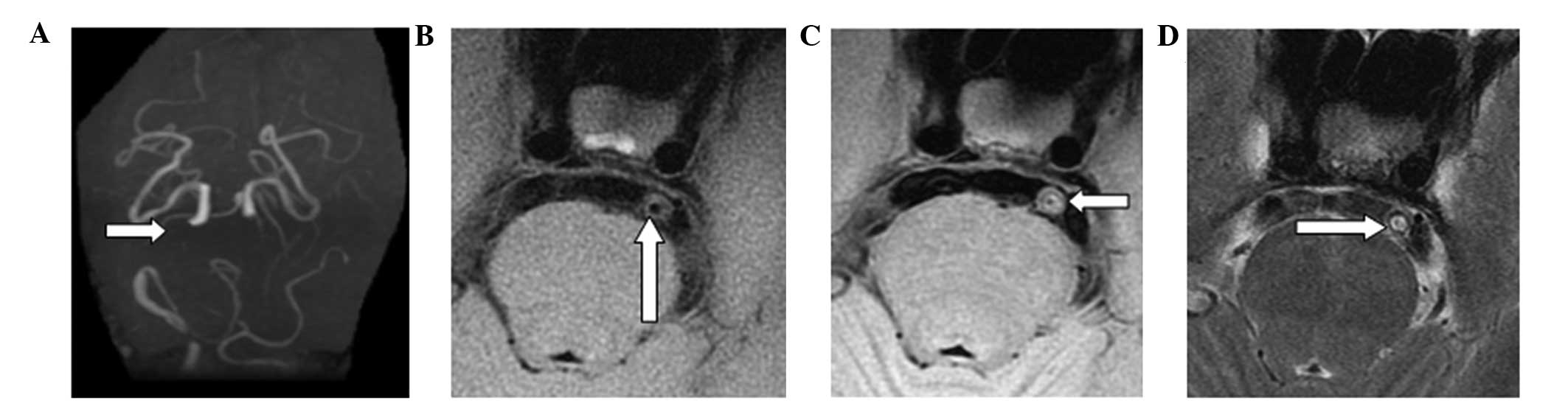
(Fig. 4). A total of 23 cases
presented with type VI plaques, in which an anabrosis on the plaque
surface, hemorrhage in the plaque or thrombosis was observed
(Fig. 5). Type VII plaques were
observed in 11 cases, and were defined as calcified plaques
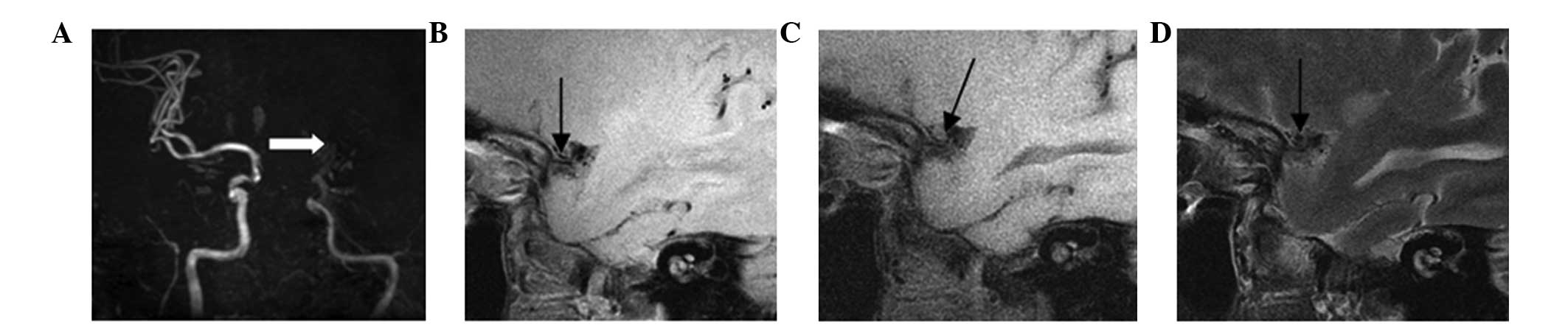
(Fig. 6), while type VIII plaques
were observed in 14 cases and presented as fibrous plaques without
a lipid core, combined with a small amount of calcification
(Fig. 7). Finally, one case
exhibited a basilar arterial occlusion, with complex plaque
components, which was classified as a mixed plaque (Fig. 8). The majority of patients included
in the present study presented with unstable ICAS plaques of types
IV, V and VI, which may be attributed to the selection of the
patient population for the study.
However, there are disadvantages to black-blood MRA
in plaque examination, including a long scanning period, no
re-establishment of images and a limited examination range of
vessels in abnormal sections. Subsequently, the effectiveness of
black-blood MRA in clinical practice is limited. Therefore, at
present, the combined application of bright- and black-blood MRA is
considered to be the most effective approach for identifying
cerebrovascular stenosis, the degree of lumen stenosis, the
atherosclerotic plaque type and the risks of the plaque. This
combined approach may provide a reliable method for the effective
individualized clinical treatment of ICAS plaques at an early
stage.
References
|
1
|
Park KY, Chung CS, Lee KH, Kim GM, Kim YB
and Oh K: Prevalence and risk factors of intracranial
atherosclerosis in an asymptomatic korean population. J Clin
Neurol. 2:29–33. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cai JM, Hatsukami TS, Ferguson MS, Small
R, Polissar NL and Yuan C: Classification of human carotid
atherosclerotic lesions with in vivo multicontrast magnetic
resonance imaging. Circulation. 106:1368–1373. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saam T, Cai JM, Cai YQ, et al: Carotid
plaque composition differs between ethno-racial groups: an MRI
pilot study comparing mainland Chinese and American Caucasian
patients. Arterioscler Thromb Vasc Biol. 25:611–616. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guntheroth WG: A critical review of the
American College of Cardiology/American Heart Association practice
guidelines on bicuspid aortic valve with dilated ascending aorta.
Am J Cardiol. 102:107–110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clarke SE, Hammond RR, Mitchell JR and
Rutt BK: Quantitative assessment of carotid plaque composition
using multicontrast MRI and registered histology. Magn Reson Med.
50:1199–1208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Honda M, Kitagawa N, Tsutsumi K, Nagata I,
Morikawa M and Hayashi T: High-resolution magnetic resonance
imaging for detection of carotid plaques. Neurosurgery. 58:338–346.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan C, Zhang SX, Polissar NL, et al:
Identification of fibrous cap rupture with magnetic resonance
imaging is highly associated with recent transient ischemic attack
or stroke. Circulation. 105:181–185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cappendijk VC, Kessels AG, Heeneman S, et
al: Comparison of lipid-rich necrotic core size in symptomatic and
asymptomatic carotid atherosclerotic plaque: Initial results. J
Magn Reson Imaging. 27:1356–1361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kantelhardt SR, Greke C, Keric N, Vollmer
F, Thiemann I and Giese A: Image guidance for transcranial Doppler
ultrasonography. Neurosurgery. 68:(Suppl 2). 257–266. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Futami K, Sano H, Misaki K, Nakada M, Ueda
F and Hamada J: Identification of the inflow zone of unruptured
cerebral aneurysms: comparison of 4D flow MRI and 3D TOF MRA data.
AJNR Am J Neuroradiol. 35:1363–1370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okuchi S, Okada T, Ihara M, et al:
Visualization of lenticulostriate arteries by flow-sensitive
black-blood MR angiography on a 1.5 T MRI system: A comparative
study between subjects with and without stroke. AJNR Am J
Neuroradiol. 34:780–784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan C, Mitsumori LM, Ferguson MS, et al:
In vivo accuracy of multispectral magnetic resonance imaging for
identifying lipid-rich necrotic cores and intraplaque hemorrhage in
advanced human carotid plaques. Circulation. 104:2051–2056. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan C, Miller ZE, Cai J and Hatsukami T:
Carotid atherosclerotic wall imaging by MRI. Neuroimaging Clin N
Am. 12:391–401. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chiu B, Shamdasani V, Entrekin R, Yuan C
and Kerwin WS: Characterization of carotid plaques on 3-dimensional
ultrasound imaging by registration with multicontrast magnetic
resonance imaging. J Ultrasound Med. 31:1567–1580. 2012.PubMed/NCBI
|
|
15
|
Millon A, Mathevet JL, Boussel L, Faries
PL, Fayad ZA, Douek PC and Feugier P: High-resolution magnetic
resonance imaging of carotid atherosclerosis identifies vulnerable
carotid plaques. J Vasc Surg. 57:1046–1051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Q, Zeng Y, Wang Y, Cai J, Cai Y, Ma L
and Xu X: Comparison of carotid arterial morphology and plaque
composition between patients with acute coronary syndrome and
stable coronary artery disease: A high-resolution magnetic
resonance imaging study. Int J Cardiovasc Imaging. 27:715–726.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chu B, Kampschulte A, Ferguson MS, et al:
Hemorrhage in the atherosclerotic carotid plaque: a high-resolution
MRI study. Stroke. 35:1079–1084. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kerwin W, Hooker A, Spilker M, Vicini P,
Ferguson M, Hatsukami T and Yuan C: Quantitative magnetic resonance
imaging analysis of neovasculature volume in carotid
atherosclerotic plaque. Circulation. 107:851–856. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kerwin WS, OBrien KD, Ferguson MS,
Polissar N, Hatsukami TS and Yuan C: Inflammation in carotid
atherosclerotic plaque: a dynamic contrast-enhanced MR imaging
study. Radiology. 241:459–468. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen XY, Wong KS, Lam WW, Zhao HL and Ng
HK: Middle cerebral artery atherosclerosis: histological comparison
between plaques associated with and not associated with infarct in
a postmortem study. Cerebrovasc Dis. 25:74–80. 2008. View Article : Google Scholar : PubMed/NCBI
|