Introduction
Ovarian cancer is the third most common female
genaital malignancy behind cervical and uterine cancer. ; however,
ovarian cancer has the highest mortality rate of all the
gynecologic cancers (1), posing a
serious threat to the health of females. Furthermore, in the United
States, there are ~21880 cases of ovarian cancer diagnosed
annually, and ~13,850 patients succumb to ovarian cancer each year
(2). At present, chemotherapeutic
agents used for the treatment of ovarian cancer can not only kill
the tumor cells, but also damage the normal cells; therefore, there
is an urgent requirement for the development of effective
chemotherapy drugs for ovarian cancer (3).
Quercetin, a flavonoid, is widespread in nature and
can be found in fruits, vegetables and plants (4,5).
Quercetin has been demonstrated to be able to inhibit the growth of
tumor cells, prevent cancer metastasis and suppress cancer cell
proliferation by inducing tumor cell apoptosis or cell cycle arrest
at a certain stage within the cycle, suggesting that the compound
has potential for use in the treatment of cancer (6–8). Our
preliminary unpublished data suggest that quercetin has inhibitory
effects on gastric and esophageal cancer; however, the effects of
quercetin on ovarian cancer require further study. The aim of the
present study was therefore to observe the effects of quercetin on
the proliferation and apoptosis of the ovarian cancer cell line
SKOV-3 in vitro, and to provide a foundation for the
treatment of ovarian cancer using this agent.
Materials and methods
Materials
The human ovarian cancer cell line SKOV-3 was
obtained from the Tumor Cell Library of the Chinese Academy of
Medical Sciences (Beijing, China). Quercetin (Sigma, St. Louis, MO,
USA) was suspended in dimethylsulfoxide (DMSO; Sigma) and stored at
−20°C. MTT was purchased from Sigma. Dulbecco's modified Eagle's
medium (DMEM), fetal calf serum (FCS) and TRIzol™ reagent were
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA),
and Hoechst 33258 was provided by Biyuntian Biotechnology Research
Institute (Haimen, China).
Methods
Cell culture
Human ovarian cancer SKOV-3 cells were cultured in
10% DMEM containing 10% FCS at 37°C under saturated humidity
conditions in 5% CO2. The medium was replaced every two
or three days.
Cell viability and proliferation
The SKOV-3 cells were harvested during the
logarithmic growth phase and digested with trypsin. The effects of
quercetin on SKOV-3 cell proliferation and viability were
investigated by plating the SKOV-3 cells (5×103/well) in
96-well plates and incubating the cells in DMEM supplemented with
10% FCS. After 24 h, the cells were washed once with medium and
treated with 0, 0.12, 0.23, 0.47, 0.94, 1.88, 3.75, 7.5, 15 or 30
mg/ml quercetin added to the medium. The control wells contained
SKOV-3 cells cultured without the addition of quercetin, and blank
wells containing only culture medium were established. Cell
proliferation and viability were assessed after 24 or 48 h of
treatment by incubating the cells in DMEM supplemented with 10% FCS
and 20 µl MTT (5 mg/ml) for 4 h. Following the swilling of the
culture solution, 150 µl DMSO was added to each well and the
solution was agitated to completely dissolve the blue-purple
precipitate obtained from the MTT. A microplate reader (OPTImax;
Molecular Dynamics, Sunnyvale, CA, USA) was used to measure the
absorbance of each well at 540 nm and the average values were
obtained. The experiments were repeated at least three times, and
data are presented as the mean ± standard deviation (SD).
Cell apoptosis analysis
The SKOV-3 cells were double-stained with Annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide (PI), and
cell apoptosis was then analyzed using flow cytometry (FCM) with an
inverted fluorescence microscope (Axiovert 200; Carl Zeiss SMT
GmbH, Oberkochen, Germany). In brief, the adherent cells treated
with quercetin at different concentrations (0, 15 or 30 mg/ml) for
24 h were collected in a centrifuge tube, and the cell density was
adjusted to 1×106/ml. Following centrifugation at 700 ×
g at 4°C for 5 min, the supernatant was discarded and the pelleted
cells were collected and washed with phosphate-buffered saline
(PBS), prior to further centrifugation. The cells were then
resuspended in 200 µl incubation buffer, 10 µl Annexin V-FITC/PI
was added and the cells were incubated for 15 min at room
temperature away from the light. Following incubation, 300 µl
incubation buffer was added to the cells and the samples were
analyzed for cell apoptosis using a BD FACSCalibur™ flow cytometer
(BD Biosciences, San Jose, CA, USA).
Hoechst staining
Cell apoptosis was determined using an
apoptosis-Hoechst staining kit according to the manufacturer's
instructions (Biyuntian Biotechnology Research Institute). The
cells were seeded into six-well plates and cultured for 24 h.
Quercetin was then added to the cells, and the cells were incubated
for a further 48 h. The cover slips were removed from the six-well
plates and the cells were washed with PBS, prior to being fixed
with the fixative for 10 min. Two further washes were performed
with PBS for 3 min. Hoechst 33258 staining solution was added to
the cells, which were then agitated for 5 min for the staining.
Following staining, the cells were washed twice with PBS for 3 min,
and the cover slips were sealed with a fluorescence-resistant
quenching sealing solution. The cell nuclei were observed under a
fluorescence microscope.
Analysis of survivin protein expression
The expression of survivin protein was analyzed
using western blotting. Following treatment of the SKOV-3 cells
with quercetin at different concentrations for 24 h, the medium was
changed and the cells were cultured for a further 6 h. Different
groups of cells were collected in order to extract and quantify the
protein. Protein separation was performed using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, and the proteins were
then transferred to a nitrocellulose membrane, which was blocked
for 1 h with 5% skimmed dry milk. The membrane was subsequently
incubated overnight at 4°C with rabbit polyclonal anti-human
survivin antibody [cat. no. ab24479, Abcam Trading (Shanghai) Co.,
Ltd., Shanghai, China] diluted 1:1,000 in blocking buffer, and
mouse monclonal anti-β-actin antibody [cat. no. ab8226, Abcam
Trading (Shanghai) Co., Ltd.] diluted 1:1,000 in blocking buffer,
prior to being washed with Tris-buffered saline with Tween 20
(TBST) three times for 10 min each time. An enhanced
chemiluminescence western blotting kit (Suzhou JiShi Biological
Technology Co., Ltd., Suzhou, China) was used for detection.
Cell cycle analysis
The cell cycle was analyzed using FCM. Briefly, the
cells in the logarithmic phase were seeded into 12-well plates and
cultured for 24 h, followed by the addition of quercetin (30 mg/ml)
and incubation for 48 h. The cells were then digested using trypsin
and after 30 min were centrifuged at 700 × g for 5 min at 4°C.
Following centrifugation, the pelleted cells were washed with PBS,
resuspended and fixed with ice-cold ethanol overnight at 4°C. The
cells were stained with PI for 1 h and analyzed using FCM.
Statistical analysis
Results are expressed as the mean ± SD. Statistical
analysis was conducted with a one-way analysis of variance using
SPSS software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Inhibitory effect of quercetin on the
proliferation of SKOV-3 cells
The proliferation of human ovarian cancer SKOV-3
cells treated with quercetin at different concentrations (0, 0.12,
0.23, 0.47, 0.94, 1.88, 3.75, 7.5, 15 and 30 mg/ml) for 24 and 48
h, respectively, was determined by an MTT assay. As shown in
Fig. 1, the growth of the SKOV-3
cells was significantly inhibited by quercetin treatment, and cell
proliferation was also suppressed. Furthermore, the inhibitory
effect of quercetin at the same dose on the growth of SKOV-3 cells
was enhanced by an increase in the incubation time. The inhibitory
rate of cell proliferation increased in a dose- and time -dependent
manner. The strongest inhibitory effect on cell growth was observed
following treatment with 30 mg/ml quercetin, when the inhibitory
rate of cell proliferation reached its peak value (58.72%).
Effect of quercetin on the apoptosis
of SKOV-3 cells
Following the double staining of the SKOV-3 cells
with Annexin V-FITC and PI, the role of quercetin in cell apoptosis
was analyzed by FCM. In Fig. 2,
normal cells were located within the lower left quadrant, while
apoptotic cells in the early stage of apoptosis were observed
within the lower right quadrant. Apoptotic cells in the late stage
of apoptosis and the dead cells were located within the upper right
quadrant. The total apoptosis rate was a sum of the early and late
apoptosis rates. The results showed that the total apoptosis rate
of the SKOV-3 cells treated with 15 mg/ml quercetin was
significantly higher than that of the control cells (33.62±1.17 vs.
7.13±0.92%, n=6, P<0.01). When the cells were treated with 30
mg/ml quercetin, the total apoptosis rate was 69.12±2.97%, which
was significantly higher than that of the low-dose (15 mg/ml)
quercetin group and the control (n=6, P<0.01).
Apoptosis determination by Hoechst
33258 staining
Chromatin condensation occurs during tumor cell
apoptosis; therefore, Hoechst 33258 staining was used in the
analysis of cell apoptosis. Following the staining of the cells
with Hoechst 33258, the cell nuclei were observed under a
fluorescence microscope. Normal cell nuclei were evenly stained
blue, while the cell nuclei of the apoptotic cells were condensed
and hyperchromatic or fragmented and hyperchromatic, so the nuclei
became bright white (Fig. 3). The
number of apoptotic cells among the cells treated with 15 or 30
mg/ml quercetin was higher than that of the control, and the
apoptosis rates in the 15 and 30 mg/ml quercetin groups were
significantly higher than the rate in the control group
(P<0.05).
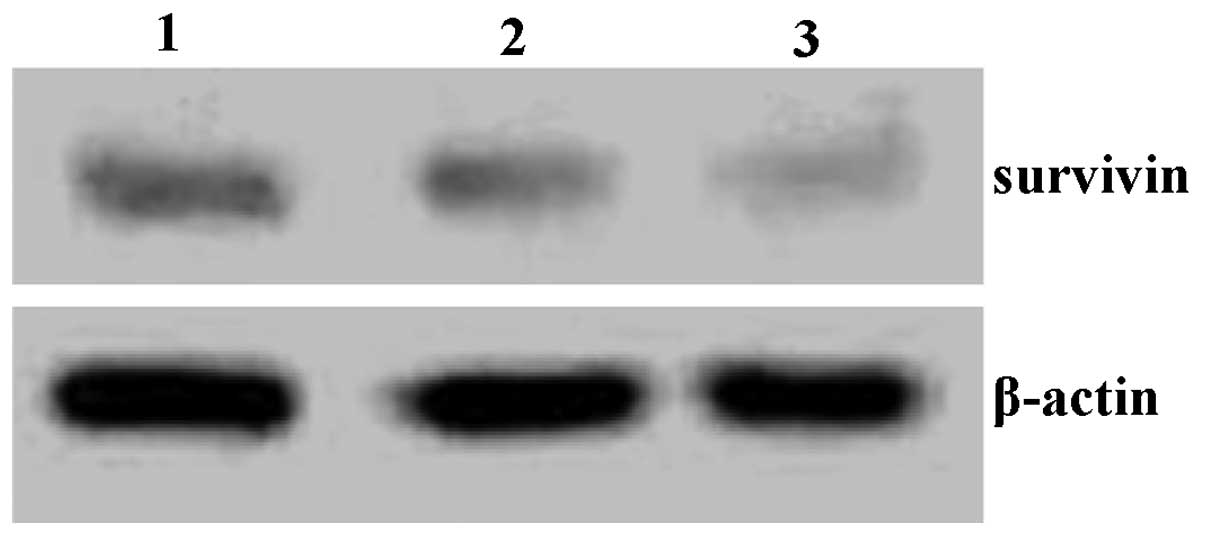
Expression of survivin protein
SKOV-3 cells were treated with quercetin for 48 h
and then survivin protein expression was analyzed using western
blotting. As shown in Fig. 4,
survivin protein levels were reduced following quercetin
treatment.
Effect of quercetin on the cell cycle
of SKOV-3 cells
The cell cycle of human ovarian cancer SKOV-3 cells
treated with 30 mg/ml quercetin for 48 h was determined using FCM.
As shown in Fig. 5, following
treatment with quercetin, the percentage of cells at
G0/G1 phase was significantly increased,
while the percentage of cells at G2/M phase was markedly
decreased compared with results for the control cells, indicating
that quercetin can induce the apoptosis of SKOV-3 cells largely by
causing cell cycle arrest in the G1 phase.
Discussion
Bioflavonoids are extracted from fruits and
vegetables due to their unique biological characteristics. It has
been reported that flavonoids exhibit numerous biological
activities, including anti-oxidative, -bacterial, -inflammatory,
-viral and -cancer effects, and may play a role in cancer
prevention (9,10). The high incidence and mortality
associated with ovarian cancer has necessitated the search for a
novel effective clinical treatment. The flavonoid quercetin has
been shown to be able to inhibit the proliferation and induce the
apoptosis of various types of cancer cells, including colon,
pancreatic, stomach, bladder and breast (11–15). In
the present study, the effects of quercetin on the proliferation
and apoptosis of ovarian cancer cells were investigated in order to
provide an experimental basis for the clinical application of
quercetin in the treatment of ovarian cancer.
In the present study, the effect of different doses
of quercetin on the growth of ovarian cancer SKOV-3 cells was
investigated at different time-points using an MTT assay. The
results showed that quercetin inhibited ovarian cancer cell growth,
and the rate of quercetin-induced cell proliferation inhibition
significantly increased in a dose- and time-dependent fashion.
Following the double staining of the SKOV-3 cells with Annexin
V-FITC and PI, cell apoptosis was analyzed using FCM. The results
showed that quercetin induced the apoptosis of ovarian cancer
cells, and the cell apoptosis rate increased in a dose-dependent
manner. In addition, Hoechst staining and morphological analysis
demonstrated that quercetin could induce the apoptosis of ovarian
cancer SKOV-3 cells. In combination, these data suggest that
quercetin can not only inhibit ovarian cancer cell proliferation,
but also induce the apoptosis of ovarian cancer cells. In the
present study, quercetin caused a concentration- and time-dependent
reduction in the viability of SKOV-3 ovarian cancer cells. The
results of the present study are concordant with the findings of Yi
et al (16).
Cell apoptosis plays a key role in the regulation of
the proliferation of tumor cells, and the genesis and development
of tumors. In the present study, the possible mechanisms by which
quercetin inhibits the proliferation of ovarian cancer SKOV-3 cells
were analyzed using FCM. The results showed that, following
treatment with quercetin, the number of cells at
G0/G1 phase was significantly increased,
while the number of cells at S and G2/M phases was
relatively decreased compared with the control group, indicating
that quercetin can cause cell cycle arrest at the G1
phase for ovarian cancer SKOV-3 cells, prevent cell cycle
progression from G1 to S phase and induce cell
apoptosis. These results suggest that cell cycle arrest is one of
the important mechanisms underlying the quercetin-induced
inhibition of ovarian cancer SKOV-3 cell growth, as well as
quercetin-induced cell apoptosis.
In conclusion, the present study has explored the
role of quercetin in the inhibition of proliferation and the
induction of apoptosis in ovarian cancer SKOV-3 cells; however, the
exact mechanism still requires further investigation. In this
study, the effects of quercetin on the proliferation and apoptosis
of ovarian cancer cells were only demonstrated through in
vitro experiments. Further studies in vivo and
associated clinical trials are necessary to elucidate the
mechanisms by which quercetin exerts its anti-ovarian cancer
properties, thus providing experimental evidence for the treatment
of ovarian cancer and contributing to the development of novel
quercetin-related drugs.
Acknowledgements
This study was financially supported by the Key
Research Areas Subject of Xinxiang Medical University (no. ZD
2011–14), the Key Laboratory for Medical Tissue Regeneration of
Henan Province (grant nos. 81301174 and 31200897), the Tumor and
Signal Transduction Laboratory of Xinxiang Medical University
(grant nos. 81272251 and 91229115) and the Scientific Research Fund
of Xingxiang Medical University (no. 2014QN118).
References
|
1
|
Gilbert L, Basso O, Sampalis J, et al DOvE
Study Group: Assessment of symptomatic women for early diagnosis of
ovarian cancer: results from the prospective DOvE pilot project.
Lancet Oncol. 13:285–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Drake B: Intraperitoneal chemotherapy: a
reemerging approach in the treatment of ovarian cancer. J Infus
Nurs. 32:314–322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Slusarz A, Shenouda NS, Sakla MS, et al:
Common botanical compounds inhibit the hedgehog signaling pathway
in prostate cancer. Cancer Res. 70:3382–3390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Comalada M, Camuesco D, Sierra S, et al:
In vivo quercitrin anti-inflammatory effect involves release of
quercetin, which inhibits inflammation through down-regulation of
the NF-kappaB pathway. Eur J Immunol. 35:584–592. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma L, Feugang JM, Konarski P, et al:
Growth inhibitory effects of quercetin on bladder cancer cell.
Front Biosci. 11:2275–2285. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beniston RG and Campo MS: Quercetin
elevates p27(Kip1) and arrests both primary and HPV16 E6/E7
transformed human keratinocytes in G1. Oncogene. 22:5504–5514.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshida M, Yamamoto M and Nikaido T:
Quercetin arrests human leukemic T-cells in late G1 phase of the
cell cycle. Cancer Res. 52:6676–6681. 1992.PubMed/NCBI
|
|
9
|
Paydar M, Wong YL, Moharam BA, et al: In
vitro anti-oxidant and anti-cancer activity of methanolic extract
from Sanchezia speciosa leaves. Pak J Biol Sci.
16:1212–1215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Psahoulia FH, Drosopoulos KG, Doubravska
L, et al: Quercetin enhances TRAIL-mediated apoptosis in colon
cancer cells by inducing the accumulation of death receptors in
lipid rafts. Mol Cancer Ther. 6:2591–2599. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lai WW, Hsu SC, Chueh FS, et al: Quercetin
inhibits migration and invasion of SAS human oral cancer cells
through inhibition of NF-κB and matrix metalloproteinase-2/-9
signaling pathways. Anticancer Res. 33:1941–1950. 2013.PubMed/NCBI
|
|
12
|
Bruning A: Inhibition of mTOR signaling by
quercetin in cancer treatment and prevention. Anticancer Agents Med
Chem. 13:1025–1031. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nguyen TT, Tran E, Nguyen TH, et al: The
role of activated MEK-ERK pathway in quercetin-induced growth
inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis.
25:647–659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hamedeyazdan S, Fathiazad F, Sharifi S and
Nazemiyeh H: Antiproliferative activity of Marrubium
persicum extract in the MCF-7 human breast cancer cell line.
Asian Pac J Cancer Prev. 13:5843–5848. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Borska S, Chmielewska M, Wysocka T, et al:
In vitro effect of quercetin on human gastric carcinoma: targeting
cancer cells death and MDR. Food Chem Toxicol. 50:3375–3383. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yi L, Zongyuan Y, Cheng G, Lingyun Z,
Guilian Y and Wei G: Quercetin enhances apoptotic effect of tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL) in
ovarian cancer cells through reactive oxygen species (ROS) mediated
CCAAT enhancer-binding protein homologous protein (CHOP)-death
receptor 5 pathway. Cancer Sci. 105:520–527. 2014. View Article : Google Scholar : PubMed/NCBI
|