Introduction
Acute promyelocytic leukemia (APL) is a type of
acute myeloid leukemia (AML) with distinct clinical, morphological,
immunophenotypic and cytogenetic characteristics (1). APL has two morphological subtypes:
Hypergranular (typical) APL and microgranular APL. Both subtypes
are associated with disseminated intravascular coagulation. Unlike
typical APL, the leukocyte count in microgranular APL is very high.
The genetic hallmark of APL is t(15;17)(q22;q21), which can be
detected in >90% of APL cases (2–5). The
reciprocal translocation results in a fusion gene product between
the retinoic acid receptor α (RARα) gene on 17q21 and the
promyelocytic leukemia (PML) gene on 15q22. This translocation is
associated with particular sensitivity to treatment with all-trans
retinoic acid (ATRA) (2) and a
favorable prognosis; however, a subset of cases with APL morphology
and variant translocations, such as t(11;17) and t(5;17), have been
reported not to respond to ATRA (6)
and have been associated with an unfavorable prognosis (7).
The present study describes a case of microgranular
APL with ins(17;15)(q12;q14q22) in a pediatric patient, along with
its clinical and biological characteristics. The case demonstrates
the importance of taking into consideration all clinical,
morphological and cytogenetic/molecular findings in the diagnosis
of APL when typical morphological characteristics and/or
cytogenetic findings are absent.
Case report
A 2-year-old boy, who had been suffering from
recurring fever and cough for 5 days and epistaxis for 1 day, was
admitted to the Affiliated Hospital of Qingdao University (Qingdao,
China). He had an upper respiratory infection 5 days prior to
admission. On admission, the patient was conscious but, according
to physical examination, had pallor. No petechiae, xanthochromia or
hemoptysis were observed and no hepatosplenomegaly or
lymphadenopathy was noted during the examination. The head, chest
and cardiovascular system examinations gave no notable results. The
complete blood count differential showed that the white blood cell
density was 4.628×1010/l with 6% neutrophils and 72%
atypical promyelocytes, the concentration of hemoglobin was 31 g/l
and the platelet density was 1.0×1010/l.
Immunophenotyping by flow cytometry revealed that the atypical
promyelocytes were positive for cluster of differentiation (CD) 13
(41.3%) and CD33 (91.6%) and negative for CD5, CD10, CD14, CD19,
CD22, CD34 and human leukocyte antigen (HLA)-DR. Prothrombin time,
partial thromboplastin time and the D-dimer test were within normal
ranges. Bone marrow aspiration was subsequently performed and the
smears showed hypocellularity with 33.5% atypical promyelocytes.
The majority of the leukemic cells were oval-shaped and slightly
larger than their normal counterparts, whereas the occasional cells
were irregular in shape, with abundant pauci-granular cytoplasm,
round or oval nuclei and 1–4 nucleoli (Fig. 1A). In addition, there was an absence
of Auer bodies. Bone marrow aspiration performed at complete
remission showed <5% immature cells (Fig. 1B). Prior written informed consent was
obtained from the guardians of the patient and the study was
approved by the Ethics Review Board of Qingdao University.
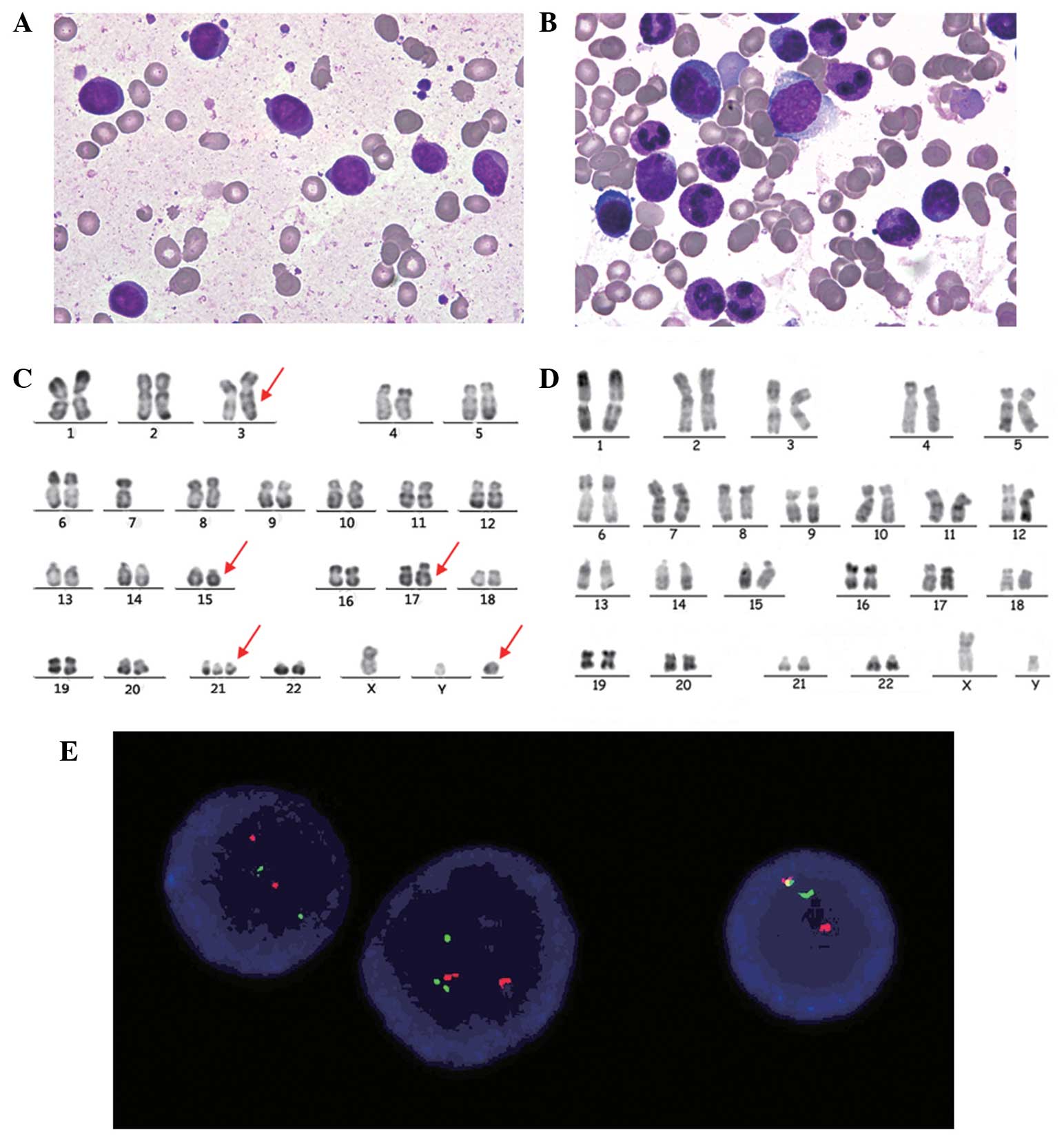 | Figure 1.(A) Bone marrow aspiration smears
showing hypoplastic and leukemic promyelocytes with round or oval
nuclei, fine chromatin and the presence or absence of sparse small
granules in the plasma at diagnosis (Wright stain; magnification,
x100). (B) Bone marrow aspiration smears showing hyperplastic and
rare promyelocytes with normal morphological characteristics when
achieving complete remission (Wright stain; magnification, x100).
(C) R-banded karyotype of a representative bone marrow cell: 47,
XY, add(3) (q29), −7, ins(17;15)(q12;q14q22),+21,+mar. Arrows
indicate derivative chromosomes. (D) R-banded karyotype of a
representative bone marrow cell at clinical remission: 46, XY. (E)
Fluorescence in situ hybridization of representative
interphase cells with a PML/RARα dual-color, dual-fusion
translocation probe at diagnosis. Red signals represent the PML
gene, green signals represent the RARα gene and yellow signals
represent the PML/RARα fusion gene. PML/RARα, promyelocytic
leukaemia/retinoic acid receptor α. |
Chromosomal analysis
Chromosomal analysis was carried out following the
standard protocol. In brief, the bone marrow aspiration samples
were cultured in medium for 24 h and R-banding analysis was then
performed. All 10 cells that were examined revealed a karyotype of
47, XY, add(3)(q29), −7, ins(17;15)(q12;q14q22),+21,+mar (Fig. 1C). A repeat karyotype analysis at
clinical remission showed a normal karyotype (Fig. 1D).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to detect the transcripts of
the PML/RARα fusion gene. Total RNA was extracted using TRIzol
reagent (Thermo Fisher Scientific, Waltham, MA, USA) from bone
marrow aspirates, and complementary DNA (cDNA) was synthesized
using total RNA (1–2 µg) in a 40-µl final reaction system
containing reverse transcriptase M-MLV (Thermo Fisher Scientific)
and random hexamers. The PCR primers were designed as previously
described (8) The final volume of
PCR was 50 µl, including cDNA (3 µl), primers (10 pmol each), dNTPs
(200 µM), MgCl2 (1.5 mM) and Taq DNA polymerase
(0.3 U). An uninvolved RARα gene segment was co-amplified as an
inter-control. The products were detected on 2% agarose gels
stained with ethidium bromide, transferred to positively charged
nylon membranes (GeneScreen Plus®; Perkin-Elmer Life Sciences,
Waltham, MA, USA) and then hybridized using the probe R4b (5′-CTC
ACA GGC GCT GAC CCC CAT-3′), as previously reported (9). No PML/RARα transcript was detected
Fluorescence in situ hybridization
(FISH)
FISH was performed on bone marrow interphases with
the PML/RARα dual-color DNA probe (obtained from Suzhou University,
Suzhou, China), in which the PML and RARα genes were labeled red
and green, respectively. The detection was executed according to
the manufacturer's instructions. Three signals (one red, one green
and one yellow) were observed, indicating the presence of the
PML-RARα gene rearrangement (Fig.
1E). This observation may support the insertion observed in the
conventional cytogenetic analysis.
Management and follow-up
The patient was initially treated with a supportive
approach, and concentrated red blood cells and platelets were
administered when necessary. Once the diagnosis of microgranular
variant M3 (M3v) APL was rendered on the basis of the clinical and
cytogenetic findings, the patient was treated with 10 mg
pirarubicin for 2 days, 30 mg cytarabine for 4 days, 2 mg arsenic
trioxide for 5 days and 5 mg ATRA twice a day. Four weeks later,
the patient achieved complete remission and continued with
consolidation therapy using pirarubicin and cytarabine.
Discussion
Hypergranular and microgranular APLs are both
associated with high risks of disseminated intravascular
coagulation and hemorrhagic manifestations, such as hematuria and
epistaxis. According to the literature, microgranular APL accounts
for 15–20% of APL cases (6,10,11).
Unlike hypergranular APL, leukemic cells in microgranular APL are
pauci-granular or contain small, sparse cytoplasmic granules that
sometimes can be mistakenly classified as monocytes (12,13). As
a result, M3v is difficult to diagnose only by morphology and is
often misdiagnosed as AML-M4 or -M5 (6). Cytogenetics and FISH studies are
therefore indispensable for the correct diagnosis of M3v. In rare
cases where typical morphological and clinical characteristics of
APL are present or negative PML-RARα rearrangement is observed in
karyotyping, RT-PCR and FISH become necessary for the
identification of rare variant translocations.
Regardless of the morphological variances, it
appears that M3 and M3v have no significant differences regarding
cytogenetic characteristics and clinical outcomes (12,14). The
PML/RARα fusion product plays an important role in the pathogenesis
of the disease by interfering with a normal cellular response
towards retinoic acid. The classical translocation and presence of
PML/RARα rearrangement are generally associated with a favorable
prognosis (2,15); however, the presence of variant
translocations and/or complex karyotypes may be associated with
uncertain outcomes and prognosis (16,17). For
example, t(11;17) and t(5;17) translocations, which result in
NUMA/RARα and NPM/RARα fusion products, respectively, are
associated with a poor response to ATRA (6) and unfavorable prognosis (7). In addition, Kurian et al
(18) reported that an M3v case
secondary to breast cancer, which carried t(15;17)(q13;12) and no
PML/RARα fusion transcripts, was resistant to ATRA. The present
report described an M3v case with variant ins(17;15)(q12;q14q22)
translocation, in which a segment of 15q, including the PML gene,
was inserted into the RARα gene on 17q. Among the reported APL
cases with insertion, ins(15;17) is more common than ins(17;15)
(19). To the best of our knowledge,
only seven APL cases with ins(17;15) have been reported and all of
these cases have been confirmed by FISH to carry insertions that
can result in RARα/PML fusion products. By conventional chromosomal
analysis, four cases were found to have no abnormalities on
chromosomes 15 and 17, and three cases had ins(17;15)(q21;q12q22),
ins(17;15)(q21;q15q22) and ins(17;15)(q21;q14q22) (20,21),
respectively. In the case with ins(17;15)(q21;q14q22) (19), the RARα/PML fusion transcripts were
not detected by RT-PCR; therefore, there may be certain
discrepancies between FISH and RT-PCR results, as found in the
present case. The aforementioned and present cases exhibited
complete remission following treatment with ATRA. The findings
suggest that the rare variant ins(17;15) has no adverse effect on
the therapeutic response to ATRA and is therefore associated with
favorable prognosis. The underlying mechanisms remain unclear and
further studies are required for their elucidation. Although RT-PCR
has higher sensitivity in the diagnosis of acute leukemia and the
monitoring of minimal residual disease than FISH, it is necessary
to compare and combine the results of RT-PCR and FISH studies when
dealing with cases with atypical characteristics.
In this case, there were three additional
chromosomal abnormalities: add(3)(q29), −7 and trisomy 21. It has
been reported that trisomy 8 and 21 and abn(17) are the three most
frequently observed aberrations, in addition to t(15;17), in APL
(22–25). To date, there has only been one case
report describing 3q29 involved in translocation changes, rather
than additional changes (26).
Monosomy 7(−7) has been identified in 40% of pediatric patients
with myelodysplastic syndrome, while only 4–5% of the AML cases
carry −7 or del(7q), which, according to the literature, suggests a
poor prognosis (27). In pediatric
patients, trisomy 21 is predominantly found in AML in association
with Down's syndrome. Only a few reported cases were not associated
with Down's syndrome, and the majority of these cases were
associated with a poor outcome (24). In the present case, the patient did
not have Down's syndrome. Spell et al (28) reported an M3v case with trisomy 21 as
an additional change but the prognostic implication of trisomy 21
was not clearly illustrated. The effect of the presence of
additional aberrations in APL on prognosis is still controversial
(18,27); however, the findings of the present
study suggest that the additional cytogenetic abnormalities appear
to have no adverse effect on the response to treatment with ATRA
and the prognosis.
References
|
1
|
Diverio D, Lo Coco F, D'Adamo F, et al:
Identification of DNA rearrangements at the retinoic acid
receptor-alpha (RAR-alpha) locus in all patients with acute
promyelocytic leukemia (APL) and mapping of APL breakpoints within
the RAR-alpha second intron. Blood. 79:3331–3336. 1992.PubMed/NCBI
|
|
2
|
Yamanouchi J, Hato T, Niiya T, et al: A
new four-way variant t(5;17;15;20)(q33;q12;q22;q11.2) in acute
promyelocytic leukemia. Int J Hematol. 94:395–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rowley JD, Golomb HM and Dougherty C:
15/17 translocation, a consistent chromosomal change in acute
promyelocytic leukaemia. Lancet. 1:549–550. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Thé H, Chomienne C, Lanotte M, Degos L
and Dejean A: The t(15;17) translocation of acute promyelocytic
leukaemia fuses the retinoic acid receptor alpha gene to a novel
transcribed locus. Nature. 347:558–561. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campbell LJ, Oei P, Brookwell R, et al:
FISH detection of PML-RARA fusion in ins(15;17) acute promyelocytic
leukemia depends on probe size. Biomed Res Int. 2013:1645012013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rizzatti EG, Portieres FL, Martins SL,
Rego EM, Zago MA and Falcão RP: Microgranular and
t(11;17)/PLZF-RARalpha variants of acute promyelocytic leukemia
also present the flow cytometric pattern of CD13, CD34 and CD15
expression characteristic of PML-RARalpha gene rearrangement. Am J
Hematol. 76:44–51. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Redner RL: Variations on a theme: The
alternate translocations in APL. Leukemia. 16:1927–1932. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng L, Xue Y, Li J, et al: Application
of metaphase-fluorescence in situ hybridization to the diagnosis of
acute promyelocytic leukemia and the detection of minimal residual
disease. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 8:180–184. 2000.(In
Chinese). PubMed/NCBI
|
|
9
|
Gallagher RE, Li YP, Rao S, et al:
Characterization of acute promyelocytic leukemia cases with PML-RAR
alpha break/fusion sites in PML exon 6: Identification of a
subgroup with decreased in vitro responsiveness to all-trans
retinoic acid. Blood. 86:1540–1547. 1995.PubMed/NCBI
|
|
10
|
Avvisati G, Lo Coco F and Mandelli F:
Acute promyelocytic leukemia: Clinical and morphologic features and
prognostic factors. Semin Hematol. 38:4–12. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guglielmi C, Martelli MP, Diverio D, et
al: Immunophenotype of adult and childhood acute promyelocytic
leukaemia: Correlation with morphology, type of PML gene breakpoint
and clinical outcome. A cooperative Italian study on 196 cases. Br
J Haematol. 102:1035–1041. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tallman MS, Kim HT, Montesinos P, et al:
Does microgranular variant morphology of acute promyelocytic
leukemia independently predict a less favorable outcome compared
with classical M3 APL? A joint study of the North American
Intergroup and the PETHEMA Group. Blood. 116:5650–5659. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshii M, Ishida M, Yoshida T, et al:
Clinicopathological features of acute promyelocytic leukemia: An
experience in one institute emphasizing the morphological and
immunophenotypic changes at the time of relapse. Int J Clin Exp
Pathol. 6:2192–2198. 2013.PubMed/NCBI
|
|
14
|
Invernizzi R, Iannone AM, Bernuzzi S, et
al: Acute promyelocytic leukemia: Morphological and clinical
features. Haematologica. 78:156–161. 1993.PubMed/NCBI
|
|
15
|
Fang J, Chen SJ, Tong JH, Wang ZG, Chen GQ
and Chen Z: Treatment of acute promyelocytic leukemia with ATRA and
As2 O3: A model of molecular target-based
cancer therapy. Cancer Biol Ther. 1:614–620. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim MJ, Cho SY, Lim G, et al: A rare case
of microgranular acute promyelocytic leukemia associated with
ider(17)(q10)t(15;17) in an old-age patient. Korean J Lab Med.
31:86–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang H, Liu ZF and Pan JL: The clinical
and experimental research of 2 cases of acute promyelocytic
leukemia with atypical t(15;17) translocation. Guo Ji Shu Xue Ji
Xue Ye Xue Za Zhi. 35:315–317. 2012.(In Chinese).
|
|
18
|
Kurian S, Hogan TF, Bleigh OC, Dowdy YG,
Merghoub T, Pandolfi PP and Wenger SL: Atypical t(15;17)(q13;q12)
in a patient with all-trans retinoic acid refractory secondary
acute promyelocytic leukemia: A case report and review of the
literature. Cancer Genet Cytogenet. 138:143–148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen SN, Xue YQ, Wu YF and Pan JL:
Cytogenetic and molecular genetic studies on a variant of t(15;17),
ins(17;15)(q21;q14q22), in an acute promyelocytic leukemia patient.
Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 21:77–79. 2004.(In Chinese).
PubMed/NCBI
|
|
20
|
Grimwade D, Gorman P, Duprez E, et al:
Characterization of cryptic rearrangements and variant
translocations in acute promyelocytic leukemia. Blood.
90:4876–4885. 1997.PubMed/NCBI
|
|
21
|
Grimwade D, Biondi A, Mozziconacci MJ, et
al: Characterization of acute promyelocytic leukemia cases lacking
the classic t(15;17): Results of the European Working Party. Groupe
Français de Cytogénétique Hématologique, Groupe de Français
d'Hematologie Cellulaire, UK Cancer Cytogenetics Group and BIOMED 1
European Community-Concerted Action ‘Molecular Cytogenetic
Diagnosis in Haematological Malignancies’. Blood. 96:1297–1308.
2000.PubMed/NCBI
|
|
22
|
Haimi M, Elhasid R, Weyl Ben-Arush M,
Brill-Zamir R, Laevski I and Gershoni-Baruch R: Derivative
(7)t(7;8)(q34;q21): An additional chromosome aberration in acute
promyelocytic leukemia-prognostic influence debated. Cancer Genet
Cytogenet. 153:81–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cervera J, Montesinos P, Hernández-Rivas
JM, et al: Additional chromosome abnormalities in patients with
acute promyelocytic leukemia treated with all-trans retinoic acid
and chemotherapy. Haematologica. 95:424–431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wan TS, Ma SK, Au WY, Liu HS, Chan JC and
Chan LC: Trisomy 21 and other chromosomal abnormalities in acute
promyelocytic leukemia. Cancer Genet Cytogenet. 140:170–173. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bastos EF, Silva LA, Ramos MC, et al:
Trisomy 11 as an additional chromosome alteration in a child with
acute promyelocytic leukemia with poor prognosis. Case Rep Genet.
2012:6590162012.PubMed/NCBI
|
|
26
|
Xue Y, Lu D, Yuan YZ, Guo Y and Xie X: A
rare variant translocation t(3;8)(q29;q22) without AML1/ETO fusion
transcript in a case of oligoblastic leukemia. Leuk Res.
22:1015–1019. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hasle H, Alonzo TA, Auvrignon A, et al:
Monosomy 7 and deletion 7q in children and adolescents with acute
myeloid leukemia: An international retrospective study. Blood.
109:4641–4647. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spell DW, Velagaleti GV, Jones DV and
Velasquez WS: Translocation (15;17) and trisomy 21 in the
microgranular variant of acute promyelocytic leukemia. Cancer Genet
Cytogenet. 132:74–76. 2002. View Article : Google Scholar : PubMed/NCBI
|















