Introduction
Cervical cancer is the third most commonly diagnosed
cancer and the fourth most common cause of mortality in women
worldwide (1). In 2014, ~12,360 new
cases of invasive cervical cancer and ~4,020 cases of mortality
from this disease were estimated for the USA (2). The development of cervical cancer is a
slow process; it typically takes 10–15 years for the pre-cancerous
condition dysplasia to develop into cancer. Although cervical
cancer is fully treatable in the early stages, once it has
metastasized, patient outcome is poor (3).
Metastasis occurs following the detachment of cancer
cells from the primary tumor. The detached cells invade through
degraded basement membrane into the surrounding stroma, enter into
the vascular or lymphatic system and are transported to distal
sites such as the liver, lungs and brain, where they undergo
extravasation, tumor cell proliferation and angiogenesis (4–8). The
extracellular matrix (ECM), which comprises collagen,
proteoglycans, fibronectin, laminin and other glycoproteins
(9–11), acts as a barrier to cancer cell
invasion, and tumor cell invasion is dependent upon its
degradation. Matrix metalloproteinases (MMPs) and urokinase
plasminogen activators (uPA) are also involved in tumor invasion
and metastasis. Clinical and experimental studies have indicated
that elevated levels of uPA and MMPs are associated with tumor
growth, cancer progression, metastasis and a reduction in the
survival time of patients (12,13). The
expression of MMP-9 at the mRNA and protein levels has been shown
to be significantly elevated in tumor and stromal cells of invasive
squamous cell carcinoma of the uterine cervix (14).
Nutrients such as lysine and ascorbic acid have been
suggested to target plasmin-mediated connective tissue degradation
as a universal approach to the prevention of tumor growth and
expansion (15). Lysine binds to the
active sites of plasminogen, thereby blocking the conversion of
plasminogen to plasmin and the plasmin-induced MMP activation
cascade (16). We have developed a
strategy to inhibit the development and spread of cancer using a
nutrient mixture (NM) comprising nutrients such as lysine, proline,
ascorbic acid and green tea extract. This mixture has exhibited
synergistic anticancer activity in vivo and in vitro
in a number of cancer cell lines through the inhibition of cancer
cell growth, MMP secretion, invasion, metastasis and angiogenesis
(17).
In previous studies, NM was found to significantly
inhibit HeLa cervical cancer cell proliferation in vitro,
MMP-2 and -9 secretion, uPA activity and Matrigel invasion, in
addition to enhancing TIMP-2 activity (18,19). In
the present study the in vivo effect of NM supplementation
on tumor growth and ECM protein markers were investigated in HeLa
cervical tumor xenografts in female nude mice. Morphological
changes in key ECM proteins associated with the tumor, including
collagen I, collagen IV, fibronectin, laminin, periodic acid-Schiff
(PAS) and elastin, were evaluated.
Materials and methods
Animals
Female athymic nude mice, ~5 weeks of age, were
purchased from Simonsen Laboratories (Gilroy, CA, USA) and
maintained in microisolator cages under pathogen-free conditions on
a 12-h light/12-h dark schedule for 1 week. All procedures were
performed according to guidelines for the humane and customary care
and use of experimental animals and followed a protocol prepared by
a veterinary consultant of Stanford University (Stanford, CA, USA)
and approved by the Animal Safety Review Committee of Dr Rath
Research Institute (Santa Clara, CA, USA).
Experimental design
After housing for 1 week, 5- or 6-week-old female
athymic nude mice (n=12) were inoculated subcutaneously with
3×106 HeLa cells in 0.2 ml phosphate-buffered saline
(PBS) and 0.1 ml Matrigel (BD Biosciences, Bedford, MA, USA).
Following the inoculation, the mice were randomly divided into two
groups. These were the control group, in which the mice were fed
regular Purina mouse chow (Laboratory Rodent Diet 5001; Purina
Mills, Richmond, IN, USA), and the NM group, in which the mice were
fed the regular diet supplemented with 0.5% NM (w/w). The mice
consumed an average of 4 g diet/day during the study. Thus, the
supplemented mice received ~20 mg NM per day. After 4 weeks, the
mice were anesthetized/sacrificed by exposure to isoflurane USP
soaked cotton balls (Piramel Healthcare Ltd., Medak, Ap, India) in
a closed environment. The tumors were excised, weighed and
processed for histological examination. The mean weight of the mice
at the initiation and termination of the study did not differ
significantly between the groups.
Immunohistochemistry
Tumors were placed in a formalin cassette and sent
to IDEXX Laboratories, Inc. (Sacramento, CA, USA) and HistoTox
Labs, Inc. (Boulder, CO, USA) for analysis. Formalin-fixed samples
of tumors were trimmed, processed, blocked, sectioned and stained
with hematoxylin and eosin (H&E) and elastic-Van Gieson stains,
and evaluated microscopically by IDEXX Laboratories, Inc. HistoTox
Labs, Inc. conducted PAS histochemistry of the tumor sections as
well as the immunohistochemical analysis of collagens I and IV,
fibronectin, laminin and elastin.
Results
Tumor growth
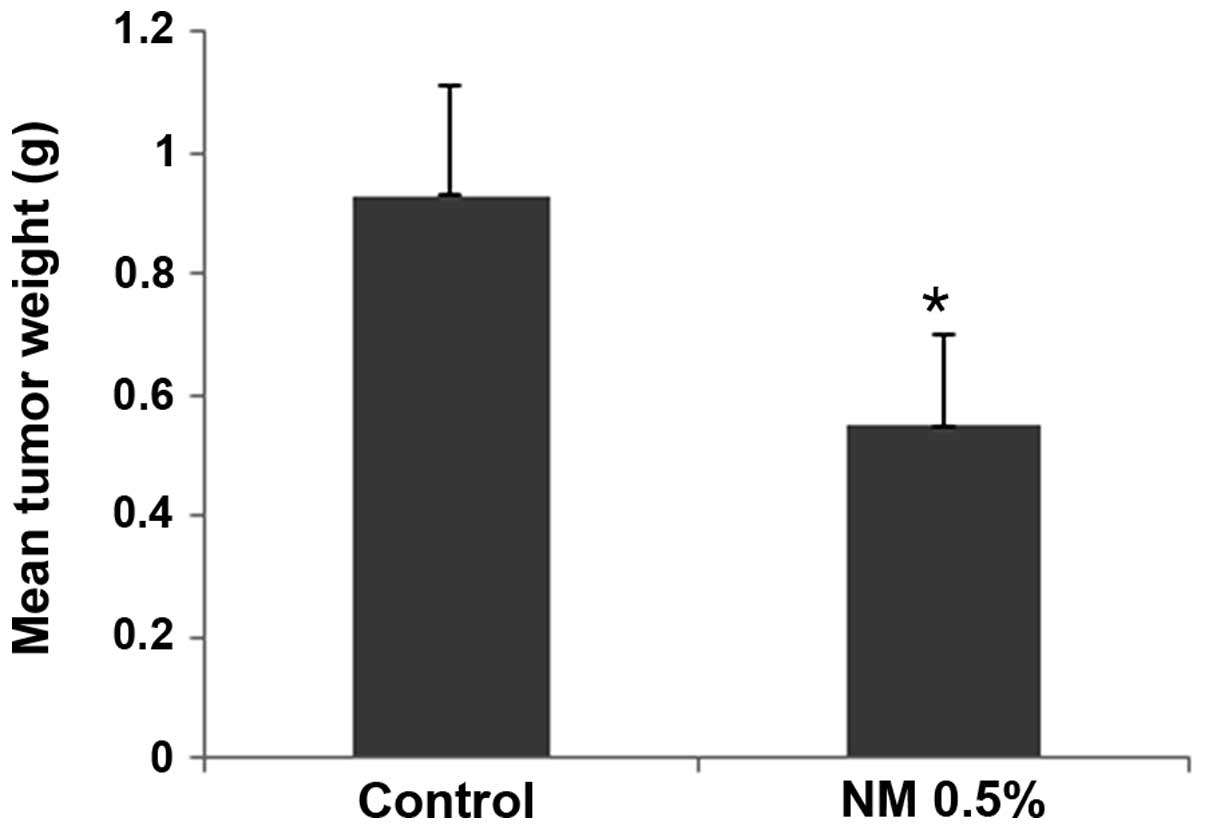
NM strongly inhibited the growth of HeLa xenografts
in nude mice. In the mice that were fed a diet supplemented with
0.5% NM, the tumor weight was inhibited by 59% (P=0.001) compared
with that in the control group mice, as shown in Fig. 1. No significant difference in initial
and final mean body weights was observed between the groups.
Histology of the tumors
Histology of the two groups was comparable. However,
the fibrous capsule in the NM-treated group was prominent while the
tumor border in the untreated group was poorly defined (not
shown).
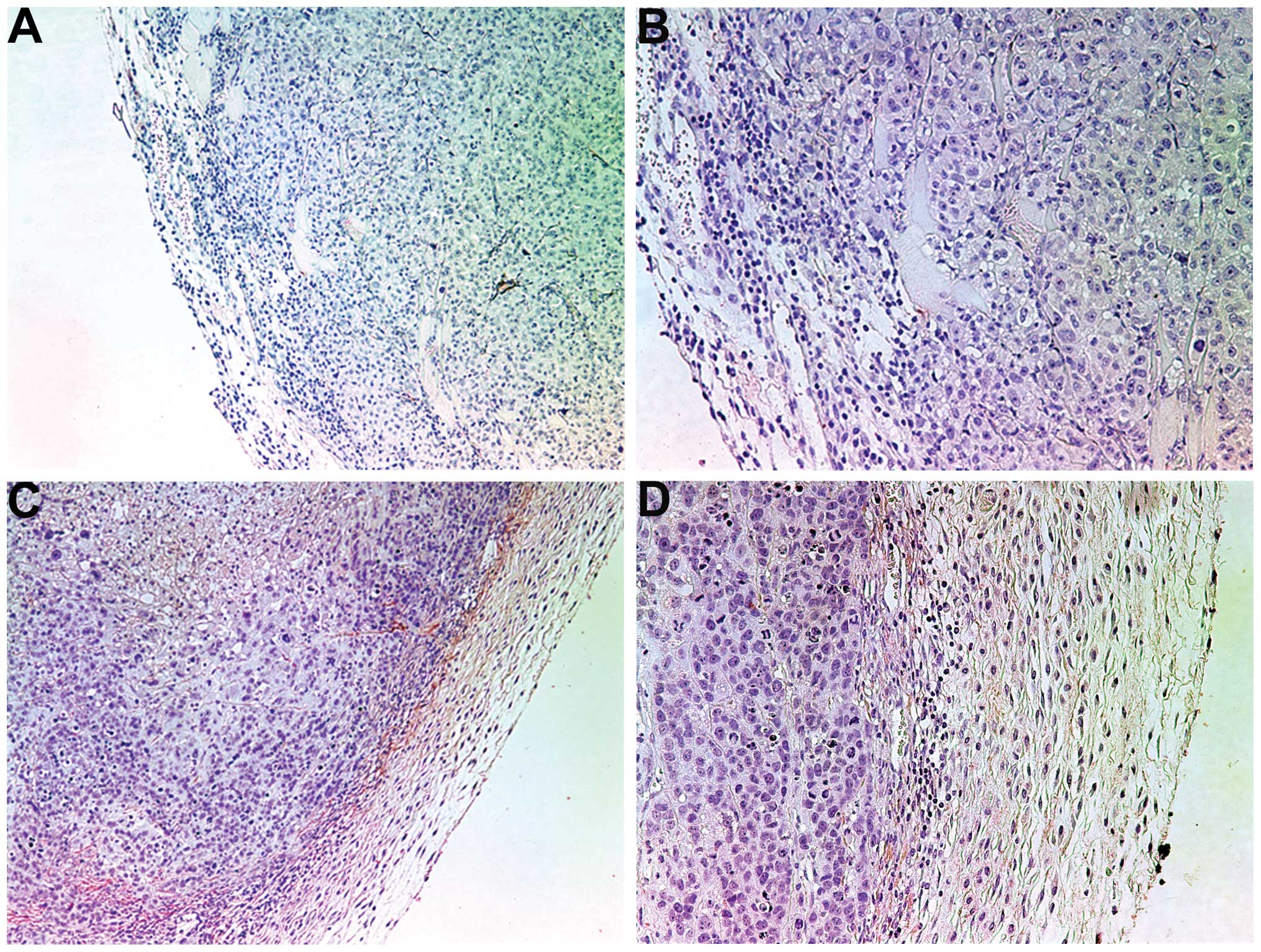
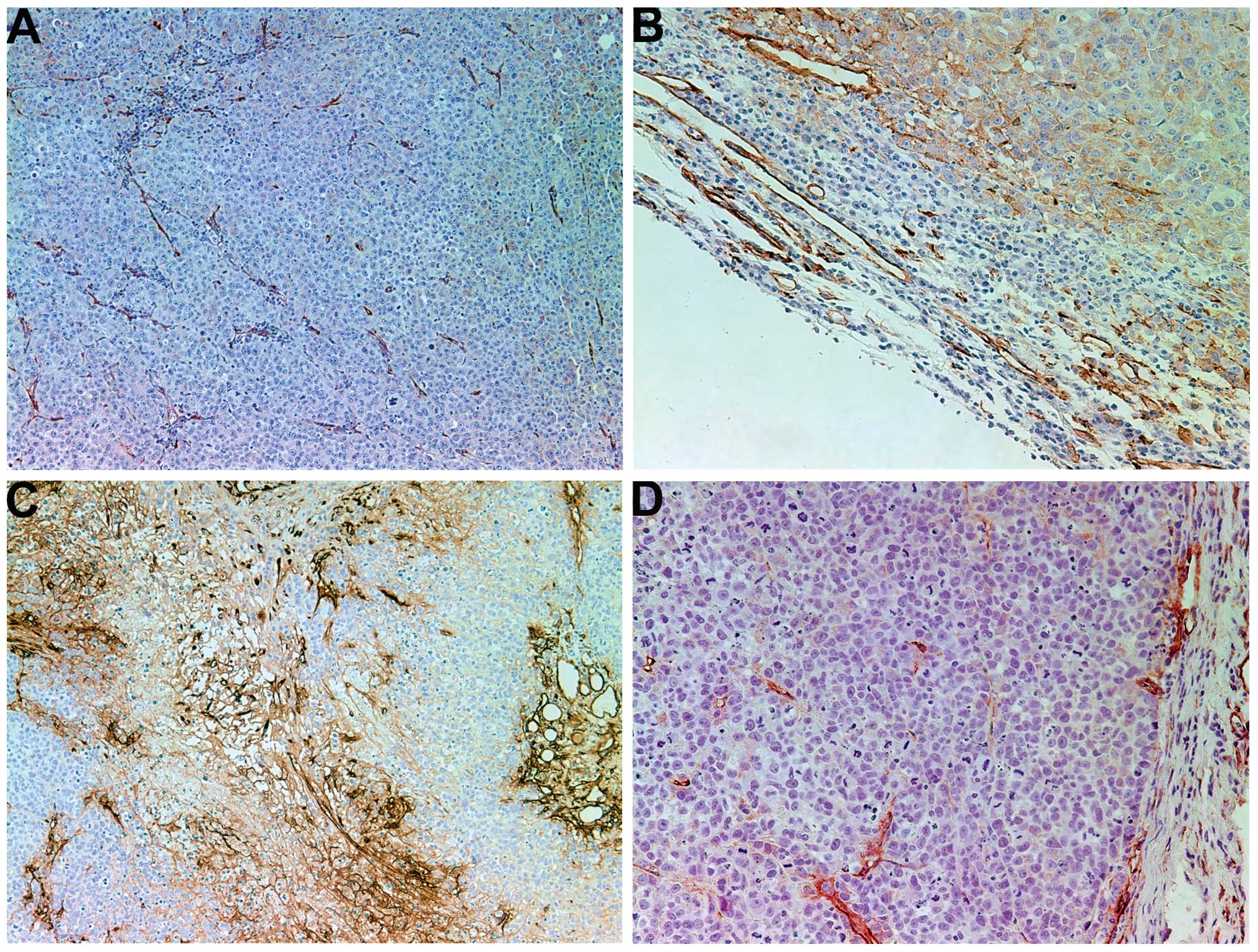
Collagen I
Tumors from control mice exhibited little to no
collagen I expression, either internally or in the fibrous capsule,
as shown in Fig. 2A and B. By
contrast, tumors from the NM-treated mice expressed notably greater
expression levels of collagen I in the fibrous capsule and some
interdigitation and lamellar structures of collagen I within the
tumor (Fig. 2C and D).
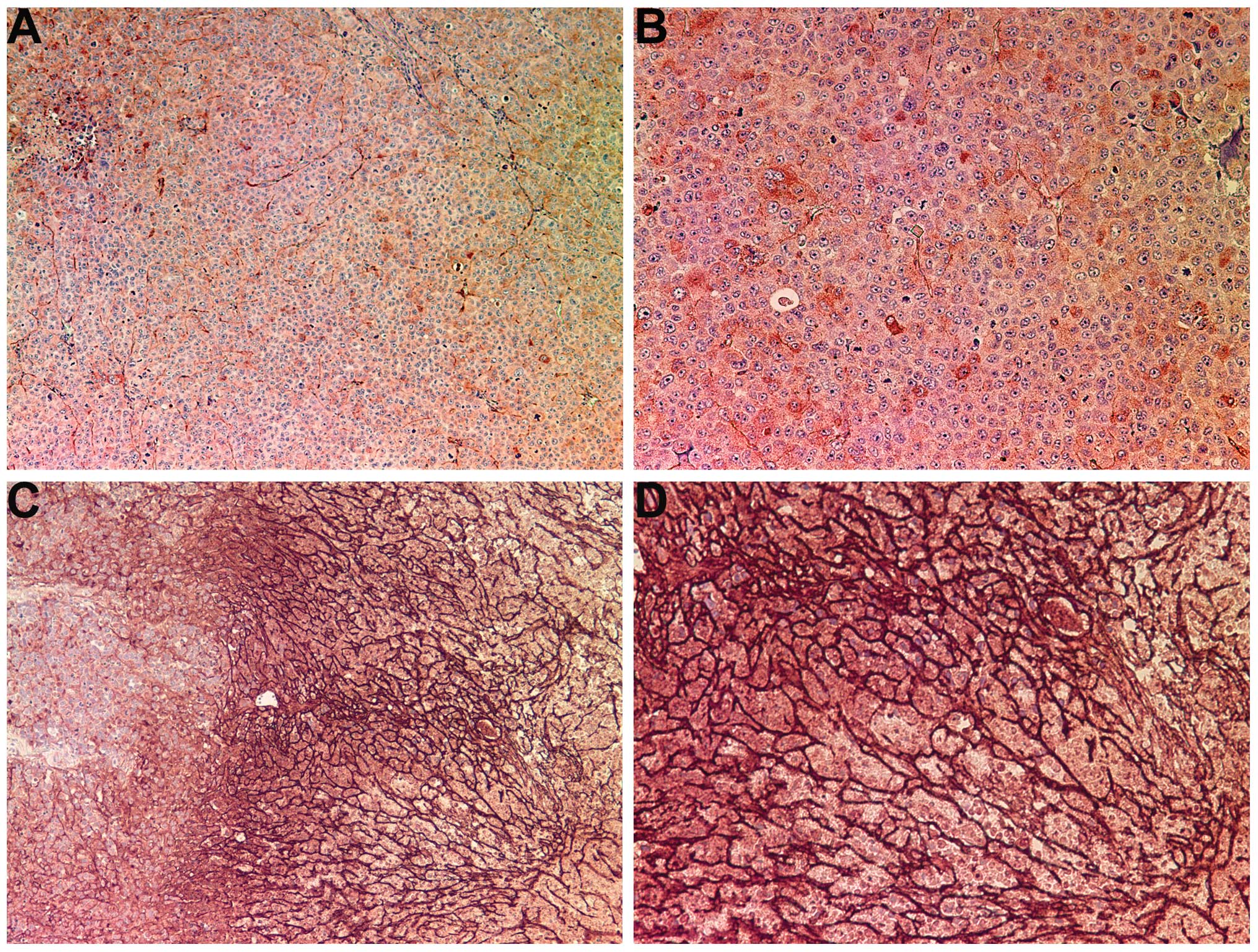
Collagen IV
The control tumors demonstrated diffuse cytoplasmic
and capsular collagen IV with abundant nucleated cells, as shown in
Fig. 3A and B. There was an intense
increase in collagen IV production within the tumors of the mice
that underwent treatment with NM. NM supplementation induced a
dense fibrous network of collagen IV, creating chambers that
surrounded live nucleated cells and large amounts of necrotic cell
debris, as shown in Fig. 3C and D.
The fibrotic network of collagen IV became denser towards the core
of the tumor and the increased density of collagen IV was
associated with a greater amount of cell necrosis, as evidenced by
a lack of nuclear staining.
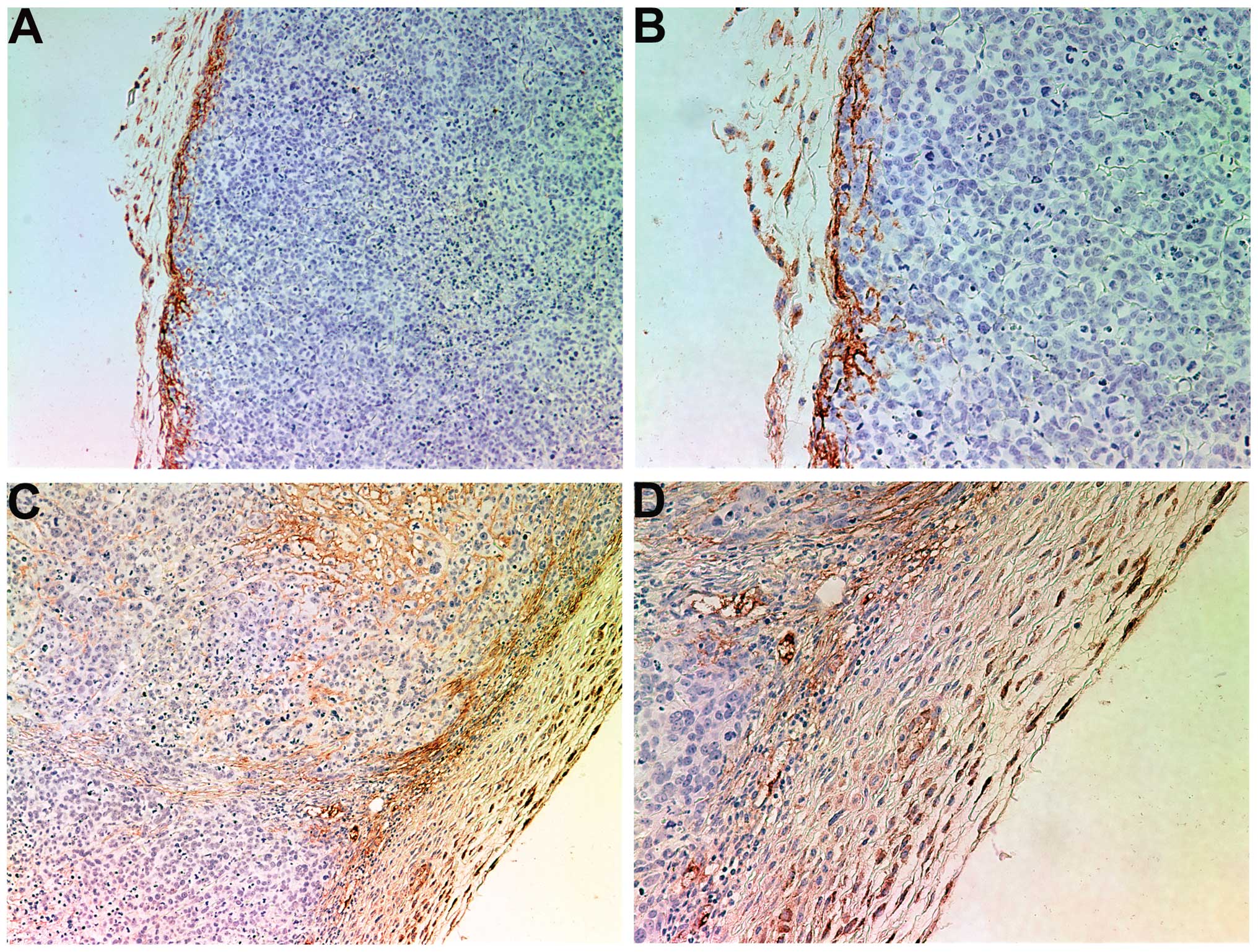
Fibronectin
Less fibronectin appeared in the control tumor
tissue than in the tumors from the NM-treated mice. Tumors from the
control group exhibited intense sporadic internal staining in a
heterogeneous interdigitating pattern with very little staining in
the fibrous capsule (Fig. 4A and B).
Tumors from the NM-treated mice exhibited a well-defined border of
fibronectin in the capsule and intense areas of staining internally
(Fig. 4C and D).
Laminin
Laminin appeared abundantly in the tumors from the
control and NM groups. In the NM group, chamber-like networks of
laminin were formed within the tumors (Fig. 5).
PAS
Tumors from the control group exhibited internal
areas of intense PAS staining (Fig. 6A
and B) whereas the tumors from the NM-treated group showed a
more uniform and diffuse pattern of PAS staining (Fig. 6C and D).
Elastin
No conclusive difference was observed between the
tumors from the control and NM-treated groups (not shown).
Discussion
Tumor cell invasion is associated with degradation
of the ECM, which is intact in normal cells. Among various types of
MMP, MMP-2 and MMP-9 are pivotal in tumor cell invasion and
metastasis as they degrade type IV collagen, a major component of
the ECM (11–13). Barsky et al demonstrated that
in invasive tumors the basement membrane, specifically collagen IV
and laminin components, was thinned, fragmented and disrupted,
while benign and in situ lesions had intact basement
membranes with linear staining of collagen IV and laminin (10). Stromal resistance to invasion is
dependent upon the encapsulation of the neoplastic cells by a
practically impenetrable barrier of dense fibrous tissue (15,20). In
the present study, NM supplementation of HeLa xenograft-bearing
female nude mice resulted in a significant reduction in mean tumor
weight compared with that in the control group. Furthermore,
immunohistochemical staining of the tumors confirmed the protective
effect of nutrient supplementation on the basement membrane.
Control group tumors showed diffuse cytoplasmic and
capsular collagen IV with abundant nucleated cells. In marked
contrast, NM supplementation induced intense internal collagen IV
production, forming a dense fibrous network of collagen IV that
surrounded live nucleated cells and large amounts of necrotic
cellular debris. The fibrotic response observed with NM
supplementation is similar to the collagen deposition reported by
Almholt et al when investigating an experimental pan-MMP
inhibiting drug, which was shown to decrease metastatic burden
100-fold (21). Furthermore, tumors
from the control group mice exhibited little to no collagen I
expression either internally or in the fibrous capsule. By
contrast, tumors from the NM-treated mice expressed greater amounts
of collagen I in the fibrous capsule, and some interdigitation and
lamellar structures of collagen I were present within the
tumor.
Fibronectin, a high molecular weight glycoprotein
binds ECM components such as collagen, fibrin and heparin sulfate
proteoglycans (22). Fibronectin
affects various cellular interactions with the ECM and plays
important roles in cell adhesion, migration, growth and
differentiation (23). Altered
fibronectin expression, degradation and organization have been
associated with cancer progression (23). Tumors from NM-treated mice exhibited
well-defined borders of fibronectin in the capsule and intense
areas of staining internally. By contrast, control tumors showed
less fibronectin staining with a sporadic internal pattern and
little in the fibrous capsule.
Laminins, major glycoproteins in the basal lamina,
influence cell differentiation, migration and adhesion, as well as
phenotype and survival (24). In the
present study, laminin appeared abundantly in tumors from the two
groups. In tumors from the NM-treated mice, chamber-like networks
of laminin were formed internally.
Nutrients such as lysine and ascorbic acid have been
hypothesized to modulate tumor growth and expansion by inhibiting
ECM degradation and MMP activity, and strengthening the integrity
of the connective tissue surrounding cancer cells (15). Based on this approach we developed a
complex of nutrients that can simultaneously affect key cancer
mechanisms through their synergistic effects. The individual
components of this mixture have various effects on certain critical
aspects of cancer. For optimization of the structure of the ECM,
adequate supplies of ascorbic acid and the amino acids lysine and
proline are essential to ensure the proper synthesis and
hydroxylation of collagen fibers. In addition, lysine contributes
to the stability of the ECM as a natural inhibitor of
plasmin-induced proteolysis (15,25).
Manganese and copper are essential cofactors for collagen
formation. Green tea extract has a well-documented ability to
modulate cancer cell growth, metastasis, angiogenesis and other
aspects of cancer progression (26–30).
N-acetyl cysteine and selenium have demonstrated the ability to
inhibit the expression of MMP-9 and invasive activities of tumor
cells, as well as the migration of endothelial cells through ECM
(31–33). Ascorbic acid has demonstrated
cytotoxic and antimetastatic actions on malignant cell lines
(34–39), and it has been observed that the
levels of ascorbic acid in cancer patients are low (40,41). Low
levels of arginine, a precursor of nitric oxide (NO), can limit the
production of NO, which has been shown to predominantly act as an
inducer of apoptosis (42).
The results of the present study demonstrated that
NM potently inhibited tumor growth and enhanced the expression of
ECM proteins in female nude mice with HeLa xenografts, suggesting
the therapeutic value of this specific nutrient complex in the
treatment of cervical cancer. Supplementation with NM has
beneficial effects in optimizing the basement membrane by
increasing the stability and thickness of collagen IV, collagen I
and the glycoproteins supporting the matrix, such as laminin and
fibronectin. Furthermore, the micronutrient mixture appears to be
safe to use. In a previous in vivo study addressing safety,
it was observed that administering NM at doses of 30, 90 or 150
mg/day to adult female osteogenic disorder Shionogi (ODS) rats
(weighing 250–300 g) for 7 days, had no adverse effects on vital
organs (heart, liver and kidney). In addition, it did not adversely
affect functional serum enzymes, indicating that the NM is safe to
use even at these high doses, which far exceed the normal
equivalent dosage of the nutrient (43).
Acknowledgements
This study was funded by Dr. Rath Health Foundation
(Santa Clara, CA, USA), a non-profit organization. The authors
would particularly like to thank Earl Rainey for maintenance of the
animal colony.
References
|
1
|
Jemal A, Bray F, Center MM, Ferley J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Cancer Society, . Cervical
cancer: What are the key statistics about cervical cancer?
http://www.cancer.org/cancer/cervicalcancer/detailedguide/cervical-cancer-key-statisticsLast
revised. January 31–2014 June 9–2014
|
|
3
|
Cancer.net, . Cervical cancer: Statistics.
http://www.cancer.net/cancer-types/cervical-cancer/statisticsLast
reviewed. April;2014 June 9–2014
|
|
4
|
Fidler IJ: Molecular biology of cancer:
Invasion and metastasisCancer Principles and Practice of Oncology.
DeVita VT Jr, Hellman S and Rosenberg SA: 5th. Lippincott-Raven;
Philadelphia, PA: pp. 135–152. 1997
|
|
5
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29:(Suppl 16). 15–18. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chambers AF and Matrisian LM: Changing
views on the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kleiner DE and Stetler-Stevenson WG:
Matrix metalloproteinases and metastasis. Cancer Chemother
Pharmacol. 43:(Suppl). 42–51. 1999. View Article : Google Scholar
|
|
9
|
Yurchenko PD and Schitny JC: Molecular
architecture of basement membranes. FASEB J. 4:1577–1590.
1990.PubMed/NCBI
|
|
10
|
Barsky SH, Siegel GP, Jannotta F and
Liotta LA: Loss of basement membrane components by invasive tumors
but not by their benign counterparts. Lab Invest. 49:140–147.
1983.PubMed/NCBI
|
|
11
|
Liotta LA, Tryggvason K, Garbisa S, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stetler-Stevenson WG: The role of matrix
metalloproteinases in tumor invasion, metastasis and angiogenesis.
Surg Oncol Clin N Am. 10:383–392. 2001.PubMed/NCBI
|
|
13
|
Stetler-Stevenson WG: Type IV collagenases
in tumor invasion and metastasis. Cancer Metastasis Rev. 9:289–303.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davidson B, Goldberg I, Koplovic J,
Lerner-Geva L, Gottlieb WH, Weiss B, Ben-Baruch G and Reich R:
Expression of matrix metalloproteinase-9 in squamous cell carcinoma
of the uterine cervix - clinicopathologic study using
immunohistochemistry and mRNA in situ hybridization. Gynecol
Oncol. 72:380–386. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rath M and Pauling L: Plasmin-induced
proteolysis and the role of apoprotein (a), lysine and synthetic
analogs. J Orthomol Med. 7:17–23. 1992.
|
|
16
|
Andreasen PA, Kjøller L, Christensen L and
Duffy MJ: The urokinase-type plasminogen activator system in cancer
metastasis: A review. Int J Cancer. 72:1–22. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niedzwiecki A, Roomi MW, Kalinovsky T and
Rath M: Micronutrient synergy - a new tool in effective control of
metastasis and other key mechanisms of cancer. Cancer Metastasis
Rev. 29:529–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roomi MW, Kalinovsky T, Rath M and
Niedzwiecki A: Modulation of u-PA, MMPs and their inhibitors by a
novel nutrient mixture in human female cancer cell lines. Oncol
Rep. 28:768–776. 2012.PubMed/NCBI
|
|
19
|
Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Suppression of human cervical cancer cell
lines Hela and DoTc2 4510 by a mixture of lysine, proline, ascorbic
acid and green tea extract. Int J Gynecol Cancer. 16:1241–1247.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cameron E, Pauling L and Leibovitz B:
Ascorbic acid and cancer: A review. Cancer Res. 39:663–681.
1979.PubMed/NCBI
|
|
21
|
Almholt K, Juncker-Jensen A, Laerum OD,
Danø K, Johnsen M, Lund LR and Rømer J: Metastasis is strongly
reduced by the matrix metalloproteinase inhibitor Galardin in the
MMTV-PymT transgenic breast cancer model. Mol Cancer Ther.
7:2758–2767. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pankov R and Yamada KM: Fibronectin at a
glance. J Cell Sci. 115:3861–3863. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ruoslahti E: Fibronectin and its integrin
receptors in cancer. Adv Cancer Res. 76:1–20. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Timpl R, Rohde H, Robey PG, Rennard SI,
Foidart JM and Martin GR: Laminin - a glycoprotein from basement
membranes. J Biol Chem. 254:9933–9937. 1979.PubMed/NCBI
|
|
25
|
Sun Z, Chen YH, Wang P, Zhang J, Gurewich
V, Zhang P and Liu JN: The blockage of high-affinity lysine binding
sites of plasminogen by EACA significantly inhibits
prourokinase-induced plasminogen activation. Biochem Biophys Acta.
1596:182–192. 2002.PubMed/NCBI
|
|
26
|
Valcic S, Timmermann BN, Alberts DS,
Wachter GA, Krutzsch M, Wymer J and Guillén JM: Inhibitory effect
of six green tea catechins and caffeine on the growth of four
selected human tumor cell lines. Anticancer Drugs. 7:461–468. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mukhtar H and Ahmed N: Tea polyphenols:
Prevention of cancer and optimizing health. Am J Clin Nutr. 71:(Sul
6). 1698S–1702S. 2000.PubMed/NCBI
|
|
28
|
Yang CY, Liao J, Kim K, Yurtow EJ and Yang
CS: Inhibition of growth and induction of apoptosis in human cancer
cell lines by tea polyphenols. Carcinogenesis. 19:611–616. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Taniguchi S, Fujiki H, Kobayashi H, Go H,
Miyado K, Sadano H and Shimikawa R: Effect of (−) epigallocatechin
gallate, the main constituent of green tea, on lung metastasis with
mouse B16 melanoma cell lines. Cancer Lett. 65:51–54. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hara Y: Green Tea - Health Benefits and
Applications. Marcel Dekker; New York, NY: 2001, View Article : Google Scholar
|
|
31
|
Kawakami S, Kageyama Y, Fujii Y, Kihara K
and Oshima H: Inhibitory effects of N-acetyl cysteine on invasion
and MMP-9 production of T24 human bladder cancer cells. Anticancer
Res. 21:213–219. 2001.PubMed/NCBI
|
|
32
|
Morini M, Cai T, Aluigi MG, Noonan DM,
Masiello L, De Floro S, D'Agostinin F, Albini A and Fassima G: The
role of the thiol N-acetyl cysteine in the prevention of tumor
invasion and angiogenesis. Int J Biol Markers. 14:268–271.
1999.PubMed/NCBI
|
|
33
|
Yoon SO, Kim MM and Chung AS: Inhibitory
effects of selenite on invasion of HT 1080 tumor cells. J Biol
Chem. 276:20085–20092. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cha J, Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Ascorbate supplementation inhibits growth
and metastasis of B16FO melanoma and 4T1 breast cancer cells in
vitamin C deficient mice. Int J Oncol. 42:55–64. 2013.PubMed/NCBI
|
|
35
|
Naidu KA, Karl RC, Naidu KA and Coppola D:
Antiproliferative and proapoptotic effect of ascorbyl stearate in
human pancreatic cancer cells: Association with decreased
expression of insulin-like growth factor 1 receptor. Dig Dis Sci.
48:230–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Anthony HM and Schorah CJ: Severe
hypovitaminosis C in lung-cancer patients: The utilization of
vitamin C in surgical repair and lymphocyte-related host
resistance. Br J Cancer. 46:354–367. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maramag C, Menon M, Balaji KC, Reddy PG
and Laxmanan S: Effect of vitamin C on prostate cancer cells in
vitro: effect on cell number, viability and DNA synthesis.
Prostate. 32:188–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Koh WS, Lee SJ, Lee H, Park C, Park MH,
Kim WS, Yoon SS, Park K, Hong SI, Chung MH and Park CH:
Differential effects and transport kinetics of ascorbate
derivatives in leukemic cell lines. Anticancer Res. 8:2487–2493.
1998.
|
|
39
|
Chen Q, Espey MG, Krishna MC, Mitchell JB,
Corpe CP, Buettner GR, Shacter E and Levine M: Pharmacologic
ascorbic acid concentrations selectively kill cancer cells: Action
as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl
Acad Sci USA. 102:13604–13609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Núñez Martín C and Ortiz de Apodaca y Ruiz
A: Ascorbic acid in the plasma and blood cells of women with breast
cancer. The effect of consumption of food with an elevated content
of this vitamin. Nutr Hosp. 10:368–372. 1995.PubMed/NCBI
|
|
41
|
Kurbacher CM, Wagner U, Kolster B,
Andreotti PE, Krebs D and Bruckner HW: Ascorbic acid (vitamin C)
improves the antineoplastic activity of doxorubicin, cisplatin and
paclitaxel in human breast carcinoma cells in vitro. Cancer Lett.
103:183–189. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cooke JP and Dzau VJ: Nitric oxide
synthase: Role in the genesis of vascular disease. Annu Rev Med.
48:489–509. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Roomi MW, Ivanov V, Netke S, Niedzwiecki A
and Rath M: Serum markers of the liver, heart and kidney and lipid
profile and histopathology in ODS rats treated with nutrient
synergy. J Am Coll Nutr. 22:4772003.
|