Introduction
Microcystic adnexal carcinoma (MAC) is a rare,
locally aggressive type of adenocarcinoma with low-grade malignancy
and extremely low metastatic ability (1–3). Since
the first report of a MAC in 1982 by Goldstein et al
(1), only ~300 cases of MAC have
been described to date. MACs are also known as sclerosing sweat
duct carcinomas, eccrine epitheliomas and syringomatous carcinomas
(4–6). MACs are most frequently located in the
head and neck area (7); however, the
occurrence of nasal MACs is extremely rare. MAC is usually
diagnosed by detection of characteristic histological features
(1–3). Histologically, MACs contain numerous
keratin cysts, basaloid cells and squamous cell islands that form
ductular and glandular structures, which often invade nerves
(2,3). These characteristics complicate the
surgical treatment of MAC (8). There
are several therapies to treat MAC, including standard excision
(SE), Mohs micrographic surgery (MMS), irradiation and chemotherapy
(3–6); however, there is no consensus regarding
the treatment of MAC due to its rarity. Furthermore, there is no
reported incidence rate of MAC since it is so rare.
In the present study, a novel case of a Chinese man
with a MAC located on the nasal dorsum and nosewing is described.
Mohs micrographic surgery (MMS) was performed to ensure the
complete removal of tumorous tissue. In addition, successful
reconstruction of the area was accomplished using an expanded
rotational forehead skin flap, which restored the normal nose shape
and skin color. There were no signs of tumor recurrence after a
three-year follow-up period, indicating the effectiveness of MMS
for the treatment of MAC.
Case report
Patient information
A 44-year-old Chinese man was admitted to the
117th Hospital of Peoples Liberation Army (Hangzhou,
China) following repeated local infections to his nose. An initial
injury had occurred 20 years previously when the nose was subjected
to blunt trauma inflicted by a brick. Treatment at a local hospital
12 years prior had resulted in a nasal scar and a gradually
enlarging mass. Aside from this complaint, the patient exhibited a
normal general condition.
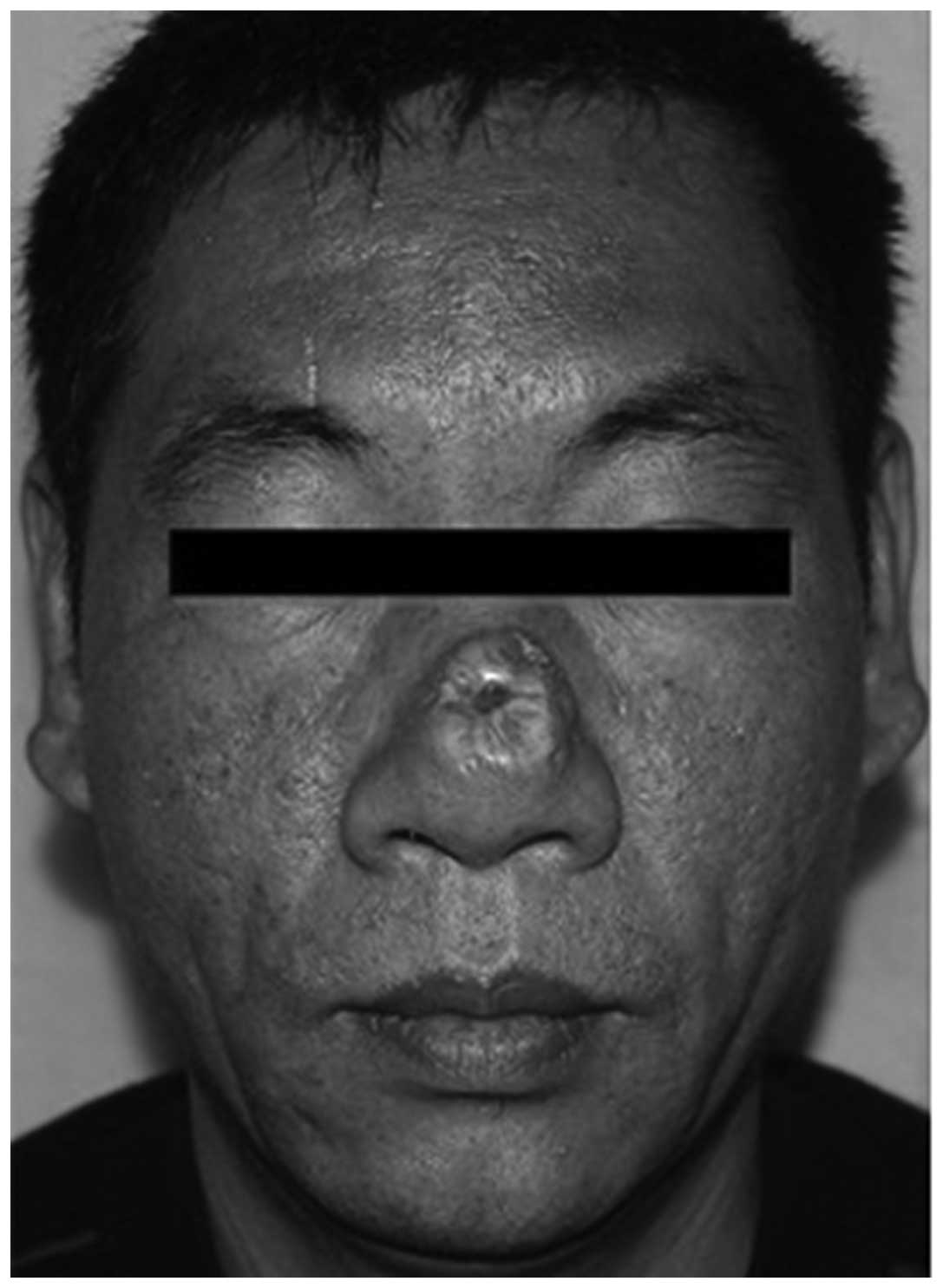
A physical examination revealed a hypertrophic
deformity of the nose and an indurated scar plaque, measuring
2.0×2.0 cm, located on the nasal dorsum and nosewing, with no
redness, pain, ulceration or scaling (Fig. 1). Compared with the surrounding skin,
the plaque was raised and slightly lighter in color. The nasal
cavity sustained normal ventilation and olfactory sensation,
without purulent discharge or nasal septum deviation. No
lymphadenopathy was observed, and routine laboratory examinations
(including routine blood and urine tests, biochemical,
electrocardiographic, chest x-ray, and abdominal B ultrasonic
examinations) produced normal results. The patient had no
significant medical history, no known incidence of similar disease
in his family history and had no known exposure to radiation or
chemicals. The patient provided informed consent, and the study was
approved by the Ethics Committee of the 117th Hospital
of People's Liberation Army.
Surgical methods
A forehead tissue expander (Kidney type, 100ml;
Shanghai Winner Plastic Surgery Products Co., Ltd, Shanghai, China)
was implanted under local anesthesia (lidocaine hydrochloride,
Shanghai Zhaohui Pharmaceutical Co. Ltd., Shanghai, China; Fig. 2). The expander was inflated with
saline (Hunan Kelun Pharmaceutical Co. Ltd., Yueyang, China) every
4–7 days for 2 months, until a final volume of ~132 ml was reached.
Following the final filling of the expander, the expander was
removed and an extensive resection of the tumor was performed. A
0.5-cm tumor-free margin surrounding the skin lesion was identified
and excised using MMS (9). Immediate
reconstruction was performed using an expanded rotational forehead
skin flap (Figs. 2 and 3). Two months after the reconstruction, the
flap was separated from its pedicle (Fig. 4). The stitches (model no. 0) were
removed the following week, and the patient was discharged from
hospital. The reconstruction of the defect maintained normal nasal
function and improved esthetics (Fig.
5). No recurrence was detected during a three-year follow-up
period.
Histology
Macroscopic observations revealed that the tumor was
round and rough, with beige coloring and a diameter of ~2.5 cm.
Microscopic examination (Olympus microscope BX43; Olympus, Tokyo,
Japan) demonstrated a heterogeneous tumor cell composition with
multiple differentiations. The external portion of the tumor
consisted of solid cell nests (Fig.
6A) and cystic structures with capsular spaces (Fig. 6B), in addition to ductal cells of an
adnexal cell origin. The medial portion of the tumor consisted of
microductal structures formed by ductal epithelial cells, which
invaded the deeper tissues without evident atypia (Fig. 6C). The tumor cells presented a normal
nuclear to cytoplasmic ratio and minimal mitotic activity (Fig. 6D). Furthermore, the tissue in the
deepest region of the tumor exhibited interstitial sclerosis and
collagenization (Fig. 6E).
Immunohistochemical analysis revealed positive staining for
epithelial membrane antigen (EMA; MAB-0581, Fuzhou Maixin
Biotechnology Development Co. Ltd., Fuzhou, China), cytokeratin
(CK; MAB-0049, Fuzhou Maixin Biotechnology Development Co. Ltd.),
B-cell lymphoma 2 (Bcl-2; MAB-0014, Fuzhou Maixin Biotechnology
Development Co. Ltd.) and S-100 proteins (MAB-0585, Fuzhou Maixin
Biotechnology Development Co. Ltd.), with negative staining for CK7
(kit-0021, Fuzhou Maixin Biotechnology Development Co. Ltd.), CK20
(MAB-0057, Fuzhou Maixin Biotechnology Development Co. Ltd.) and
smooth muscle actin (SMA; MAB-0575, Fuzhou Maixin Biotechnology
Development Co. Ltd.) proteins. Based on these results, a diagnosis
of MAC (low-grade malignancy) was confirmed.
Discussion
Although the etiology of MACs is unclear, previous
studies have reported an association between MACs and exposure to
radiation (2,10). MACs generally affect the lips and
facial regions in adult patients, and are more prevalent among
females. The appearance of a MAC is usually similar to a depressed
scar with no clinical symptoms, but may present with occasional
swelling and numbness, and rarely producing ulcerations (1–3). These
tumors are able to develop gradually for a number of months or
years in a locally aggressive mode. MACs have a 50% postoperative
local recurrence rate, with very rare instances of metastasis
(1,2,11). The
macroscopic characteristics of the MAC case reported in the present
study were similar to those reported in previous literature
(2,3,7,9,10). Since
there was no incidence of similar disease in the patient's family
history and no radioactive or chemical exposure, the cause of the
disease was presumed to be associated with the repeated local
infections.
A diagnosis of a MAC is usually established based on
the detection of characteristic histological features (1–3,12). Microscopic examinations show numerous
keratocysts above the dermis layer of the tumor. In addition, the
tumor cells are basal-like, and certain tumor cells have
transparent cytoplasm, forming squamous cell clusters, island or
cords, or are arranged in a grid. Furthermore, in the medial
portion of the tumor, there are ductular and glandular structures.
Mitotic structures are rare in these tumor cells, and they lack
significant atypia. MAC tumor cells invade the whole dermis,
frequently invading the striated muscle, nerves, vascular
adventitia, cartilage tissue and periosteum (1–3,13). Although MACs have the ability to
locally invade tissues, very few cases have reported systemic
metastasis and mortality. In the present case, the histological
characteristics of the MAC were similar to those detected in
previous studies (1,4,7,8).
Although a diagnosis of MAC may be confirmed based
on the detection of certain histological characteristics,
immunohistochemical confirmation may be a useful ancillary
diagnostic tool. In the present case, immunohistochemical staining
was positive for EMA, CK, Bcl-2 and S-100 proteins, and negative
for CK20, CK7 and SMA. By contrast, in the study by Smith et
al (14), 10 MAC cases were
analyzed and all the MAC tumor cells were shown to exhibit a
distinctive pattern of staining for CK7 and α-SMA, in addition to
CD34, EMA, Ber-EP4 and S-100 proteins. However, the tumor cells
were negative for c-erbB-2. In the present case, <2% of the
tumor cells were Ki-67-positive, which was in accordance with the
results of Smith et al (14),
who found that <5% of tumor cells were Ki-67-positive in MAC.
These results indicate that the low level of Ki-67 reflects the low
proliferative rate, while the additional immunohistochemical
markers support the divergent patterns of adnexal differentiation
in MAC. Additionally, Hoang et al (15) reported that CK15 was a useful marker
for distinguishing MAC from cases of infiltrative basal cell
carcinoma and squamous cell carcinoma with ductal differentiation.
However, further studies are required to identify a reliable
immunohistochemical marker specific to MACs.
Therapy for MACs remains a challenge, as the
tumor-free margin is significantly more extensive than can be
determined clinically and the tumors exhibit a marked tendency for
perineural invasion (3–5). Numerous treatment modalities have been
used to date, including standard excision (SE), MMS, irradiation
and chemotherapy (6,16); however, a number of studies have
found that MAC is not sensitive to radiotherapy and chemotherapy
(5,17). Although the extent of resection using
SE is reduced and the technique results in improved esthetics, the
tumor recurrence rate is higher. Chiller et al (18) observed that 30% of MAC patients that
initially underwent SE treatment were required to submit to SE or
MMS at least once again, since the margins of the specimens were
positive for tumor cells. In addition, Chow et al (19) reported that 40–60% of patients
experienced one or more local recurrences following standard wide
local excision.
MMS is a therapeutic approach for skin cancer
removal that aims to achieve the highest possible cure rates, while
minimizing the size of the wound and consequent distortions. MMS
involves the removal of the tumor in stages by histologically
confirming clear margins on frozen sections and by treating the
resultant defect. A number of studies have been published regarding
the treatment of MACs with MMS. Chiller et al (18) observed that the size of the affected
surface area after full excision with MMS was four times larger
compared with the clinically apparent size. Therefore, this method
ensures complete excision of the cancerous tissue, and subsequently
the recurrence rates following MMS are low (0–12%) (11). In the present study, the patient was
treated with MMS following reconstruction, and no tumor recurrence
was detected during the three-year follow-up period.
These results indicate that the therapeutic and
esthetic advantages of MMS may enable the surgical technique to
become a useful therapeutic option for the complete eradication of
MAC. However, the repair of the resulting defect is a considerable
reconstructive challenge, particularly in the centrofacial region.
In the present study, a 0.5-cm tumor-free margin surrounding the
skin lesion was identified using MMS and the contained area was
excised, with the incision depth reaching into the deep fasciae.
The defect was too large to directly suture or repair with a local
flap. Therefore, an innovative reconstruction approach was
attempted using an expanded rotational forehead skin flap, which
resulted in normal function and esthetics. Based on the present
case, a key factor for successful reconstruction is the full
expansion of the skin flap with a sufficient volume of saline. A
similar procedure was reported by Rustemeyer et al (20), who used a rotational flap from the
cheek and lower lip in combination with an Abbe flap for philtrum
and vermilion reconstruction, which resulted in a successful
reconstruction following the complete resection of the MAC.
In conclusion, the present study was the first to
report the case of a Chinese man with a MAC located on the nasal
dorsum and nosewing. For similar cases of MAC, complete surgical
resection with MMS is recommended as the most effective therapeutic
option. An expanded rotational forehead skin flap may be used for
reconstruction to achieve normal function and esthetics.
Acknowledgements
The authors thank the Ethics Committee of the
117th Hospital of People's Liberation Army for their
assistance.
References
|
1
|
Goldstein DJ, Barr RJ and Santa Cruz DJ:
Microcystic adnexal carcinoma: A distinct clinicopathologic entity.
Cancer. 50:566–572. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu JB, Blitzblau RC, Patel SC, Decker RH
and Wilson LD: Surveillance, Epidemiology, and End Results (SEER)
database analysis of microcystic adnexal carcinoma (sclerosing
sweat duct carcinoma) of the skin. Am J Clin Oncol. 33:125–127.
2010.PubMed/NCBI
|
|
3
|
Bier-Laning cm, Hom DB, Gapany M, Manivel
JC and Duvall AJ III: Microcystic adnexal carcinoma: Management
options based on long-term follow-up. Laryngoscope. 105:1197–1201.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lipper S and Peiper SC: Sweat gland
carcinoma with syringomatous features: A light microscopic and
ultrastructural study. Cancer. 44:157–163. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Glatt HJ, Proia AD, Tsoy EA, Fetter BF,
Klintworth GK, Neuhaus R and Font RL: Malignant syringoma of the
eyelid. Ophthalmology. 91:987–990. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alessi E and Caputo R: Syringomatous
carcinoma of the scalp presenting as a slowly enlarging patch of
alopecia. Am J Dermatopathol. 15:503–505. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beltramini GA, Baj A, Moneghini L, Poli T,
Combi VA and Giannì AB: Microcystic adnexal carcinoma of the
centrofacial region: A case report. Acta Otorhinolaryngol Ital.
30:2132010.PubMed/NCBI
|
|
8
|
Wetter R and Goldstein GD: Microcystic
adnexal carcinoma: A diagnostic and therapeutic challenge. Dermatol
Ther. 21:452–458. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Leibovitch I, Huilgol SC, Selva D, Lun K,
Richards S and Paver R: Microcystic adnexal carcinoma: Treatment
with Mohs micrographic surgery. J Am Acad Dermatol. 52:295–300.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abbate M, Zeitouni NC, Seyler M, Hicks W,
Loree T and Cheney T: Clinical course, risk factors, and treatment
of microcystic adnexal carcinoma: A short series report. Dermatol
Surg. 29:1035–1038. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Snow S, Madjar DD, Hardy S, Bentz M,
Lucarelli MJ, Bechard R, Aughenbaugh W, McFadden T, Sharata H,
Dudley C, et al: Microcystic adnexal carcinoma: Report of 13 cases
and review of the literature. Dermatol Surg. 27:401–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sirikanjanapong S, Seymour AW and Amin B:
Cytologic features of microcystic adnexal carcinoma. Cytojournal.
8:52011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamsch C and Hartschuh W: Microcystic
adnexal carcinoma - aggressive infiltrative tumor often with
innocent clinical appearance. J Dtsch Dermatol Ges. 8:275–278.
2010.(In English and German). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith KJ, Williams J, Corbett D and
Skelton H: Microcystic adnexal carcinoma: An immunohistochemical
study including markers of proliferation and apoptosis. Am J Surg
Pathol. 25:464–471. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoang MP, Dresser KA, Kapur P, High WA and
Mahalingam M: Microcystic adnexal carcinoma: An immunohistochemical
reappraisal. Mod Pathol. 21:178–185. 2008.PubMed/NCBI
|
|
16
|
Pugh TJ, Lee NY, Pacheco T and Raben D:
Microcystic adnexal carcinoma of the face treated with radiation
therapy: A case report and review of the literature. Head Neck.
34:1045–1050. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stein JM, Ormsby A, Esclamado R and Bailin
P: The effect of radiation therapy on microcystic adnexal
carcinoma: A case report. Head Neck. 25:251–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiller K, Passaro D, Scheuller M, Singer
M, McCalmont T and Grekin RC: Microcystic adnexal carcinoma:
Forty-eight cases, their treatment, and their outcome. Arch
Dermatol. 136:1355–1359. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chow WC, Cockerell CJ and Geronemus RG:
Microcystic adnexal carcinoma of the scalp. J Dermatol Surg Oncol.
15:768–771. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rustemeyer J, Zwerger S, Pörksen M and
Junker K: Microcystic adnexal carcinoma of the upper lip
misdiagnosed benign desmoplastic trichoepithelioma. Oral Maxillofac
Surg. 17:141–144. 2013. View Article : Google Scholar : PubMed/NCBI
|