Introduction
Brucella organisms are facultative,
intracellular bacteria of animals and humans that can cause
diseases of worldwide significance (1,2).
Brucella infections can result in a variety of acute
diseases, such as epididymitis or abortion in animals, and fever,
arthritis, dementia and meningitis in humans (3–5).
Currently, an effective and safe vaccine targeting Brucella
for animals and humans does not exist. Therefore, low virulence and
high protective vaccines are important to prevent the spread of
disease.
Brucella melitensis M5-90 is the only
approved vaccine currently available for protection against B.
melitensis infection in China (6). Vaccination with M5-90 induces
significant protection in sheep and goats. In addition, M5-90
administration has decreased the incidence of brucellosis in
animals and humans, and is routinely administered to sheep and
goats to prevent brucellosis. However, the M5-90 vaccine has a
number of disadvantages. For example, the vaccination has been
found to cause abortions if administered to pregnant animals.
Furthermore, M5-90 can cause local hypersensitivity reactions in
cases of accidental inoculation. Therefore, the development of a
less virulent and more efficient vaccine to prevent and control
brucellosis is crucial. The deletion of virulence genes is required
for the development of live vaccines against B. melitensis
infection that are superior to M5-90 (7).
The two-component regulatory system (TCS) is one of
the most important virulence regulatory systems in Brucella,
and genome sequencing has revealed 21 putative TCSs in the
Brucella genus (8). TcfSR is
one of TCSs, and is located in chromosome II (9). TCSs can coordinate an intricate network
of virulence genes to allow the host cells to sense environmental
varieties and to subsequently exert an appropriate response in
Brucella.
In the present study, the effect of the B.
melitensis 16M TcfSR promoter mutant (16MΔTcfSR) on virulence
was investigated. The aim of the current study was to determine
whether 16MΔTcfSR may be useful as an attenuated live B.
melitensis vaccine.
Materials and methods
Bacterial strains, plasmids, cells and
mice
B. melitensis strain 16M and the M5-90
vaccine strain were obtained from the Center of Chinese Disease
Prevention and Control (Beijing, China). Brucella was
cultured in tryptone soya agar (TSA) or tryptone soya broth
(Sigma-Aldrich, St. Louis, MO, USA), while Escherichia coli
strain DH5α cells were grown on Luria-Bertani medium. The
pGEM-7Zf+ plasmid was purchased from Promega Corporation
(Madison, WI, USA) and a RAW 264.7 murine macrophage cell line was
purchased from the Cell Resource Center at the Institute of Basic
Medical Sciences of the Chinese Academy of Medical Sciences/Peking
Union Medical College (Beijing, China). A total of 290 BALB/c
female mice (age, 6 weeks) were obtained from the Experimental
Animal Center of the Academy of Military Medical Science (Beijing,
China). All experimental procedures and animal care protocols were
performed in compliance with institutional animal care regulations.
The present study was approved by the ethics committee of Shihezi
University (Shihezi, China).
Construction of the 16MΔTcfSR
mutant
The sequence of the TcfSR promoter region was
predicted using Neural Network Promoter Prediction software
(http://www.fruitfly.org/seq_tools/promoter.html). The
specific DNA sequences for TcfSR and homologous arms were screened
from GenBank (http://www.ncbi.nlm.nih.gov/nuccore/179
86243?from=1053312&to=1054655&sat=4&sat_key=105779
979), and Primer 5.0 software (Premier Biosoft, Palo Alto, CA,
USA) was used to design all polymerase chain reaction (PCR)
primers. Two pairs of primers with restriction sites at the 5′ ends
were designed for amplification of the upstream (1,026 bp) and
downstream (1,024 bp) arms of the B. melitensis 16M TcfSR
promoter, in which the XhoI, KpnI and SacI
(underlined) sites were integrated into the two PCR fragment ends.
The primer sequences were as follows: TcfSR-N-terminal forward, CTC
GAG AGC CGC TAT TAT ACC GGA, and reverse, GGT ACC TTG GCC GAT AAT
GAT TGC; TcfSR-C-terminal forward, GGT ACC ATG AGA ATT ATC CTC ATC
GAA G, and reverse, GAG CTC GTC TGG AAA CCC ATG GTG. The two arms
of the 16M TcfSR promoter were cloned into a T-Vector pMD19 simple
vector (Takara Bio, Inc., Tokyo, Japan) for sequencing, and
subsequently subcloned into the pGEM-7Zf+ plasmid to
generate the suicide plasmid, pGEM-7Zf+-TcfSR. In
addition, one pair of primers with restriction sites at the 5′ end
were designed for SacB DNA fragment amplification. The
primer sequences were as follows: SacB forward, GAG CTC GGG
CTG GAA GAA GCA GAC CGC TA (XhoI site), and reverse, GAG CTC
GCT TAT TTG TTA ACT GTT AAT TGT CC (XhoI site). The 1,475-bp
fragment was amplified using a PCR method from Bacillus
subtilis. Briefly, genomic DNA was isolated from B
subtilis using a commercial kit (Omega Bio-Tek, Norcross, GA,
USA), according to the manufacturer's instructions. The PCR
reaction system contained the following: 1.5 µl 10X buffer, 0.2 µl
dNTP (10 mmol/l), 1 µl DNA (20 ng/µl), 0.2 µl Taq enzyme, 0.2 µl
primers x2 (20 µmol/l) 0.6 µl MgCl2 (25 mmol/l) (TIANGEN
Biotech Co., Ltd., Beijing, China) and 11.1 µl H2O. The
total volume was 15 µl (60°C; 30 cycles). The PCR reaction
conditions were as follows: 5 min at 95°C, followed by 30 cycles at
65°C for 40 sec and 72°C for 1 min, and 10 min at 72°C. The PCR
products were analyzed using 2% agarose gel electrophoresis
(voltage, 150 V; 15 min). Next, the SacB fragment was
subcloned into the pGEM-7Zf+-TcfSR plasmid to generate
the pGEM-7Zf+-TcfSR-SacB plasmid. The competent B.
melitensis 16M strain was subjected to electroporation with
pGEM-7Zf+-TcfSR-SacB, and the potential TcfSR deletion
mutant, 16MΔTcfSR, was isolated using its ampicillin resistance and
sucrose phenotypes. The mutant was further confirmed by PCR
amplification using the following primers: TcfSR-I forward, GCT CTG
CGG GTT GAT CTT GG, and reverse, TGA CAG GCG TGG AAC AGC, which
were located on the upstream and downstream homologous arm of the
TcfSR promoter, respectively. The PCR products were sequenced by
Shanghai Sangon Biotech Co., Ltd. (Shanghai, China), to confirm the
sequence. In addition, the deletion mutant was further confirmed by
PCR amplification and reverse transcription PCR sequencing, as
described previously (10). The RNA
of parental 16M and mutant 16MΔTcfSR was extracted using RNAprotect
Bacteria Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA),
according to the manufacturer's instructions. RNA was reverse
transcribed into cDNA using an Omniscript RT kit (Invitrogen Life
Technologies), according to the manufacturer's instructions. The
mutant was detected and confirmed as correct using PCR. The primer
sequences were as follows: TcfSR forwad, GGCGGCTTGTGGCGCAG, and
reverse, GCCTTGGTCGTTCCTGCTTG. Briefly, total RNA was isolated from
Brucella parental strain and mutant strain using a
commercial kit (Omega Bio-Tek), according to the manufacturer's
instructions. RNA concentration and purity were determined using 2%
agarose gel electrophoresis, and the RNA was measured at an optical
density of 260/280, with an absorption ratio of >1.8 (ELx808;
Bio-Tek Instruments, Inc., Winooski, VT, USA). cDNA was synthesized
in a 20 µl reaction mixture, containing 2 µg total RNA, using the
Omniscript Reverse Transcription kit (Takara Cio, Inc.) and
oligo(dT) primers (forward, ATGATGCGCCCGCGCAG and reverse,
CTAATGCAGCACGCGCCC), according to the manufacturer's instructions.
The total PCR reaction volume was 15 µl. The PCR reaction
conditions were as follows: 5 min at 95°C, followed by 30 cycles at
65°C for 40 sec and 72°C for 1 min, and 10 min at 72°C.
Evaluation of the 16MΔTcfSR mutant
survival capacity in RAW 264.7 macrophages
A RAW 264.7 murine macrophage cell line was used to
assess the survival capability of 16MΔTcfSR, M5-90 or the B.
melitensis 16M parental strain. RAW 264.7 murine macrophages at
a density of 2×106 cells/well were cultured in a
six-well plate for 24 h at 37°C and 5% CO2. The cells
were infected with Brucella at a multiplicity of infection
(MOI) of 100. At 45 min post-infection, the cells were washed three
times with phosphate-buffered saline (PBS) and incubated with 50
µg/ml gentamicin (Invitrogen Life Technologies) for 1 h to
eliminate any extracellular bacteria. Subsequently, the culture was
replaced with Dulbecco's modified Eagle's medium (Gibco Life
Technologies, Carlsbad, CA, USA) containing 25 µg/ml gentamicin. At
0, 4, 8, 12 and 24 h post-infection, the supernatant was discarded
and the cells were lysed by incubation in PBS containing 0.1% (v/v)
Triton X-100. The number of colony forming units (CFU) was
determined by plating serial dilutions of the lysates on TSA
plates. All assays were performed in triplicate and repeated at
least three times (11).
Evaluation of the 16MΔTcfSR mutant
survival capability in mice
BALB/c mice (age, 6 weeks; n=50 per group) were
inoculated intraperitoneally (i.p.) with a 200-µl sample of
1×106 CFU 16MΔTcfSR, M5-90 or 16M, or 200 µl PBS for the
control mice. The virulence of the bacteria in the mice was
evaluated by enumeration of the bacteria in the spleens at
different time points post-inoculation. At weeks 2, 4, 6, 8 and 10
post-inoculation, the mice (n=10/time point per group) were
euthanized by CO2 asphyxiation and the spleens were
removed aseptically. The splenocytes were homogenized in 1 ml PBS
containing 0.1% (v/v) Triton X-100, serially diluted and plated on
TSA plates. All the assays were repeated twice with similar
results.
Evaluation of the protection
efficiency induced by 16MΔTcfSR in mice
Groups of female BALB/c mice (age, 6 weeks; n=20 per
group) were injected i.p. with 1×106 CFU (200 µl)
16MΔTcfSR (experimental vaccine group) or M5-90 (reference vaccine
control group), or with 200 µl PBS (unvaccinated control group). At
week 11 post-vaccination, the mice were challenged i.p. with
1×106 CFU per mouse (200 µl) of the 16M virulent strain.
The mice (n=10/time point per group) were euthanized at weeks 2 and
4 after the challenge, and bacterial CFU in the spleens were
determined, as aforementioned. A mean value for each spleen count
was obtained following logarithmic conversion. Log units of
protection were obtained by subtracting the mean log CFU for the
experimental group from the mean log CFU for the control group, as
previously described (12). The
experiments were repeated twice.
Evaluation of antibody production
To determine the antibody production of sera from
the inoculated mice, serum samples were obtained from the mice at
2, 4, 6, 8, and 10 weeks post-immunization. Immunoglobulin G (IgG)
levels were determined using the ELISA Quantikine Mouse kit
(R&D Systems, Inc., Minneapolis, MN, USA) (13). Briefly, heat-killed and sonicated
B. melitensis 16M whole-cell antigen was used to coat
96-well plates at a concentration of 25 µg protein/well. Following
overnight incubation at 4°C, the plates were washed once with 100
µl PBS containing 0.05% Tween-20, and blocked with 200 µl blocking
buffer [10% heat-inactivated fetal bovine serum (Gibco Life
Technologies) in PBS, pH 7.4] for 2 h at 37°C. Mice serum samples
in dilution buffer (1:300) were added to the wells in triplicate
and incubated for 2 h at 37°C. Following 2 h incubation, 100 µl
rabbit anti-mouse IgG-horseradish peroxidase (1:3,000) was added,
and the plates were incubated at 37°C for 30 min. After two washes
with wash solution, 100 µl TMB substrate solution was added to each
well and incubated at 37°C in the dark for 15 min. The reaction was
terminated following the addition of 50 µl
H2SO4 and the absorbance was measured at 450
nm (Scan 500; Interscience, Saint-Nom-la-Bretèche, France). All
assays were performed in triplicate and repeated at least three
times.
Cytokine production assay
Briefly, 10 weeks post-vaccination, the BALB/c mice
were sacrificed and their spleens were aseptically removed. Single
cell suspensions were obtained from the spleens by homogenization,
as described previously. The cells were suspended in complete RPMI
1640 medium (Gibco Life Technologies) supplemented with 2 mM
L-glutamine (Solarbio Science & Technology, Co., Ltd., Beijing,
China) and 10% (v/v) heat-inactivated fetal bovine serum.
Splenocytes were cultured in 96-well plates (4×105
cells/well); the cultures were stimulated by adding 25 µg
heat-killed B. melitensis 16M lysate/well, 0.5 µg ConA
(positive control), or medium alone (negative control). The cells
were then incubated at 37°C with 5% CO2 for 72 h. The
plates were centrifuged at 350 × g for 10 min, and the clear
culture supernatants were collected and stored at −20°C. Interferon
(IFN)-γ levels were estimated using an iELISA. The detection of
IFN-γ was conducted as previously described (14). IFN-γ levels were determined using an
ELISA Quantikine Mouse kit (R&D Systems, Inc.), according to
the manufacturer's instructions.
Cloning, expression and purification
of the recombinant protein
The open reading frames of TCFS and L7/L12 were
amplified by PCR using the DNA from the B. melitensis 16M
strain (14). Subsequently, the
amplified DNA fragments were cloned into the pET-32a vector
(Novagen®; EMD Biosciences, Inc., Madison, WI, USA) and expressed
in E. coli BL21 (DE3) cells (Novagen®; EMD Biosciences,
Inc.,) as an N-terminal His-tagged recombinant protein. The
recombinant proteins were separated and analyzed with SDS-PAGE
(12%). The recombinant proteins, TCFS and L7/L12, were purified
using affinity chromatography with Ni2+-conjugated
Sepharose (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA).
Western blot analysis
Cell lysates of the recombinant proteins, TCFS and
L7/L12, were analyzed by western blot analysis, as previously
described (15). The purified
recombinant TCFS and L7/L12 proteins were separated by 12% SDS-PAGE
and electrotransferred to nitrocellulose membranes (Solarbio
Science & Technology, Co., Ltd.) using a Mini Trans-Blot Cell
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 200 mA for 1 h.
The membranes were blocked for 2 h with 5% nonfat milk in TBST (100
mM trus-HCl; 150 mM NaCl; 0.05% Tween 20, pH 7.2) at 37°C. The
membranes were then washed three times with TBST and incubated with
a primary Brucella-vaccinated sera (1:300) for 1 h at 37°C,
and a sheep anti-mouse IgG horseradish peroxidase (HRP)-conjugated
secondary antibody (1:5,000; cat. no. ab6808; Abcam, Cambridge, UK)
for 1 h at 37°C. The membrane was developed using an enhanced
HRP-3,3′-diaminobenzidine substrate color kit (Beyotime Institute
of Biotechnology, Haimen, China).
TCFS iELISA
Serum samples were obtained from the mice infected
with the various Brucella strains. Antibody responses to the
purified recombinant TCFS protein were estimated using a TCFS-based
indirect ELISA (R&D Systems, Inc.), as previously described
(16).
Statistical analysis
Bacterial survival in the macrophage cell line and
in the mice was expressed as the mean ± standard deviation (SD) of
the log CFU. Furthermore, the antibody response and cytokine
production results are expressed as the mean ± SD of the optical
density value at 450 nm. The differences between groups were
analyzed by analysis of variance using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
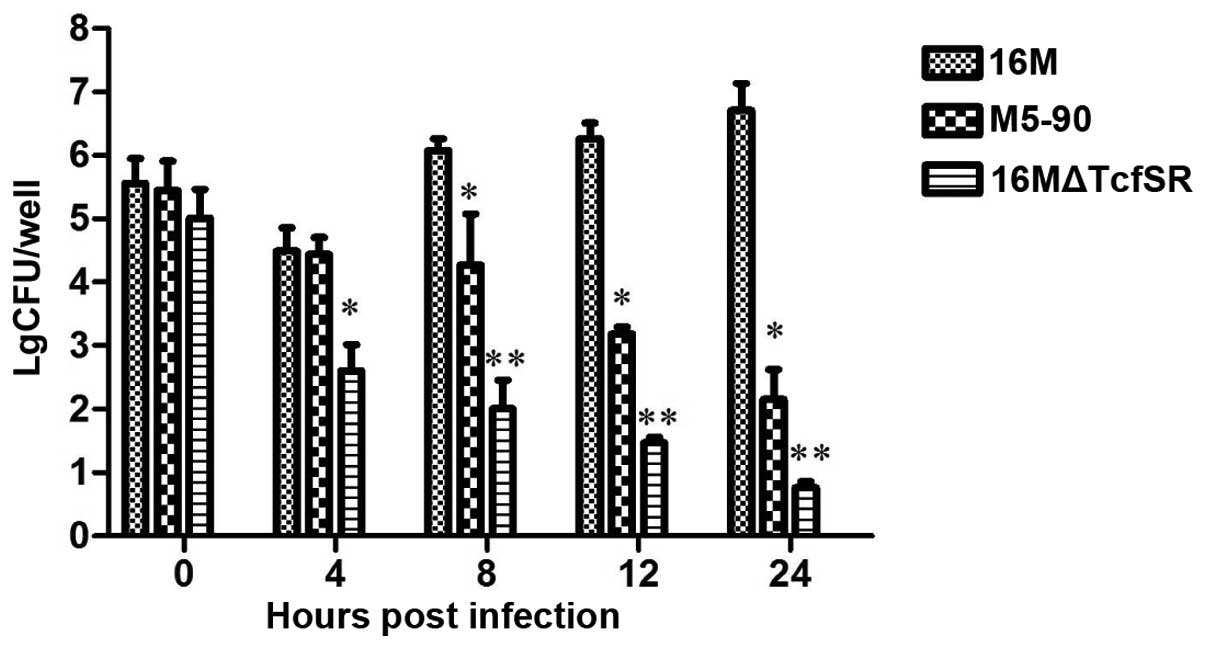
16MΔTcfSR is attenuated compared with
B. melitensis 16M for survival in RAW 264.7 murine macrophages
RAW 264.7 murine macrophages were infected with
16MΔTcfSR, M5-90 and B. melitensis 16M, and the survival
capacity and replication capability of the Brucella strains
in the macrophage cell line were determined. The macrophages were
infected with the three strains at a MOI of 100, and the surviving
bacteria were calculated. At 0 h post-infection, no statistically
significant difference in the amount of bacteria was observed among
the three strains. However, at 4 h post-infection, there was a
1.89-log and 1.84-log decrease (P<0.01) in the bacteria number
of 16MΔTcfSR when compared with that of 16M and M5-90,
respectively. By 8 h post-infection, a 4.08-log and 2.27-log
decrease (P<0.01) was observed in the bacteria number of
16MΔTcfSR when compared with that of 16M and M5-90, respectively.
Furthermore, at 12 h post-infection, there was a 4.79-log and
1.71-log decrease (P<0.01) in the bacteria number of 16MΔTcfSR
compared with that of 16M and M5-90, respectively. Finally, at 24 h
post-infection, a 5.94-log and 1.38-log decrease (P<0.01) was
observed in the bacteria number of 16MΔTcfSR when compared with
that of 16M and M5-90, respectively (Fig. 1). These results indicated that the
16MΔTcfSR mutant had a decreased survival capability in RAW 264.7
murine macrophages compared with the 16M and M5-90 strains,
indicating that 16MΔTcfSR was attenuated compared with B.
melitensis 16M for survival in RAW 264.7 murine
macrophages.
16MΔTcfSR is attenuated in BALB/c
mice
To determine the survival capability of the various
Brucella strains in the BALB/c mice, the mice were
inoculated i.p. with 1×106 CFU 16MΔTcfSR or M5–90. When
compared with M5-90 and 16M, the number of splenic CFU in the
16MΔTcfSR-infected mice was significantly reduced (P<0.01) at
weeks 2, 4, 6, 8 and 10. In addition, at week 10 post-inoculation,
16MΔTcfSR was shown to be completely cleared in the spleens of the
mice (Fig. 2). Thus, the results
demonstrated that the 16MΔTcfSR mutant was attenuated in the BALB/c
mice.
16MΔTcfSR induces immune protection
against a challenge with B. melitensis 16M
In order to determine the protection efficiency of
16MΔTcfSR, the mice were vaccinated i.p. with 1×106 CFU
16MΔTcfSR or M5-90, or PBS as the control. At week 11
post-vaccination, the mice were challenged i.p. with
1×106 CFU (200 µl) of the 16M virulent strain. The mice
immunized with 16MΔTcfSR exhibited significantly fewer splenic
Brucella colonies when compared with the non-immunized mice
at weeks 2 (2.02-log) and 4 (1.76-log) following the challenge
(P<0.05; Table I). In addition, a
similar CFU of protection was observed in the mice immunized with
16MΔTcfSR compared with those immunized with M5-90 (P<0.05). The
16MΔTcfSR vaccination exhibited a similar protective efficacy
compared with that of the M5-90 vaccination (Table I). Thus, the results indicated that
16MΔTcfSR was able to provide a similar protection efficacy against
the challenge with 16M to that of the M5-90 vaccine strain.
 | Table I.Evaluation of the protective efficacy
of 16MΔTcfSR and M5-90 vaccinations against Brucella melitensis 16M
infection in BALB/c mice. |
Table I.
Evaluation of the protective efficacy
of 16MΔTcfSR and M5-90 vaccinations against Brucella melitensis 16M
infection in BALB/c mice.
|
| Log CFU
spleena | Units of
protectionb | Uninfected/total
micec |
|---|
|
|
|
|
|
|
|---|
| Vaccination | Week 2 | Week 4 | Week 2 | Week 4 | Week 2 | Week 4 |
|---|
| 16MΔTcfSR |
5.10±0.13d |
4.83±0.11d | 2.02 | 1.76 | 2/10 | 2/10 |
| M5-90 |
5.48±0.16d |
4.98±0.11d | 1.44 | 1.63 | 1/10 | 1/10 |
| PBS |
7.12±0.19 |
6.61±0.15 | – | – | 0/10 | 0/10 |
16MΔTcfSR induces humoral and cytokine
responses
Serum samples from the mice inoculated with
16MΔTcfSR, M5-90 or PBS were obtained from the immunized mice at
selected intervals following immunization to monitor the total IgG
levels using an ELISA. For the mice inoculated with 16MΔTcfSR and
M5-90, the total IgG levels peaked at week 8 post-inoculation, and
there was no statistically significant difference between these two
groups (P>0.05). However, these two groups demonstrated
significantly higher IgG levels when compared with the control
group (P<0.01; Fig. 3).
To characterize the cellular immune response, the
IFN-γ levels in the splenocytes of the 16MΔTcfSR- and
M5-90-vaccinated mice were evaluated at week 10 following the
vaccination. Eight weeks after immunization, splenocytes were
obtained from the mice and the levels of IFN-γ in the culture
supernatants were determined in triplicate. As a positive control,
the nonspecific mitogen ConA was used. Spleen cells from 16MΔTcfSR
or M5-90 vaccinated animals were induced to secrete high levels of
IFN-γ after stimulation. As expected, ConA stimulation induced the
production of IFN-γ in spleen cells from all three groups, and no
cytokine production was induced by PBS stimulation in any of the
groups. The IFN-γ levels in the splenocytes of the
16MΔTcfSR-vaccinated mice were shown to be significantly higher, as
compared with those in the PBS-injected mice, and slightly higher
as compared with those in the M5-90-vaccinated mice (Fig. 4).
Differentiation of 16MΔTcfSR
immunization from infection using the protein TCFS as a test
antigen
To consider whether the TCFS protein may be used as
a diagnostic marker antigen for the differentiation between
vaccinated and infected mice, the recombinant purified protein,
TCFS, was interacted with 16MΔTcfSR-, 16M- and M5-90-inoculated
sera. Western blot analysis was performed using immunogenic L7/L12
protein as positive control to determine whether antibodies against
TCFS and L7/L12 were induced in these sera. For the positive
control, an L7/L12 reaction band was observed in the serum of the
16MΔTcfSR-, 16M- and M5-90-infected mice. In addition, the TCFS
protein was shown to react with the 16M and M5-90-inoculated mice
serum to produce specific bands. However, the TCFS protein was not
shown to react with the 16MΔTcfSR-inoculated mice serum (Fig. 5). Antibodies against the two proteins
were also detected using an iELISA, and the results from the iELISA
were similar to that from the western blot analysis (Fig. 6). Furthermore, antibodies against
L7/L12 were detected in the sera of the 16MΔTcfSR-, M5-90- and
16M-vaccinated mice, whereas antibodies against TCFS were only
detected in the sera of the M5-90- and 16M-vaccinated mice. These
results indicated that the TCFS protein had good reactogenicity;
thus, TCFS may be used to differentiate the vaccination from a
natural infection.
Discussion
The majority of the currently licensed vaccines have
numerous drawbacks, including residual virulence, induction of
splenomegaly, and interference with serodiagnosis (17–20).
With regard to these limitations, significant effort has been made
to develop novel vaccines. The TCS, TcfSR, is a regulatory system
that controls gene expression and is involved in the virulence for
Brucella. In the present study, the 16MΔTcfSR mutant was
constructed and the virulence and protection efficacies were
evaluated in a macrophage cell line and mice to assess the ability
of 16MΔTcfSR in maintaining protective efficacy.
Thus, a deletion mutant of TcfSR was constructed
with the aim to confirm that the reduced survival capability of the
mutant was directly associated with the deletion of the promoter
for TcfSR. The 16MΔTcfSR was evaluated for survival and attenuation
in a RAW 264.7 murine macrophage cell line and BALB/c mice. As
demonstrated by the present study, the 16MΔTcfSR mutant was much
more susceptible to eradication in the macrophage cell line
compared with the wide-type 16M strain. Moreover, clearance of
16MΔTcfSR was observed within 10 weeks in the BALB/c mice, which
was faster compared with M5-90. These results are consistent with
hypothesis that TcfSR is involved in the virulence of
Brucella.
An ideal Brucella live attenuated vaccine
combines survival capability with persistence in the host (21). Therefore, in the present study, the
protective efficacy of the 16MΔTcfSR mutant was investigated. The
results demonstrated that vaccination with 16MΔTcfSR was able to
provide good protective efficacy against a challenge with the
wild-type 16M strain. In addition, the 16MΔTcfSR vaccination
conferred a level of protection that was equivalent to that
conferred by the M5-90 vaccination.
The cytokine profiles and antibody responses were
also investigated to evaluate the protection conferred by the
16MΔTcfSR vaccination. Brucella is a facultative,
intracellular parasitic pathogen. The organism infects the host
cells and primarily provokes cell-mediated immunity. IFN-γ is
produced by T lymphocytes and is a potent macrophage-activating
factor. The T helper 1 immune responses characterized by IFN-γ
production are known to be associated with the protective immunity
against Brucella, and these responses are stimulated most
effectively by live vaccines (22).
IFN-γ plays an important role in eradicating intracellular
Brucella (23). IFN-γ exerts
antibacterial effects; thus, the current study detected the host
secretion levels of IFN-γ in order to evaluate the antimicrobial
capacity and cellular immunity of the host. A previous study
demonstrated that IFN-γ is a critical cytokine required for
macrophage bactericidal activity (24). The results of the present study
demonstrated that treatment with 16MΔTcfSR induced a higher
secretion of IFN-γ compared with that observed following treatment
with M5-90. In addition, high levels of IgG in the host humoral
response can prevent Brucella from entering the cells,
thereby reducing the injury on the body. Levels of specific IgG
antibodies in the serum are important for evaluating the
immunogenicity of brucellosis. In the present study, the results
with regard to the humoral immune response revealed that mice
infected with 16MΔTcfSR produced anti-Brucella IgG. In
addition, vaccination with 16MΔTcfSR conferred levels of IgG that
were at least similar to that conferred by the M5-90
vaccination.
Serological diagnosis using a variety of techniques,
such as the Rose Bengal plate test, serum agglutination test and
iELISA, is the most convenient method for brucellosis diagnosis.
These methods use hot saline extract and lipopolysaccharide (LPS)
as antigens of smooth Brucella. Brucella LPS is the
most important antigen during the immune response in brucellosis
(25). However, differentiating
between the serum of vaccinated animals and the serum of infected
animals using LPS-based serological tests is difficult. Thus, the
present study evaluated the possibility of using TCFS protein as a
diagnostic antigen marker. Recombinant protein expression of TCFS
was conducted, and the protein was used to detect the antibody
profiles in the different serum samples. The results revealed that
a humoral immune response to TCFS was detected in the serum of mice
infected with 16M and M5-90; however, a reaction was not observed
in the 16MΔTcfSR-vaccinated serum or in the PBS-treated controls.
These results indicated that TCFS may be used as a diagnostic
marker antigen for the serological diagnosis of brucellosis.
Furthermore, the presence of antibodies against TCFS following
16MΔTcfSR vaccination was investigated using an iELISA. The results
indicated that the mice infected with the 16M and M5-90 strains
tested positive for the presence of TCFS antibodies, whereas the
mice infected with 16MΔTcfSR exhibited negative expression.
Therefore, vaccination with 16MΔTcfSR enables the differentiation
between vaccination and infection. The TCFS protein may allow for
the distinction and differentiation of the vaccination from
infection; however, confirmation is required in further
studies.
In the present study, the 16MΔTcfSR mutant of the
TcfSR TCS in Brucella was successfully constructed. The
16MΔTcfSR mutant exhibited a reduced survival capacity in the
macrophage RAW 264.7 cell line and BALB/c mice, while providing a
level of protection similar to that provided by the M5-90 vaccine
strain against a B. melitensis virulence 16M challenge. In
addition, immunization with the 16MΔTcfSR vaccination induced
humoral and cytokine responses. Furthermore, the present study
demonstrated that TCFS protein is an ideal diagnostic antigen for
the differentiation of immunization from infection using an iELISA.
Therefore, 16MΔTcfSR enables the differentiation between the
vaccination and infection. In conclusion, 16MΔTcfSR is a potential
vaccine candidate with reduced virulence that provides high
protection efficiency. In addition, TCFS protein may be used to
differentiate between infected and vaccinated animals by
serological diagnosis.
Acknowledgements
This study was supported by grants from the National
Basic Research Program of China (973 Program; no. 2010CB530203),
the International Science and Technology Cooperation Project of
China (no. 2013DFA32380), the Outstanding Youth Science and
Technology Talent Cultivation Fund (no. 20132RKXJQ06) and the
Startup Fund for Advanced Talents (no. RCZX201228).
References
|
1
|
Lacerda TL, Cardoso PG, Augusto de Almeida
L, et al: Inactivation of formyltransferase (wbkC) gene generates a
Brucella abortus rough strain that is attenuated in
macrophages and in mice. Vaccine. 28:5627–5634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pappas G, Akritidis N, Bosilkovski M and
Tsianos E: Brucellosis. N Engl J Med. 352:2325–2336. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elzer PH, Hagius SD, Davis DS, DelVecchio
VG and Enright FM: Characterization of the caprine model for
ruminant brucellosis. Vet Microbiol. 90:425–431. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Godfroid J, Cloeckaert A, Liautard JP, et
al: From the discovery of the Malta fever's agent to the discovery
of a marine mammal reservoir, brucellosis has continuously been a
re-emerging zoonosis. Vet Res. 36:313–326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hamdy ME, El-Gibaly SM and Montasser AM:
Comparison between immune responses and resistance induced in
BALB/c mice vaccinated with RB51 and Rev. 1 vaccines and challenged
with Brucella melitensis bv. 3. Vet Microbiol. 88:85–94.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cosivi O and Corbel MJ: WHO consultation
on the development of new/improved brucellosis vaccines. 17
December 1997, Geneva, Switzerland. Biologicals. 26:361–363. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang F, Hu S, Gao Y, Qiao Z, Liu W and Bu
Z: Complete genome sequences of Brucella melitensis strains
M28 and M5-90, with different virulence backgrounds. J Bacteriol.
193:2904–2905. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Viadas C, Rodríguez MC, Sangari FJ, Gorvel
JP, García-Lobo M and López-Goñi I: Transcriptome analysis of the
Brucella abortus BvrR/BvrS two-component regulatory system.
PLoS One. 5:e102162010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lavín JL, Binnewies TT, Pisabarro AG,
Ussery DW, García-Lobo M and Oguiza JA: Differences in
two-component signal transduction proteins among the genus
Brucella: Implications for host preference and pathogenesis.
Vet Microbiol. 144:478–483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Chen Z, Qiao F, et al: Comparative
proteomics analysis reveal the virB of B. melitensis affects
expression of intracellular survival related proteins. PLoS One.
4:e53682009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hernández-Castro R, Verdugo-Rodríguez A,
Puente JL and Suárez-Güemes F: The BMEI0216 gene of Brucella
melitensis is required for internalization in HeLa cells.
Microb Pathog. 44:28–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adone R, Ciuchini F, Marianelli C, et al:
Protective properties of rifampin-resistant rough mutants of
Brucella melitensis. Infect Immun. 73:4198–4204. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goel D and Bhatnagar R: Intradermal
immunization with outer membrane protein 25 protects Balb/c mice
from virulent B. abortus 544. Mol Immunol. 51:159–168. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Bai Y, Qu Q, et al: The 16MΔvjbR
as an ideal live attenuated vaccine candidate for differentiation
between Brucella vaccination and infection. Vet Microbiol.
151:354–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Guo F, Chen C, et al: Brucella
melitensis 16MΔhfq attenuation confers protection against
wild-type challenge in BALB/c mice. Microbiol Immunol. 57:502–510.
2013.PubMed/NCBI
|
|
16
|
Liu B, Teng D, Wang X, Yang Y and Wang J:
Expression of the soybean allergenic protein P34 in Escherichia
coli and its indirect ELISA detection method. Appl Microbiol
Biotechnol. 94:1337–1345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schurig GG, Sriranganathan N and Corbel
MJ: Brucellosis vaccines: past, present and future. Vet Microbiol.
90:479–496. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davis DS and Elzer PH: Brucella
vaccines in wildlife. Vet Microbiol. 90:533–544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berkelman RL: Human illness associated
with use of veterinary vaccines. Clin Infect Dis. 37:407–414. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ashford DA, di Pietra J, Lingappa J, et
al: Adverse events in humans associated with accidental exposure to
the livestock brucellosis vaccine RB51. Vaccine. 22:3435–3439.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ko J and Splitter GA: Molecular
host-pathogen interaction in brucellosis: current understanding and
future approaches to vaccine development for mice and humans. Clin
Microbiol Rev. 16:65–78. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Golding B, Scott DE, Scharf O, et al:
Immunity and protection against Brucella abortus. Microbes
Infect. 3:43–8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding JB, Cheng JS, Mu W, Mao KR, Zhang EL
and Jiang YW: Construction of a WboA-deficient Brucella suis
S2 strain and its immune effect. Zhongguo Nong Ye Ke Xue.
8:2448–2453. 2008.(In Chinese).
|
|
24
|
Sathiyaseelan J, Goenka R, Parent M, et
al: Treatment of Brucella-susceptible mice with IL-12
increases primary and secondary immunity. Cell Immunol. 243:1–9.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiménez de Bagüés MP, Marín CM, Blasco JM,
Moriyón I and Gamazo C: An ELISA with Brucella
lipopolysaccharide antigen for the diagnosis of B.
melitensis infection in sheep and for the evaluation of
serological responses following subcutaneous or conjunctival B.
melitensis strain Rev 1 vaccination. Vet Microbiol. 30:233–241.
1992. View Article : Google Scholar : PubMed/NCBI
|