Introduction
Propofol is the most widely used intravenous general
anaesthetic worldwide. Hyperthyroidism is a common endocrine system
disease (1–3). In clinical terms, additional propofol
anaesthesia is required during surgery for patients with
hyperthyroidism compared with those with normal thyroid function
(4–7). Propofol is a primary intravenous
general anaesthetic that is accepted and used worldwide due to its
ability to enhance γ-aminobutyric acid (GABA)-mediated inhibition
in the nervous system (8,9). GABA is a natural inhibitory
neurotransmitter, and the GABA receptor (GABAR) comprises
GABAAR, GABABR and GABACR
subclasses. Considering that GABAAR is a ligand-gated
ion channel receptor, GABA can inhibit presynaptic neurotransmitter
release and generate analgesia through a primary afferent
depolarisation process by acting on GABAAR localised on
primary afferent neurons (10,11).
Approximately 19 known subunits (α1–6, β1–3, γ1–3, δ, ε, θ, π and
ρ1–3) constitute the GABAAR (10,12), and
all these subunits share an integral channel, which is permeable to
Cl− ions. Dorsal root ganglions (DRGs) are nociceptive
primary afferent sensory neurons, and the GABA
neurotransmitter/receptor system has an important role in the
modulation of spinal nociceptive information.
As basal hormones, thyroid hormones (THs) have an
essential role in maintaining the functional activity of the body
and in heat production, metabolism, tissue differentiation and
organ growth. The majority of the biological effects of TH are
mediated by nuclear TH receptors (TRs). Two kinds of receptor,
namely TRα and TRβ, have been found (13). Similar to other nuclear transcription
factor families, TR can combine with other nuclear transcription
factors to mediate the target gene expression. In a previous study
it was found that the biological effects of THs are rapid and
unaffected by inhibitors associated with genetic transcription.
This fact demonstrates the importance of the non-genetic effects of
THs, which can affect the functions of the GABAA
receptors (14).
Materials and methods
Animals and experimental
procedures
With the permission and using the protocol of the
Committee of Animal Use for Research and Education of Wuhan
University (Wuhan, China), male Sprague-Dawley rats (weighing
200–250 g) were purchased from the Center for Animal Experiments,
Wuhan University and housed under a temperature- and
light-controlled environment (22±1 h on a 12-h light/dark cycle at
60% humidity). Standard rat chow diet and water were given ad
libitum. Rats were randomly divided into two groups: Control
(n=30) and hyperthyroid (n=30). Over 14 days, hyperthyroidism was
induced in the rats of the hyperthyroid group with daily injections
of 3,3′,5-L-triiodothyronine (T3) [7 µg/100 g body
weight (BW) in 0.01 mM NaOH, intraperitoneally], whilst only a
daily injection of the vehicle was given to the control rats.
Approximately 24 h following the last dose of T3, the
rats were sacrificed by decapitation without other special
treatment. To evaluate the TH serum levels and confirm the
hyperthyroid status of the animals, blood samples were carefully
collected. Sixty rats were used in the final experiment: 20 and 10
rats from each group were utilised for the electrophysiology and
immunofluorescence experiments, respectively. Propofol was
dissolved in soybean oil at a concentration of 10 mg/ml. The rats
in the two groups were tested to determine the propofol dosage
required for successful anesthesia after 14 days of treatment. The
success of the anaesthesia was monitored by righting reflex and the
propofol dosage was calculated by measuring the volume of soybean
oil that was administered. Chemicals were purchased from
Sigma-Aldrich (St. Louis, MO, USA) and stored according to the
manufacturer's instructions unless otherwise specified.
Enzyme-linked immunosorbent assay of
T3 and T4 concentrations
An enzyme-linked competitive immunosorbent assay
with biotin-avidin amplification was employed (Beijing North
Institute of Biological Technology Co., Ltd., Beijing, China).
Serum T3 levels were determined by a competitive binding assay in
which T3 in the sample and biotin-labeled T3
competitively bound with anti-T3 antibodies on
96-well-microtiter plates. Once reaction equilibrium was reached
after 45 min at 37°C, horseradish peroxidase-labeled avidin was
added and complexes were formed. Following the addition of
substrate for colour development, optical density values were
determined at 450 nm using a Bio-Rad 680 microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Their values are inversely
proportional to the concentration of T3 in the sample.
The levels of T4 were evaluated by a similar assay.
Electrophysiological recordings of DRG
neurons
The DRG neurons were pulled out from the spines of
the rats, immersed in extracellular fluid, cut into pieces and then
soaked in the extracellular fluid with collagenase and trypsin at
37°C for 15 min and then centrifuged at 111.8 g for 5 min. The
supernatant was then removed and the neurons were stood for 30 min
to reach adherence. With the aid of a whole-cell patch clamp
amplifier, perforated patch-clamp recordings in the whole-cell mode
were performed. Using an Axon 700B amplifier (Axon, San Jose, CA,
USA) and pCLAMP 0.2 hardware and software (Axon), currents were
recorded from the DRG neurons in vitro. The room temperature
was set at 22–24°C. The internal solution was added to
micropipettes containing 150 mM KCl and 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES).
Osmolarity was regulated at 320 mOsm/l with glucose. The pH was
maintained at 7.2 with KOH. On the day of the experiment,
amphotericin B was prepared as a stock solution by dissolving in
dimethyl sulphoxide. The recording electrodes were backfilled with
amphotericin B-containing solution, and the tip of the electrode
was filled with amphotericin B-free solution. The experiment
required 15–30 min to obtain a stable series resistance and 5–10
min to perforate the membrane. Cells were immersed in an external
solution containing 2.5 mM CaCl2, 2 mM MgCl2,
10 mM HEPES, 10 mM D-glucose, 150 mM NaCl and 5 mM KCl. The pH was
maintained at 7.4 with NaOH, and the osmolarity of the solution was
maintained at 330 mOsm/l with glucose. The resistance of the
recording pipette ranged from 3 to 5 MΩ. The membrane currents were
recorded following adjustment of the capacitance and series
resistance compensations. Without other specific indication, the
holding potential was adjusted to −60 mV and the membrane currents
were filtered at 10 kHz. GABA, GABA + T3, GABA +
propofol and GABA + propofol + T3 were successively
injected in the same cell at various concentrations to detect the
effect of T3 and propofol on DRG neurons.
Immunofluorescence
Following anaesthetization with sodium pentobarbital
(60 mg/kg), the rats in the control and hyperthyroid groups were
subjected to cardiac perfusion with physiological saline followed
by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4).
Lumbar1–5 DRGs were bilaterally removed. For 4 h, DRGs
were kept in a fixative state at 4°C, and then separately soaked in
10, 20 and 30% sucrose at 4°C until the DRGs settled at the bottom.
The DRGs were then embedded in an optimal cutting temperature
compound (OCT; Sakura Finetek USA, Inc., Torrance, CA, USA) and
segmented using a cryostat at 10-µm thickness. The sections were
kept at −80°C prior to use. The sections were pretreated with
acetone (at 4°C), 0.3% Triton X-100 and 5% normal foetal calf serum
prior to incubating overnight at 4°C in a humid chamber with goat
polyclonal primary antibodies against GABAAα2 (sc-7350;
1:50; Santa Cruz Biotechnology, Dallas, TX, USA) and
GABAAβ2 (sc-7362; 1:50; Santa Cruz Biotechnology). The
sections were incubated respectively with donkey anti-goat
immunoglobulin (Ig)G conjugated with tetramethylrhodamine
isothioscyanate (1:100; Santa Cruz Biotechnology) or donkey
anti-goat IgG conjugated with fluorescein isothiocyanate (1:100;
Santa Cruz Biotechnology) at 37°C for 1 h following a thorough
rinsing with phosphate-buffered saline (PBS). The antibodies were
diluted in 0.01 M PBS. The primary antibodies were excluded from
the control experiments, leading to the negative staining of all
examined sections. Sections were examined under a laser confocal
microscope (LSM 710; Carl Zeiss Microscope, Jena, Germany).
Analytical software was used to quantitatively analyse the
immunofluorescence (hp9001; Carl Zeiss Microscope).
Statistical analysis
Data are presented as mean ± standard error of the
mean and were analysed and using SPSS software (version 17.0; SPSS,
Inc., Chicago, IL, USA). In cases of homogeneity of variance, the
least significant difference t-test, one-way analysis of variance
and two-group comparison were performed. P<0.05 was considered
to indicate a statistically significant difference.
Results
Validation of hyperthyroidism in
experimental rat models
The serum levels of free T3 and
T4 were measured to confirm whether hyperthyroidism was
successfully induced in the T3-treated rats. The
increased T3 and decreased T4 serum levels
observed in the T3-treated rats are in accordance with
hyperthyroidism (Table I).
 | Table I.Experimental groups at baseline and
following 14 days of T3-treatment. |
Table I.
Experimental groups at baseline and
following 14 days of T3-treatment.
|
| Control | Hyperthyroid |
|---|
|
|
|
|
|---|
| Parameter | Day 0 | Day 14 | Day 0 | Day 14 |
|---|
| Body weight
(g) |
241.5±9.6 |
306.9±14.8 |
242.6±9.3 |
233.9±12.1 |
| Free T3
(ng/ml) |
|
0.41±0.10 |
|
1.32±0.50a |
| Free T4
(ng/ml) |
|
30.8±3.26 |
|
8.9±0.71a |
Anaesthetic dose of control and
hyperthyroid groups
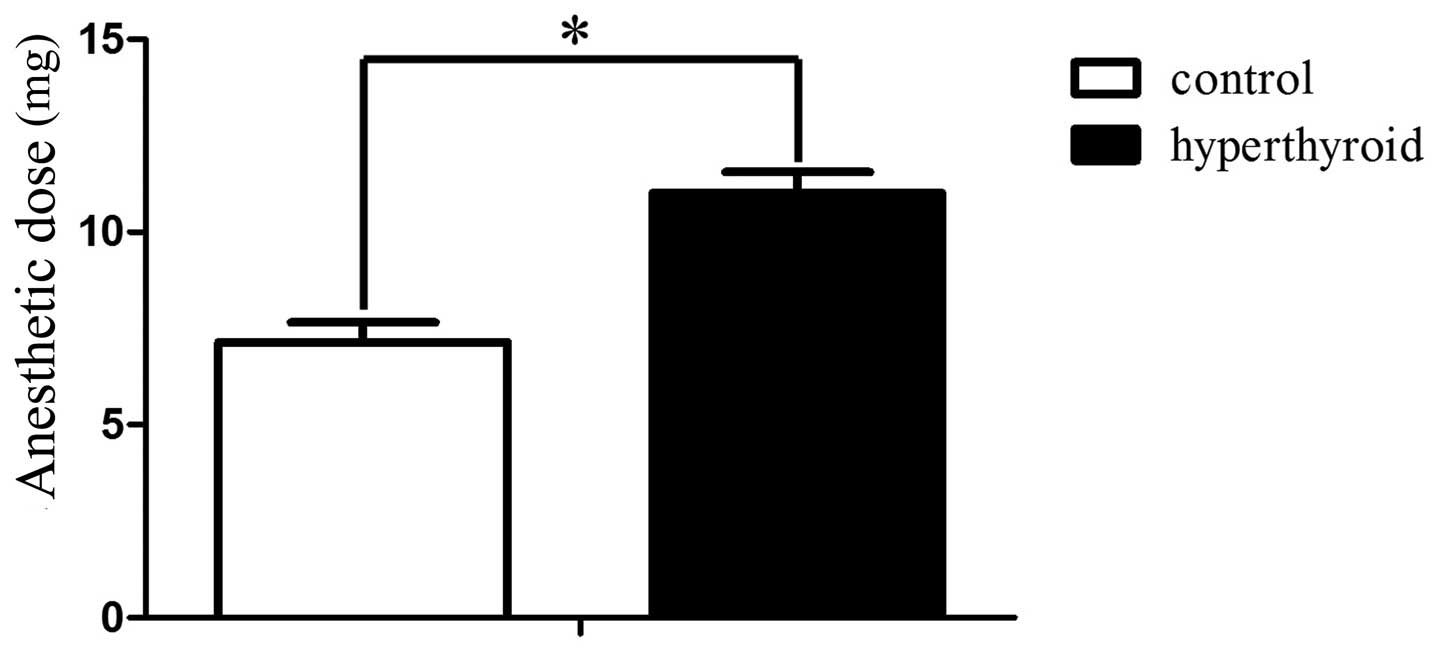
The anaesthetic dose for the control and
hyperthyroid groups was 7.14±0.51 and 11.02±0.53 mg/100 g BW
(P<0.05), respectively (Fig. 1).
The intraperitoneal injections of propofol anaesthesia to the rats
in the control and hyperthyroid groups were successful since the
righting reflex was absent.
Subunit expression differences of
GABAA receptors α2 and β2
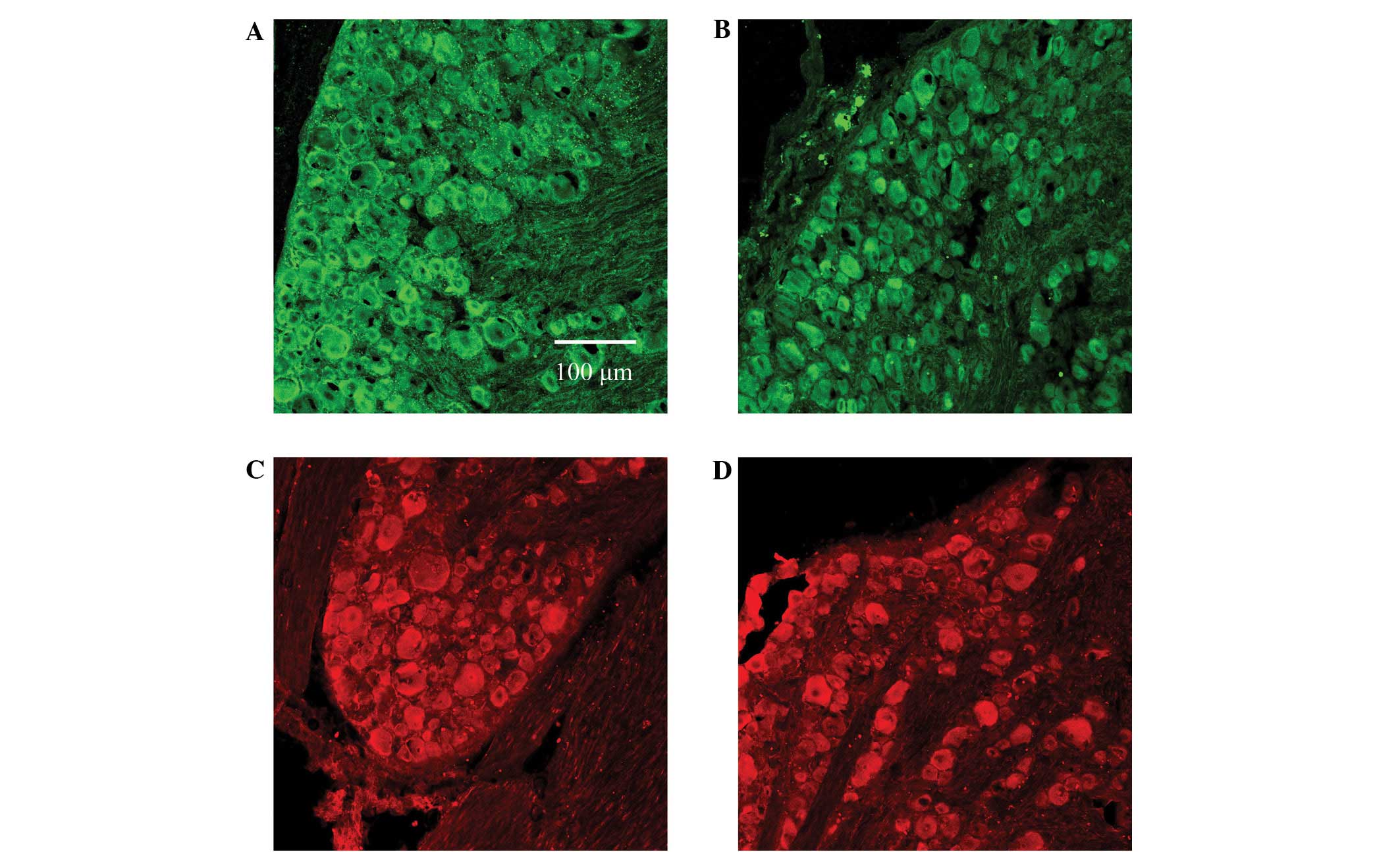
Immunofluorescence staining was used to indicate the
expression of DRG GABAA receptor α2 and β2 subunits in
rats in the control and hyperthyroid groups, and any differences in
their expression levels were determined using quantitative
analysis.
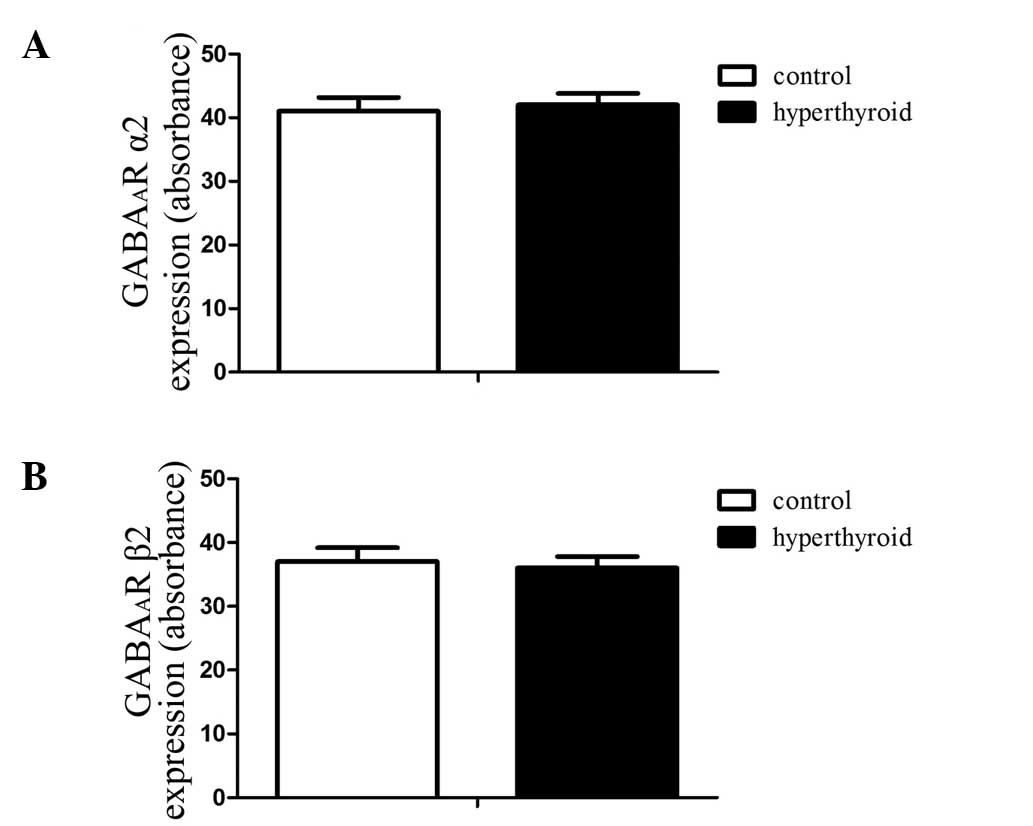
The results showed no statistical difference in the
expression of either GABAA receptor subunit α2, marked
by green immunofluorescence, or in GABAA receptor
subunit β2, marked by red immunofluorescence (Figs. 2 and 3). The absorbance values of the DRG
GABAA receptor subunits α2 and β2 of the control group
were 40.5±2.05 and 38.2±1.95, respectively, whilst those of the
hyperthyroid group were 41.1±2.17 and 37.8±1.84, respectively.
Comparison of GABA-activated
currents
GABA induced a concentration-dependent (0.01–1,000
µM) inward current in the DRG neurons of rats in the control and
hyperthyroid groups (Fig. 4).
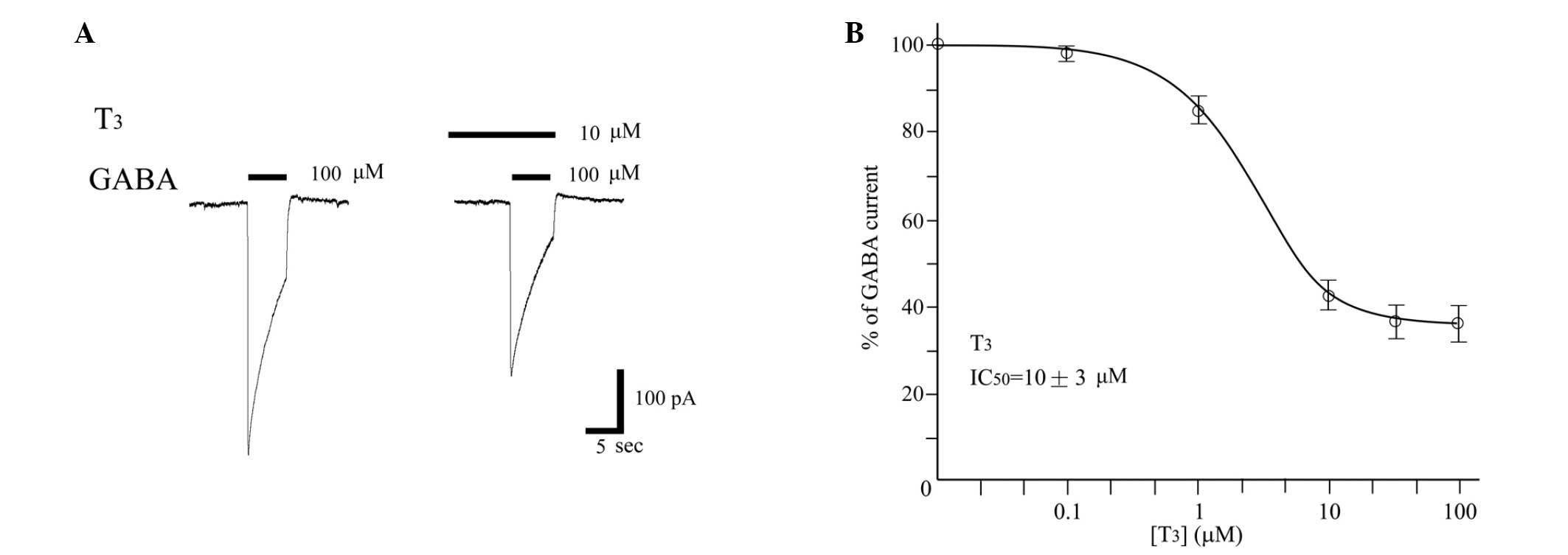
THs inhibit GABA-evoked currents in
DRGs
Using the patch clamp technique in the whole-cell
configuration, the effect of T3 on the GABA-induced
currents recorded in DRG was tested. T3 reduced the
currents in a dose-dependent manner (Fig. 5). The concentration response curves
showed an approximate IC50 of 10±3 µM for T3.
No direct T3 channel gating was observed at any of the
concentrations investigated.
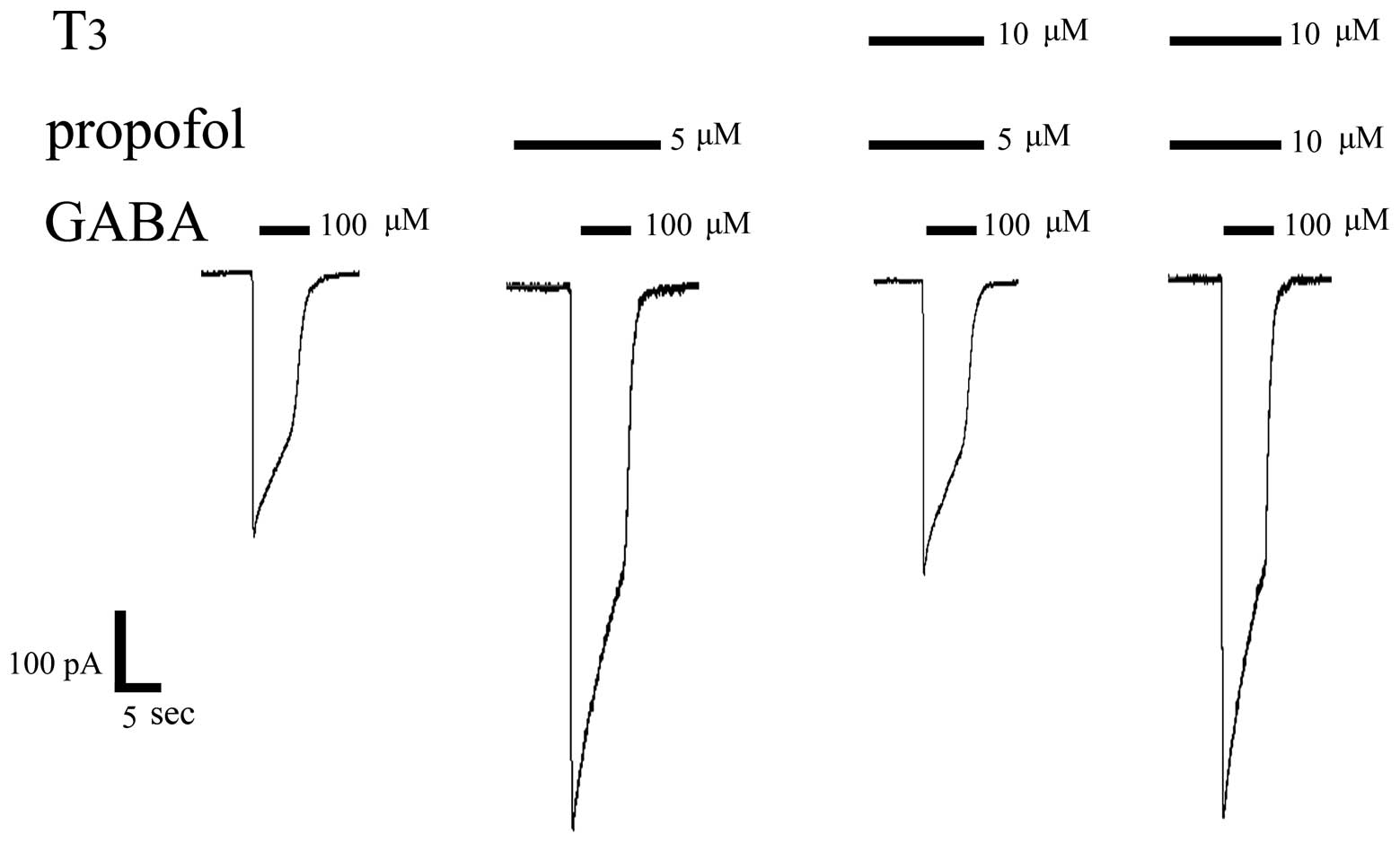
T3 inhibits the
augmentation effect of propofol on the GABA-activated currents
Since T3 inhibits GABA-activated currents
in DRG, in order to determine if T3 inhibits or
minimises the augmentation effect of propofol on the GABA-activated
currents, GABA, GABA + propofol and GABA + propofol + T3
were successively injected in the same cell (Figs. 6 and 7). The following procedures were performed
in the same-cell experiments: i) 100 µM GABA, which can induce an
inward current, was injected. The activated current for the
GABAAR was 450±35 pA; ii) 5 µM propofol was pre-perfused
followed by 100 µM GABA, which induced an increase of the inward
current, demonstrating that propofol has an inductive effect on
GABA-activated currents and that this is the anaesthetic mechanism.
The activated current for GABAAR was 860±41 pA; iii) a
mixture of 10 µM T3 and 5 µM propofol was pre-perfused,
followed by 100 µM GABA, which then clearly showed that the
augmentation effect of propofol on the GABA-activated currents was
significantly reduced by T3. The activated current for
the GABAAR was 470±43 pA; iv) the propofol concentration
was increased, and detection of the 100 µM GABA-activated currents
following the pre-perfusion of the mixture of 10 µM T3
and 10 µM propofol demonstrated that the inward current increased.
This result indicated that an increased propofol concentration can
partially offset the inhibitory effect of T3 on
GABA-activated currents. The activated current for the
GABAAR was 856±39 pA. The results presented in Fig. 7 show that a statistically significant
difference exists in the GABA-induced current amplitude between
GABA + propofol (5 µM) and GABA + propofol (5 µM) + T3
(10 µM; P<0.05). A statistically significant difference also
exists between GABA + propofol (5 µM) + T3 (10 µM) and
GABA + propofol (10 µM) + T3 (10 µM; P< 0.05).
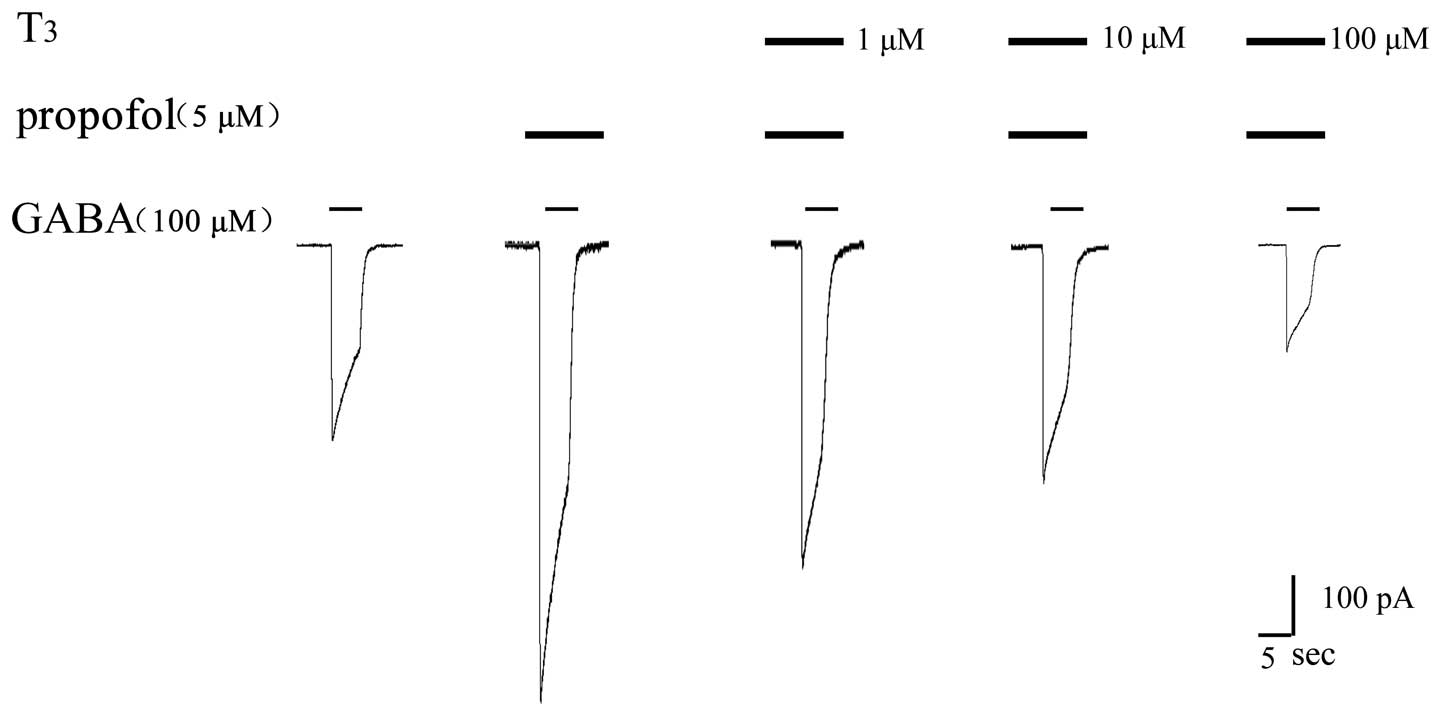
Further experiments were carried out to investigate
whether a higher concentration of T3 produced a stronger
inhibitory effect on the anaesthetic effect of propofol. GABA, GABA
+ propofol and GABA + propofol + T3 at various
concentrations were successively injected into the same cell to
validate the effect of T3. The results are shown in
Figs. 8 and 9, and demonstrate that a higher
concentration of T3 produced a stronger inhibitory
effect on the propofol-augmented increase in GABA-induced
current.
Perfusion of 100 µM GABA produced an activated
current for the GABAAR of 450±35 pA. When pre-perfusion
was conducted with 5 µM propofol, the activated current for
GABAAR was 860±41 pA. The activated current for the
GABAAR was 628±46 pA following the perfusion of 1 µM
T3 and 5 µM propofol, and was 470±43 pA following the
pre-perfusion of 10 µM T3 and 5 µM propofol. Finally,
the activated current for GABAAR was 326±38 pA following
the pre-perfusion of 100 µM T3 and 5 µM propofol.
The bar chart in Fig.
9 shows that a statistically significant difference exists
between GABA + propofol (5 µM) and GABA + propofol (5 µM) +
T3 (1 µM; P<0.05), and between GABA + propofol (5 µM)
and GABA + propofol (5 µM)+T3 (10 µM; P<0.05). In
addition, a statistically significant difference exists between
GABA + propofol (5 µM) and GABA + propofol (5 µM) + T3
(100 µM; P<0.05).
Discussion
THs, as basal hormones, have important roles in
energy utilisation, tissue differentiation, metabolism and organ
growth, as well as in maintaining the functional activities of the
body (15). THs are mainly involved
in the development and functioning of the central nervous system
(16–18). The direct transcriptional effects of
TH bound to nuclear TRs mediate the majority of the TH effects. A
new mechanism of TH action had been previously identified, and is a
novel development of the traditional view that THs mediate their
effects by controlling gene expression by binding to nuclear
receptors TRα and TRβ. According to the study (19), this novel mechanism of TH action is
rapid and unaffected by RNA and protein synthesis inhibitors. These
facts are indicative of a non-classical nuclear TR-mediated action
(19,20). Furthermore, the non-classical nuclear
TR-mediated action of TH has been verified in human fibroblasts,
human glioma, cardiomyocytes and osteoblasts. The GABAergic system
is also an important target for the non-genomic action of THs
(20–26).
Propofol is the most widely used short-acting
intravenous general anaesthetic. In the classical view, the
antalgic mechanism of propofol is mainly associated with an
increase in the function of the GABAA receptors and the
inhibitory post-synaptic potential. In clinical terms, additional
propofol general anaesthesia is required during surgery for
patients with hyperthyroidism compared with those with normal
thyroid function. A representation of the result was provided in
the present study using a hyperthyroidism model.
Rats with hyperthyroidism require propofol
administration at 11.02 mg/100 g BW and control rats at 7.14 mg/100
g BW. To determine why the two groups require different doses of
propofol anaesthetic, a DRG immunofluorescent assay was performed.
This assay was conducted to evaluate if any changes in the
distribution and number of the GABAA receptors in the
DRG neuron occur and to determine if THs alter the functions of the
GABAA receptors by changing their distribution and
number. The results showed that the expression levels of
GABAA receptor subunits α2 and β2 did not differ between
the control and hyperthyroid groups (Figs. 2 and 3). Considering that the subunits α2/β2/γ2
constitute the GABAA receptor, it may be assumed that
the hyperthyroid model does not change the distribution and number
of the GABAA receptors. The changes in the
GABA-activated currents in the DRG neurons of the hyperthyroid and
control rats were then investigated. Whole-cell patch clamp
recording tests were performed on an acutely isolated DRG neuron;
however, no statistical difference was found between the two groups
at various doses. The results indicated that there was no
significant difference between the GABA-activated currents in the
TH-free interstitial fluids of the hyperthyroid and control rats
(Fig. 4). The non-genomic effects of
THs on the GABAA receptors were considered, and the
inhibitory effect of T3 on the GABA-activated currents
in DRG neurons was examined (Fig.
5). T3 inhibits the current-augmenting and
anaesthetic effect of propofol on GABAA receptors
(Fig. 6). Propofol can increase the
amplitude of GABA-activated currents. The current amplitude
decreased when propofol was simultaneously pre-perfused with
T3 at various concentrations, and a higher concentration
of T3 induced stronger inhibition; therefore, it appears
that the non-genomic effects of THs were achieved by inhibiting the
activities of the GABAA receptors to mitigate the
anaesthetic effect of propofol. With increasing concentrations of
THs, the inhibitory effects strengthened. Fig. 7 shows that 5 µM propofol enhanced the
GABA-activated currents; however, with 10 µM T3, the
effect of propofol on the GABA-activated currents was inhibited. A
higher concentration of propofol was shown to offset the inhibitory
effect of T3 through the simultaneous use of 10 µM
propofol and T3 with the same concentration.
The ideal situation is to have effective anaesthesia
with less anaesthetic, but additional propofol anaesthesia during
surgery is necessary for patients with hyperthyroidism compared
with those with a normal thyroid function. Additional anaesthetic
suggests greater anaesthesia risks and adverse reactions;
therefore, according to the current study, higher levels of THs in
patients with hyperthyroidism inhibit the augmentation effect of
propofol on GABAA receptors. This inhibitory effect
leads to less propofol-induced anaesthesia. Given that the
mechanism through which T3 inhibits the GABAA
receptors is unclear, the optimum method of anaesthesia for
patients with hyperthyroidism is the control of hyperthyroidism
prior to surgery in order to reduce TH levels. It is expected that
the mechanism through which T3 inhibits the
GABAA receptors will be determined in future research.
By preventing this inhibition using a specific blocking agent, the
optimum anaesthesia effect of propofol can be achieved.
Acknowledgements
The authors would like to express their thanks to
all those who helped in the writing of this paper.
Glossary
Abbreviations
Abbreviations:
|
GABA
|
γ-aminobutyric acid
|
|
GABAAR
|
γ-aminobutyric acid type A
receptor
|
|
T3
|
3,3′,5-L-triiodothyronine
|
|
T4
|
thyroxine
|
|
DRG
|
dorsal root ganglion
|
References
|
1
|
Greenhill C: Thyroid function:
Hyperthyroidism - psychiatric issues. Nat Rev Endocrinol.
10:652014. View Article : Google Scholar
|
|
2
|
Brandt F, Thvilum M, Almind D, et al:
Hyperthyroidism and psychiatric morbidity: Evidence from a Danish
nationwide register study. Eur J Endocrinol. 170:341–348. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ertek S and Cicero AF: Hyperthyroidism and
cardiovascular complications: A narrative review on the basis of
pathophysiology. Arch Med Sci. 9:944–952. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bajwa SJ and Sehgal V: Anesthesia and
thyroid surgery: The never ending challenges. Indian J Endocrinol
Metab. 17:228–234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsubokawa T, Yamamoto K and Kobayashi T:
Propofol clearance and distribution volume increase in patients
with hyperthyroidism. Anesth Analg. 87:195–199. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang YG, Song XJ, Feng SW, Ge YL, Yang JJ
and He LL: Hyperthyroidism patients have shorter onset and duration
time of rocuronium than euthyroidism patients. J Pharm Pharm Sci.
10:53–60. 2007.PubMed/NCBI
|
|
7
|
Kumar VV and Kaimar P: Subclinical
hypothyroidism: A cause for delayed recovery from anaesthesia?
Indian. J Anaesth. 55:433–434. 2011.
|
|
8
|
Trapani G, Altomare C, Liso G, Sanna E and
Biggio G: Propofol in anesthesia. Mechanism of action,
structure-activity relationships, and drug delivery. Curr Med Chem.
7:249–271. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hara M, Kai Y and Ikemoto Y: Enhancement
by propofol of the gamma-aminobutyric acid A response in
dissociated hippocampal pyramidal neurons of the rat.
Anesthesiology. 81:988–994. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olsen RW and Sieghart W: International
Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid (A)
receptors: classification on the basis of subunit composition,
pharmacology, and function. Update. Pharmacol Rev. 60:243–260.
2008. View Article : Google Scholar
|
|
11
|
Duveau V, Laustela S, Barth L, et al:
Spatiotemporal specificity of GABAA receptor-mediated
regulation of adult hippocampal neurogenesis. Eur J Neurosci.
34:362–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Olsen RW and Sieghart W: GABA A receptors:
Subtypes provide diversity of function and pharmacology.
Neuropharmacology. 56:141–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gupta MK and Misra K: Atom-based 3D-QSAR,
molecular docking and molecular dynamics simulation assessment of
inhibitors for thyroid hormone receptor α and β. J Mol Model.
20:22862014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Puia G and Losi G: Thyroid hormones
modulate GABA(A) receptor-mediated currents in hippocampal neurons.
Neuropharmacology. 60:1254–1261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li M, Iismaa SE, Naqvi N, Nicks A, Husain
A and Graham RM: Thyroid hormone action in postnatal heart
development. Stem Cell Res (Amst). 13:582–591. 2014. View Article : Google Scholar
|
|
16
|
Wirth EK, Schweizer U and Köhrle J:
Transport of thyroid hormone in brain. Front Endocrinol (Lausanne).
5:982014.PubMed/NCBI
|
|
17
|
Morte B and Bernal J: Thyroid hormone
action: Astrocyte-neuron communication. Front Endocrinol
(Lausanne). 5:822014.PubMed/NCBI
|
|
18
|
Bhumika S and Darras VM: Role of thyroid
hormones in different aspects of nervous system regeneration in
vertebrates. Gen Comp Endocrinol. 203:86–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Davis PJ, Tillmann HC, Davis FB and
Wehling M: Comparison of the mechanisms of nongenomic actions of
thyroid hormone and steroid hormones. J Endocrinol Invest.
25:377–388. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bergh JJ, Lin HY, Lansing L, et al:
Integrin alphaVbeta3 contains a cell surface receptor site for
thyroid hormone that is linked to activation of mitogen-activated
protein kinase and induction of angiogenesis. Endocrinology.
146:2864–2871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davis PJ, Davis FB and Cody V: Membrane
receptors mediating thyroid hormone action. Trends Endocrinol
Metab. 16:429–435. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin HY, Sun M, Tang HY, et al: L-Thyroxine
vs. 3,5,3′-triiodo-L-thyronine and cell proliferation: Activation
of mitogen-activated protein kinase and phosphatidylinositol
3-kinase. Am J Physiol Cell Physiol. 296:C980–C991. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cohen K, Ellis M, Khoury S, Davis PJ,
Hercbergs A and Ashur-Fabian O: Thyroid hormone is a MAPK-dependent
growth factor for human myeloma cells acting via αvβ3 integrin. Mol
Cancer Res. 9:1385–1394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luidens MK, Mousa SA, Davis FB, Lin HY and
Davis PJ: Thyroid hormone and angiogenesis. Vascul Pharmacol.
52:142–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sukocheva OA and Carpenter DO:
Anti-apoptotic effects of 3,5,3′-tri-iodothyronine in mouse
hepatocytes. J Endocrinol. 191:447–458. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hausenloy DJ and Yellon DM: New directions
for protecting the heart against ischaemia-reperfusion injury:
Targeting the reperfusion injury salvage kinase (RISK)-pathway.
Cardiovasc Res. 61:448–460. 2004. View Article : Google Scholar : PubMed/NCBI
|