Introduction
Systemic lupus erythematosus (SLE) is an autoimmune
disease with multi-systemic clinical symptoms. The regions that are
most affected by SLE are the skin and the musculoskeletal,
hematopoietic and cardiopulmonary systems (1). Digestive disorders are less common, but
potentially much more serious, and perforation of the intestinal
tract is one of the most severe complications of SLE. SLE is caused
by gastrointestinal vasculitis and thrombosis, and is considered to
be life-threatening (2,3). The clinical symptoms of lupus enteritis
are nonspecific, but generally include abdominal pain, vomiting,
diarrhea and fever. Typical computed tomography (CT) features of
SLE include bowel wall edema, mesenteric abnormalities and ascites.
Lupus enteritis is steroid responsive and typically has an
excellent overall prognosis. If not treated correctly, SLE may
develop to intestinal necrosis and perforation of the intestinal
tract, which is among the most severe complications of SLE
(4,5). The present study reported a case of SLE
with intestinal perforation, which required surgical intervention.
The patient was successfully treated.
Case report
A 44-year-old female patient was admitted to the
Taicang Hospital Affiliated to Soochow University (Taicang, China)
in November 9, 2013, presenting with abdominal pain and bloating,
nausea and vomiting for 24 h, as well as no bowel movements for 2
days. The patient had been diagnosed with systemic lupus
erythematosus (SLE) and lupus nephritis (LN) 16 years prior to
admission, and received steroid therapy (prednisone, 10 mg/day).
Written informed consent was obtained from the patient. In November
2010, April 2011 and June 2013, the patient had been hospitalized
in the Department of Gastroenterology of the Taicang Hospital for
chronic intestinal pseudo-obstruction (CIP). The patient had been
successfully treated using nonsurgical treatment, which included
fasting, gastrointestinal decompression, gastromotor drug
administration and parenteral nutrition. In addition, the patient
had a history of high blood pressure for 3 years and received
amlodipine at 5 mg/day for 3 years.
In November 9, 2013, the patient was again admitted
to the hospital and treated with conservative therapy; however, 10
h after admission, the patient complained of gradually increasing
abdominal pain. The results of physical examination were within
normal ranges, as follows: Temperature, 36.7°C; blood pressure,
90/45 mmHg; heart rate, 72/min. Abdomen palpation revealed muscular
defense with diffuse tenderness and rebounding pain, particularly
in the lower area. No palpable mass was observed and bowel sounds
were hypoactive.
The data collected from the laboratory examinations
are summarized in Table I. The
patient's peripheral blood showed leucopenia, but the percentage of
neutrophils was markedly increased. Blood chemistry tests revealed
hypoproteinemia and hypoalbuminemia, while liver function test
results were normal and renal function tests revealed a slight
increase in blood urea nitrogen and marked levels of C-reactive
protein. The erythrocyte sedimentation rate was markedly elevated.
Immunological tests revealed positive results for anti-SSB
antibodies, weakly positive for anti-dsDNA and negative for
anti-ribonucleoprotein, anti-Smith and Ro antibodies. In addition,
the levels of serum immunoglobulin (Ig)G, IgM and complement
component (C)3 were decreased, while those of serum IgA and C4
levels were normal. The test results from the coagulo-fibrinolytic
system were all normal.
 | Table I.Laboratory data gathered following
admission and prior to discharge. |
Table I.
Laboratory data gathered following
admission and prior to discharge.
| Tests | Results at
admission | Results at
discharge |
|---|
| CBC |
|
|
| WBC
(3.7–9.2×109/l) | 3.0 | 4.7 |
| Neutro.
(45–80%) | 87.7 | 72.2 |
| Lymph.
(20–40%) | 9.3 | 21.4 |
| RBC
(3.7–5.1×1012/l) | 4.63 | 3.67 |
| Hb
(113–151 g/l) | 126 | 111 |
| Hct
(33.5–45.0) | 40.6 | 34.1 |
| Plt
(101–320×109/l) | 168 | 257 |
| Blood chemistry |
|
|
| TP (60–83
g/l) | 38.0 | 48.6 |
| Alb
(35–54 g/l) | 16.1 | 27.3 |
| Na
(135–145 mmol/l) | 139.3 | 141.0 |
| K
(3.5–5.2 mmol/l) | 3.7 | 3.9 |
| Cl
(96–108 mmol/l) | 109.9 | 109.8 |
| BUN
(2.9–8.2 mmol/l) | 11.2 | 6.06 |
| Cr
(40–120 umol/l) | 97.0 | 38.0 |
| T-Bil
(2–25 umol/l) | 20.7 | 7.5 |
| AST (0–40
U/l) | 24.0 | 17.2 |
| ALT (0–40
U/l) | 16.0 | 16.1 |
| γGT (5–50
U/l) | 14.0 | 154.5 |
| LDH
(109–245 U/l) | 117.0 | 165.9 |
| ESR (0–20
mm/H) | 100 | 5 |
| CRP (0–10
mg/l) | 172 | 5.9 |
| Serological test |
|
|
| IgG
(6.94–16.20 g/l) | 3.9 | 12.0 |
| IgA
(0.68–3.78 g/l) | 0.72 | 1.57 |
| IgM
(0.60–2.63 g/l) | 0.44 | 2.35 |
| C3
(0.88–2.01 g/l) | 0.28 | 0.94 |
| C4
(0.1–0.4 g/l) | 0.14 | 0.30 |
|
Anti-dsDNA (negative) | (±) | (−) |
| Anti-RNP
(negative) | (−) | (−) |
| Anti-Sm
(negative) | (−) | (−) |
| SSA
(negative) | (−) | (−) |
| SSB
(negative) | (+) | (−) |
| Hemostatic date |
|
|
| PT
(9.6–14.3s) | 12.9 | 14.0 |
| APTT
(23.7–36.4s) | 29.3 | 27.8 |
| AT3
(75.0–130.0%) | 105.7 | 113.0 |
| Fgb
(1.7–4.1 g/l) | 2.60 | 2.52 |
| INT
(0.85–1.25) | 1.09 | 1.06 |
| Tumor marker |
|
|
| CEA
(0.00–6.00 ng/ml) | 2.55 | Not performed |
| AFP
(0.00–8.00 ng/ml) | 1.91 | Not performed |
| CA199
(0.00–30.00 U/ml) | 0.6 | Not performed |
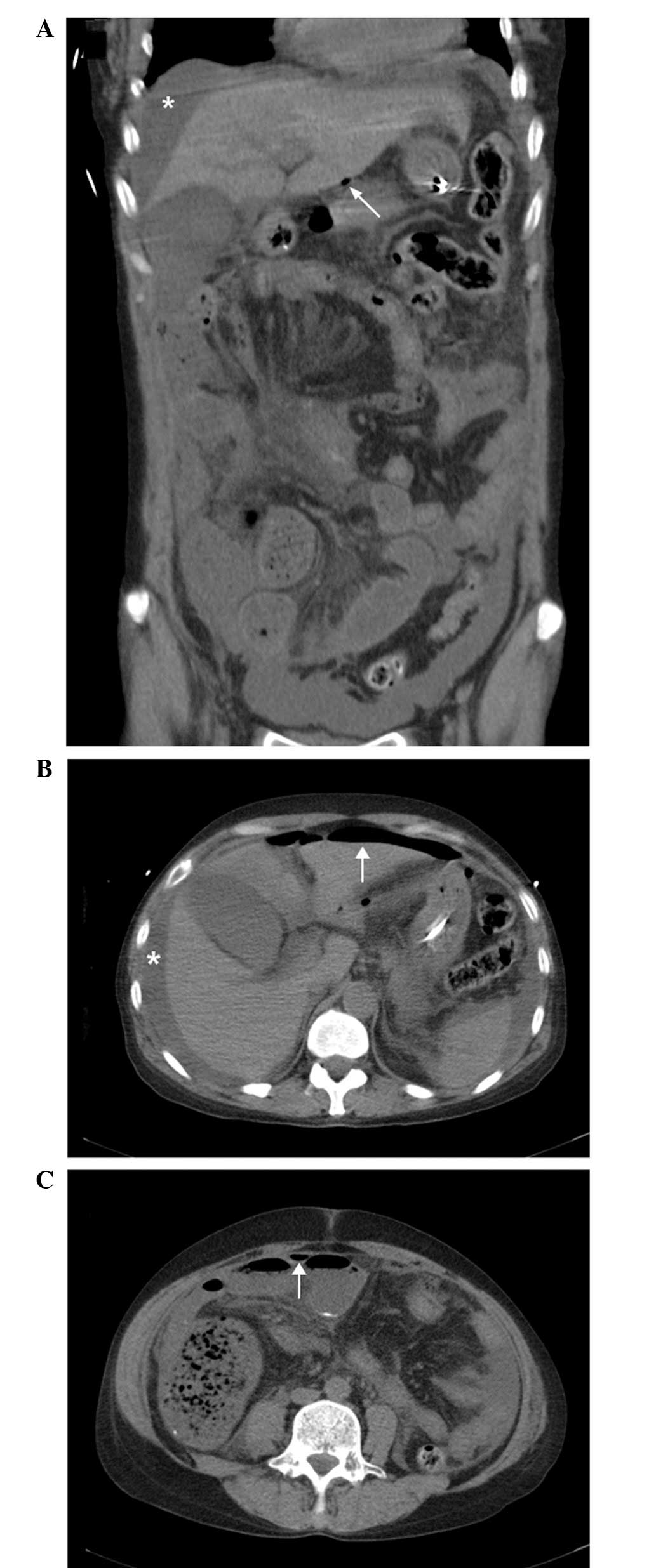
An abdominal CT scan was performed (Fig. 1), which revealed a large volume of
free fluid and gas in the abdominal cavity and an area suspicious
for intestinal perforation. Diagnostic abdominocentesis was
performed and 5 ml muddy straw-colored peritoneal fluid was
aspirated without any obstructions.
The patient underwent an emergency exploratory
laparotomy and the intraoperative findings confirmed the initial
diagnosis of intestinal perforation. Large feces and intestinal
contents were observed in the abdominal cavity, as well as severe
adhesions between the ileum (40–80 cm from the ileocecal valve) and
adjacent parietal peritoneum. A crevasse with a diameter of ~3 cm
was detected in the ileum, ~60 cm from the ileocecal valve. Several
other small perforations of the ileum, ~50 cm from the ileocecal
valve, were observed, and the bowel wall in that area was overly
thin. The appendix was found to have chronic inflammation, since
fecal stone blocked the opening of the cavity. Inspection of the
entire abdominal cavity revealed no other evident lesions. An
enterolysis, appendectomy and distal ileectomy (50 cm of the
diseased small bowel) with an ileostomy were performed.
Histological examination (Fig. 2) of
the resected small bowel tissue revealed necrosis, perforation and
inflammatory cell infiltration with bleeding observed on the
full-thickness of the bowel wall, while the removed appendix
confirmed the diagnosis of chronic appendicitis.
In addition, the patient suffered decannulation
failure and difficult weaning from the ventilator. Following four
failed attempts of decannulation, tracheotomy was perform on
December 2, 2013. Mechanical ventilation support was used
discontinuously and the tracheal incision was sealed on January 22,
2014. Continuous anesthesia of midazolam and fentanyl was
administered intravenously for sedation and pain control.
The patient also suffered from septic shock and
received a cardiac dose of norepinephrine for approximately half a
month, until November 26, 2013, and antibiotics were administered
according to the bacterial culture and drug sensitivity results. At
the end of December 2013, the patient's fever and white blood cell
count had alleviated, and the inflammation had decreased. At
postoperative day 4, the patient started drinking small volumes of
water via a nose-jejunum nutrition tube, and a few days later she
was able to consume a liquid diet. During the liquid diet phase,
the patient was treated with 10 mg prednisolone daily. On December
04, 2013, the patient developed abdominal pain, nausea and
vomiting; subsequently, the dose of prednisolone was increased to
40 mg/day for pulse-dose therapy and the symptoms were relieved.
Following the termination of the pulse-dose therapy, the
prednisolone dose was decreased and the patient developed
intestinal obstruction again; however, the patient's condition
improved soon after fasting for 3 days. Right lower extremity deep
vein thrombosis was observed on November 20, 2013, and heparin
therapy was performed for 2 weeks. The postoperative clinical
course of the patient is shown in Fig.
3. Treatment was successful and the patient was discharged on
March 3, 2014 and has exhibited no further complications to
date.
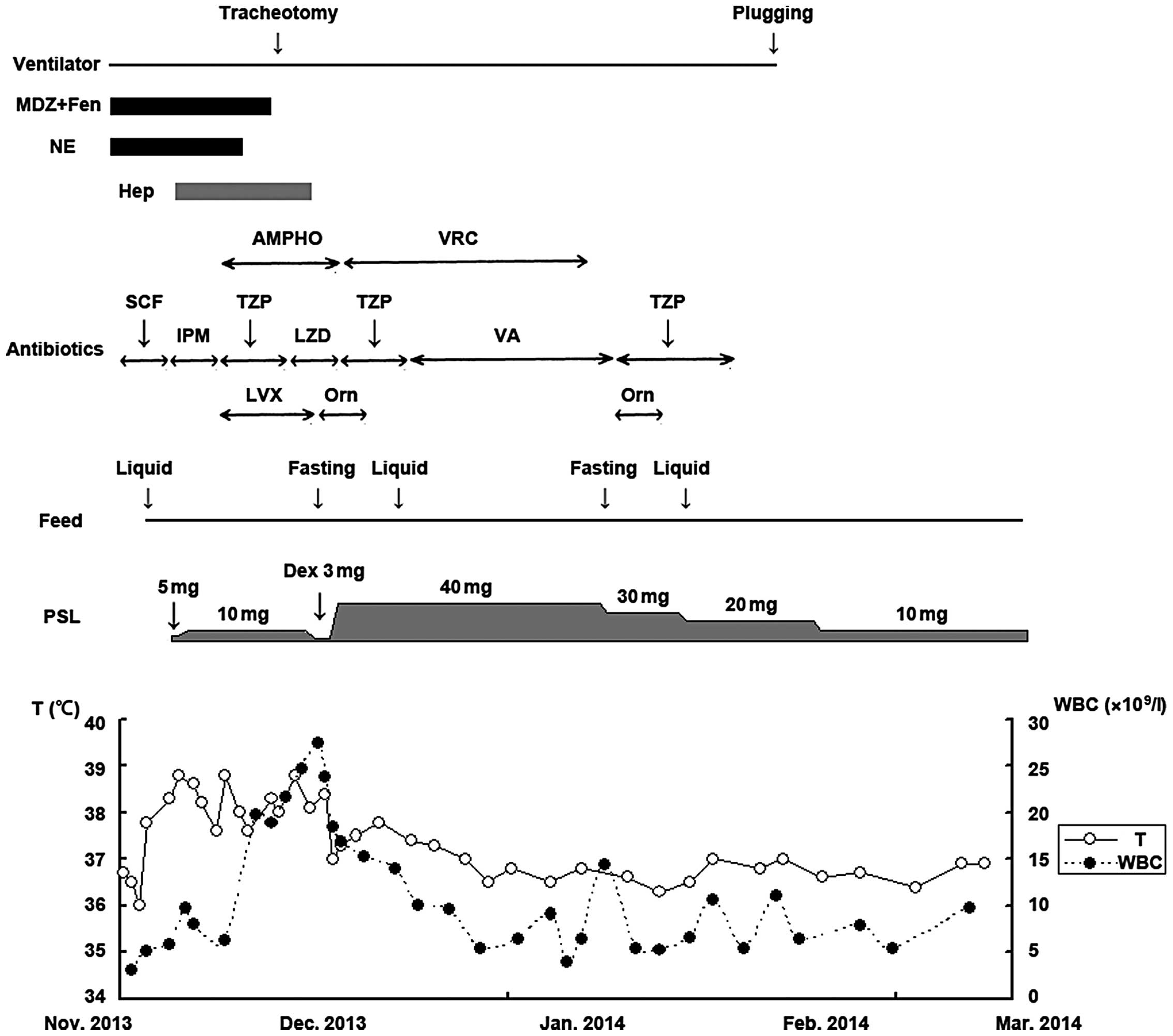 | Figure 3.Post-operative clinical course of the
patient. MDZ, midazolam; Fen, fentanyl; NE, norepinephrine; Hep,
heparin; AMPHO, amphotericin B; VRC, voriconazole; SCF,
cefoperazone/sulbactam; IPM, imipenem; TZP,
piperacillin/tazobactam; LZD, linezolid; VA, vancomycin; LVX,
levofloxacin; Orn, ornidazole; PSL, prednisolone; Dex,
dexamethasone; WBC, white blood cell count; T, T cells. |
Discussion
Systemic lupus erythematosus (SLE) is a systemic
autoimmune inflammatory disease that can affect almost every organ
and system of the human organism. Gastrointestinal disorder is one
of the most noteworthy complications of SLE, since it can be
life-threatening if not treated promptly (6,7). The
most common SLE-associated gastroenteropathies include
protein-losing enteropathy, lupus mesenteric vasculitis, acute
pancreatitis, intestinal pseudo-obstruction and other rare
complications, such as inflammatory bowel diseases and celiac
disease (8).
Chronic intestinal pseudo-obstruction (CIP) is
characterized by ineffective intestinal propulsion without any
mechanical obstruction of the gut (9). Symptoms of CIP include abdominal pain
and distension, nausea and vomiting, constipation and weight loss.
Radiographs of the abdomen demonstrate fluid in the bowel loops and
a thickened intestinal wall. CIP is considered to be caused by the
autoimmune inflammatory involvement of the visceral smooth muscle
and enteric nervous system (10).
Nonsurgical treatments that are often effective against CIP include
high doses of corticosteroids, fasting, nasogastric drainage, total
parenteral nutrition and administration of gastromotor or
anti-infective drugs (11). In the
present case, CIP was diagnosed and, although the patient had
previously been treated by conservative therapy, the disease
reappeared three more times.
At the fourth time of hospitalization, conservative
therapy was no longer effective. The patient complained of
gradually increasing abdominal pain, and abdomen palpation revealed
muscular defense and diffuse pain with rebound tenderness. The
abdominal CT scan revealed a large amount of free fluid and gas in
the abdominal cavity and an area suspicious for intestinal
perforation. An emergency exploratory laparotomy confirmed the
diagnosis of bowel infarction and perforation. Bowel infarction and
perforation in patients with SLE are often caused by acute ischemic
enteritis of the small intestine, which is also described as lupus
mesenteric vasculitis (12,13). Lupus mesenteric vasculitis is an
uncommon, but severe complication occurring in patients with SLE.
In Asia, the overall prevalence of lupus mesenteric vasculitis in
patients with SLE has been reported to be 2.2–9.7% (14,15).
Lupus mesenteric vasculitis is considered to be caused in patients
with SLE by circulating autoantibodies that form an immune complex
deposition in blood vessels, which can lead to the development of
vasculitis and thrombosis of the vessels supplying the intestine.
An inadequate blood supply to the intestine results in ulceration,
infarction and perforation (16,17).
The prognosis of SLE patients with intestinal
perforation is poor. Therefore, early diagnosis and appropriate
interventions are crucial to the management of the disease
(18). The majority of SLE cases
have been found to be long-term users of corticosteroids and
immunosuppressants, which are known to weaken the immune system
(19,20). Any surgical intervention is more
risky in SLE patients compared with individuals without SLE
(21,22). In the present case, the patient faced
three major problems following surgery. The patient suffered from
decannulation failure and difficult weaning from the ventilator,
and thus tracheotomy was performed. In addition, the patient
suffered from septic shock for which she received a cardiac dose of
norepinephrine for approximately half a month, and antibiotics were
administered according to the bacterial culture and drug
sensitivity results, until the white blood cell count and
temperature returned to normal. Finally, a correlation was observed
between prednisolone dose and intestinal obstruction: At
postoperative day 25, the patient developed intestinal obstruction;
thus, the dose of prednisolone was increased for pulse-dose therapy
and the symptoms were relieved. However, at ~2 months
postoperatively, the dose of prednisolone was gradually reduced and
intestinal obstruction reappeared. The patient then received
conservative treatment once again, including fasting and parenteral
nutrition.
In conclusion, intestinal perforation in SLE
patients is extremely rare; however, it can potentially be
life-threatening. Early diagnosis and prompt treatment are crucial
to the management of this rare complication of SLE.
References
|
1
|
Meszaros ZS, Perl A and Faraone SV:
Psychiatric symptoms in systemic lupus erythematosus: A systematic
review. J Clin Psychiatry. 73:993–1001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Janessens P, Arnaud L, Galicier L, Mathian
A, Hie M, Sene D, Haroche J, Veyssier-Belot C, Huynh-Charlier I and
Grenier PA: Lupus enteritis: From clinical findings to therapeutic
management. Orphanet J Rare Dis. 8:672013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malaviya AN, Sharma A, Agarwal D, Kapoor
S, Garg S, Singh S and Rawat R: Acute abdomen in SLE. Int J Rheum
Dis. 14:98–104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chng HH, Tan BE, Teh CL and Lian TY: Major
gastrointestinal manifestations in lupus patients in Asia: Lupus
enteritis, intestinal pseudo-obstruction and protein-losing
gastroenteropathy. Lupus. 19:1404–1413. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu D, Yang H, Lai CC, Li P, Zhang X, Yang
XO, Zhang FC and Qian JM: Clinical analysis of systemic lupus
erythematosus with gastrointestinal manifestations. Lupus.
19:866–869. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Medina F, Ayala A, Jara LJ, Becerra M,
Miranda JM and Fraga A: Acute abdomen in systemic lupus
erythematosus: The importance of early laparotomy. Am J Med.
103:100–105. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al-Hakeem MS and McMillen MA: Evaluation
of abdominal pain in systemic lupus erythematosus. Am J Surg.
176:291–294. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tian XP and Zhang X: Gastrointestinal
involvement in systemic lupus erythematosus: Insight into
pathogenesis, diagnosis and treatment. World J Gastroenterol.
16:2971–2977. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mok MY, Wong RW and Lau CS: Intestinal
pseudo-obstruction in systemic lupus erythematosus: An uncommon but
important clinical manifestation. Lupus. 9:11–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garcia López CA, Laredo-Sánchez F,
Malagón-Rangel J, Flores-Padilla MG and Nellen-Hummel H: Intestinal
pseudo-obstruction in patients with systemic lupus erythematosus: A
real diagnostic challenge. World J Gastroenterol. 20:11443–11450.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khairullah S, Jasmin R, Yahya F, Cheah TE,
Ng CT and Sockalingam S: Chronic intestinal pseudo-obstruction: A
rare first manifestation of systemic lupus erythematosus. Lupus.
22:957–960. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahn E, Luk A, Chetty R and Butany J:
Vasculitides of the gastrointestinal tract. Semin Diagn Pathol.
26:77–88. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang DF and Chen WS: Images in clinical
medicine. Lupus-associated intestinal vasculitis. N Engl J Med.
361:e32009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee CK, Ahn MS, Lee EY, Shin JH, Cho YS,
Ha HK, Yoo B and Moon HB: Acute abdominal pain in systemic lupus
erythematosus: Focus on lupus enteritis (gastrointestinal
vasculitis). Ann Rheum Dis. 61:547–550. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lian TY, Edwards CJ, Chan SP and Chng HH:
Reversible acute gastrointestinal syndrome associated with active
systemic lupus erythematosus in patients admitted to hospital.
Lupus. 12:612–616. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Passam FH, Diamantis ID, Perisinaki G,
Saridaki Z, Kritikos H, Georgopoulos D and Boumpas DT: Intestinal
ischemia as the first manifestation of vasculitis. Semin Arthritis
Rheum. 34:431–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ju JH, Min JK, Jung CK, et al: Lupus
mesenteric vasculitis can cause acute abdominal pain in patients
with SLE. Nat Rev Rheumatol. 5:273–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsu CL, Chen KY, Yeh PS, et al: Outcome
and prognostic factors in critically ill patients with systemic
lupus erythematosus: A retrospective study. Crit Care. 9:R177–R183.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan TC, Wansaicheon GK and Thong BY: Acute
onset of systemic lupus erythematosus with extensive
gastrointestinal and genitourinary involvement. Lupus.
21:1240–1243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Majithia R, Joy G, Liang J and Olden K:
Acute abdominal pain in systemic lupis erythematosus. Gastrointest
Endosc. 75:1267–1268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee J, Jung HS, Nam HC, Kwok SK, Ju JH,
Park KS, Kim HY and Park SH: Fulminant amoebic colitis mimicking
intestinal vasculitis in a patient with systemic lupus
erythematosus. Lupus. 21:1351–1355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kishimoto M, Nasir A, Mor A and Belmont
HM: Acute gastrointestinal distress syndrome in patients with
systemic lupus erythematosus. Lupus. 16:137–141. 2007. View Article : Google Scholar : PubMed/NCBI
|