Introduction
Acute promyelocytic leukemia (APL) is a subtype of
acute myelogenous leukemia, accounting for 6.2–40.2% of acute
myelogenous leukemia cases. APL manifests rapidly, causing serious
illness, and is often accompanied by severe bleeding and
disseminated intravascular coagulation. In the past, APL was
considered to be ‘the most malignant form of acute leukemia’
(1,2). APL cells are relatively sensitive to
chemotherapy (CT); however, it has been frequently observed that CT
aggravates bleeding disorders, leading to high early-mortality
rates. Despite the sensitivity of APL to CT, the median duration of
remission ranges between 11 and 25 months, and only 35–45% of the
patients are cured by CT alone (3,4).
Arsenic trioxide (As2O3) is
the primary active component in arsenic. Arsenic is a common,
naturally occurring substance that exists in organic and inorganic
forms. It has been demonstrated that As2O3
can induce cell differentiation and apoptosis.
As2O3 yields a remission rate of as high as
90% in treating APL (5,6). The traditional
As2O3 solution, however, has numerous side
effects, such as hyperleukocytosis, liver and kidney dysfunction,
and effusion (7,8). These side effects increase the
suffering of patients with APL, which sometimes results in patients
decreasing the dose of As2O3 or even stopping
halfway through therapy, thus seriously affecting its curative
effects. The aforementioned reasons limit the use of
As2O3 in clinical practice; therefore,
developing new methods of As2O3
administration that avoid these side effects is imperative.
Nanomedicine has attracted considerable focus due to its beneficial
characteristics, including targeted drug delivery and slow drug
release (9). Employing
nanotechnology in cancer treatment is currently one of the most
cutting-edge fields of biotechnology research (10,11). In
the present study, the traditional As2O3
preparation technology was modified, and
As2O3 nanoparticles were prepared using
modern nanotechnology. The aim of the present study was to evaluate
the properties of the prepared As2O3
nanoparticles and investigate their antitumor effects. We
hypothesized that the modified preparation technique would improve
bioavailability, which would reduce the drug dosage and toxicity
and enhance the associated curative effects.
Materials and methods
Cells and cell culture
NB4 cells, a human APL cell line, were provided by
Dr Jifan Hu at Stanford University Medical School (Palo Alto, CA,
USA) and maintained in the laboratory of The First Affiliated
Hospital of Jilin University (Changchun, China). The cells were
cultured at 37°C in Iscove's Modified Dulbecco's Medium (Gibco-BRL,
Grand Island, NY, USA), supplemented with 10% heat-inactivated
fetal bovine serum (Hanzhou Sijiqing Biological Engineering
Materials Co., Ltd., Hanzhou, China), 100 U/ml penicillin and 100
µg/ml streptomycin in an atmosphere of 5% CO2 and 100%
humidity.
Preparation of
As2O3 nanoparticles using the sol-gel
method
For the preparation method, the following formulae,
in which
M represents the metal element and R represents
CmH2m+1, were used (12):
The specific preparation method was as follows: All
items used in the tests were sterilized, and
As2O3 powder and hydrochloric acid were
magnetically stirred and mixed at a mass/volume ratio of 1:0.02-0.1
for 10–30 min. Ethanol was then added at a volume ratio of 1:5-10,
the solution was stirred for 20–30 min at 50–60°C and the mixture
was sonicated for 5 min. Finally, distilled water was added at a
volume ratio of 1:4-5, and the mixture was sonicated for 10–20 min.
Following preparation, a few drops of the sample were placed on a
copper mesh, dried and then characterized with transmission
electron microscopy (TEM; JEM-2010, JEOL Ltd., Tokyo, Japan),
scanning electron microscopy (JSM-840, JEOL Ltd.) and energy
dispersive spectrometry (EDS; JEOL Ltd.).
Cytotoxicity analysis
The cytotoxicity and sensitivity of
As2O3 (Institute for Drug Control of the
Ministry of Health of China) and As2O3
nanoparticles were measured using the MTT cell viability method
(13). Cells were divided into three
groups: NB4, NB4 + As2O3 and NB4 +
As2O3 nanoparticles. NB4 cells in the
logarithmic growth phase were seeded on 96-well plates in
quadruplicate at a density of 1×105/well in 100 µl.
As2O3 solution and
As2O3 nanoparticles (7 different final
concentrations: 0.25, 0.5, 1.0, 1.5, 3.0, 6.0 and 12.0 µmol/l) were
added at the appropriate time points according to the group
setting. After 24, 48, 72 and 96 h of treatment, the cells were
incubated for 4 h with MTT (Changchun Biotech Co., Ltd., Changchun,
China) and then lysed with acidified isopropanol. Absorbance was
measured at 570 nm. The inhibition rate was calculated using the
following formula: Inhibition rate = [(absorbance value of control
group - absorbance value of test group)/absorbance value of control
group] ×100%. All experiments were repeated three times.
Flow cytometric analysis
Cell apoptosis was quantified using flow cytometry
(FCM; FACSCalibur™, BD Biosciences, San Jose, CA, USA). Cells were
grouped as previously described for the cytotoxicity analysis. Both
the As2O3 solution and
As2O3 nanoparticles were tested at two
concentrations: 1.5 and 3.0 µmol/l. Following incubation at 37°C in
an atmosphere of 5% CO2 and 100% humidity for 48 h, the
cells were washed with cold phosphate-buffered saline (PBS) twice
and resuspended in cold PBS. The apoptosis rate of NB4 cells after
treatment was examined using an Annexin V/Propidium Iodide
Apoptosis Detection Assay kit (Beyotime Institute of Biotechnology
Co., Shanghai, China). All experiments were repeated three
times.
Western blot analysis
Cells were grouped and pretreated as previously
described in the FCM. The total protein was extracted, separated
using SDS-PAGE and transferred to a nitrocellulose membrane. The
membrane was blocked with 5% skimmed milk at room temperature for 2
h, and then stained with rabbit polyclonal anti-B-cell lymphoma 2
(Bcl-2; 1:200; BA0412) or mouse monoclonal anti-β-actin antibodies
(1:200; BM0627; Wuhan Boster Biotechnology Co., Ltd., Wuhan, China)
for 2 h at room temperature. Following washing, the membrane was
incubated with horseradish peroxidase-labeled goat-anti-rabbit IgG
(Sigma-Aldrich, St. Louis, MO, USA) at 1:1,000 for 1 h at room
temperature. The membrane was then washed and developed. All
experiments were repeated three times.
Statistical analysis
All data were analyzed using SPSS 13.0 software
(SPSS Inc., Chicago, IL, USA). A t-test was adopted to
analyze inter-group differences. P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristics of the
As2O3 nanoparticles
As observed using TEM, the
As2O3 powder was square, polygonal or in the
form of anomalistic crystals with high electron density (Fig. 1A). The average diameter of the
As2O3 powder was >1 µm. By contrast, the
As2O3 nanoparticles were well dispersed,
approximately spherical or elliptical and ~40 or <10 nm in
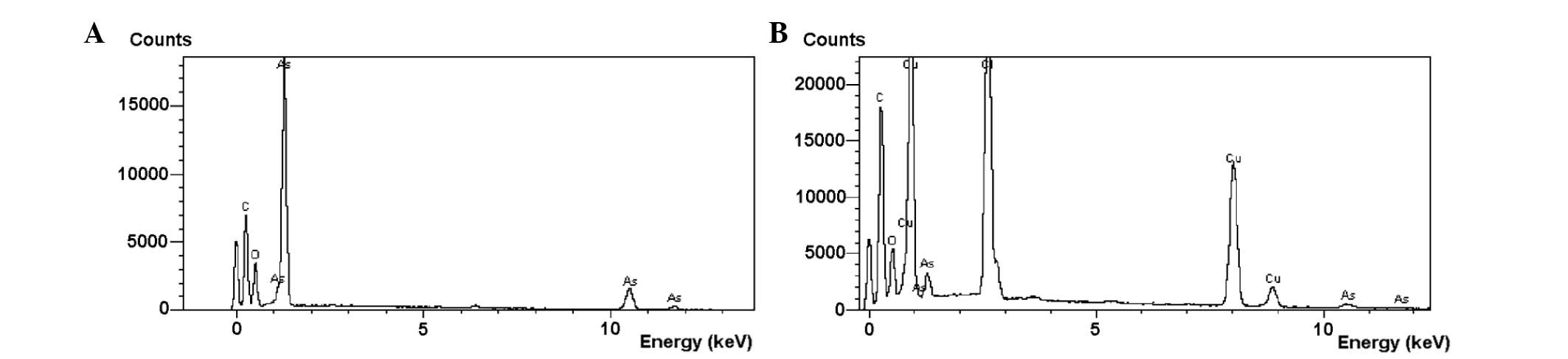
diameter (Fig. 1B). The EDS results
confirmed that these nanoparticles were As2O3
(Fig. 2).
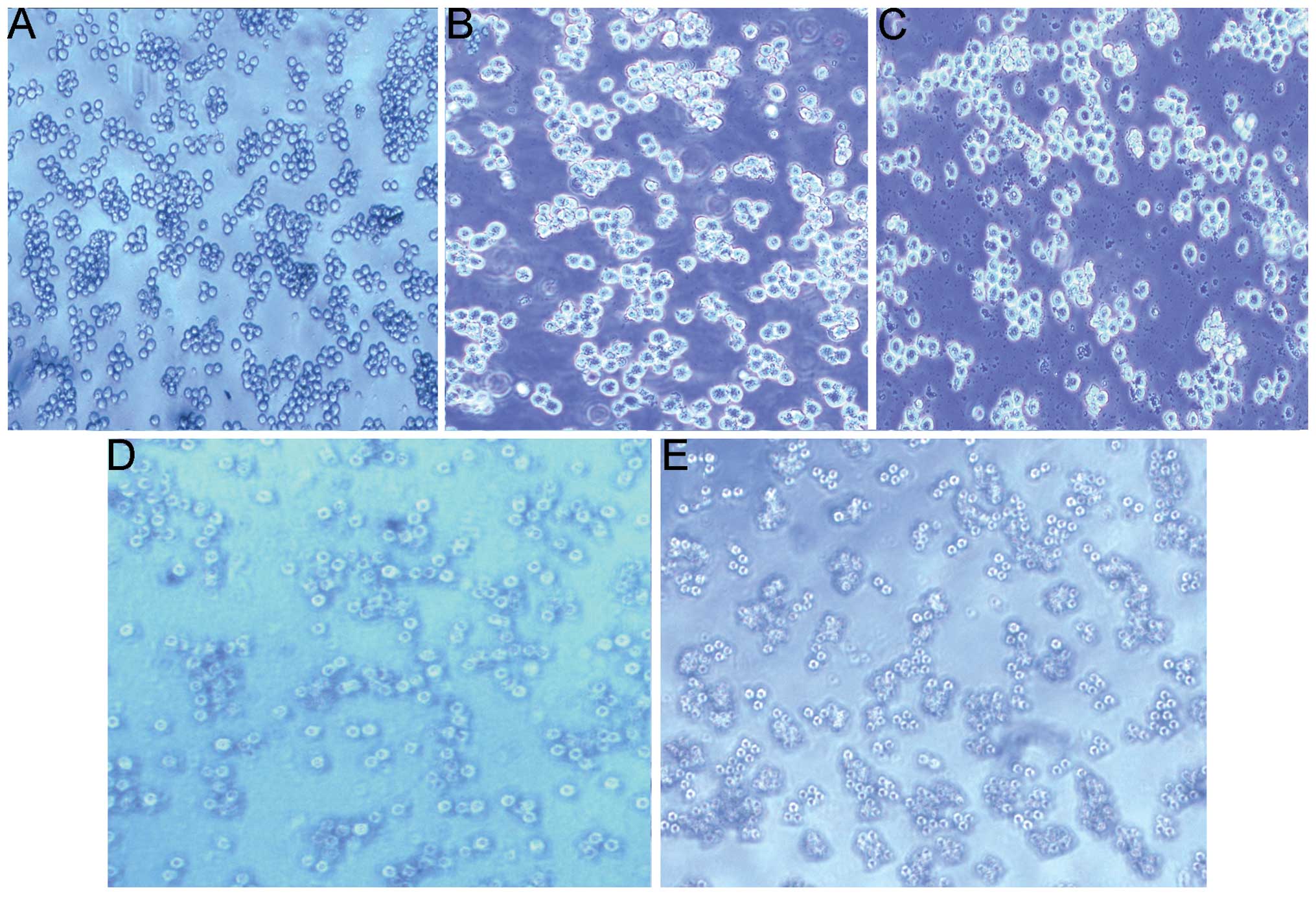
NB4 cell morphological changes
The morphological changes of the NB4 cells were
compared following treatment with As2O3 or
As2O3 nanoparticles, and the results are
shown in Fig. 3. The NB4 cells in
the control group exhibited a normal shape with similar sizes and
clear edges (Fig. 3A). No cell
fragmentation was observed (Fig.
3A). Forty-eight hours after As2O3
solution treatment (1.5 µmol/l), the NB4 cells were found to be
reduced in number and volume and to exhibit irregular shapes
(Fig. 3B). In addition to the
changes described above, the number of necrotic cells and cell
fragments increased during incubation at 3.0 µmol/l (Fig. 3D). Using the same concentrations and
incubation time, the morphological changes were more marked
following treatment with As2O3 nanoparticles
(Fig. 3C and E).
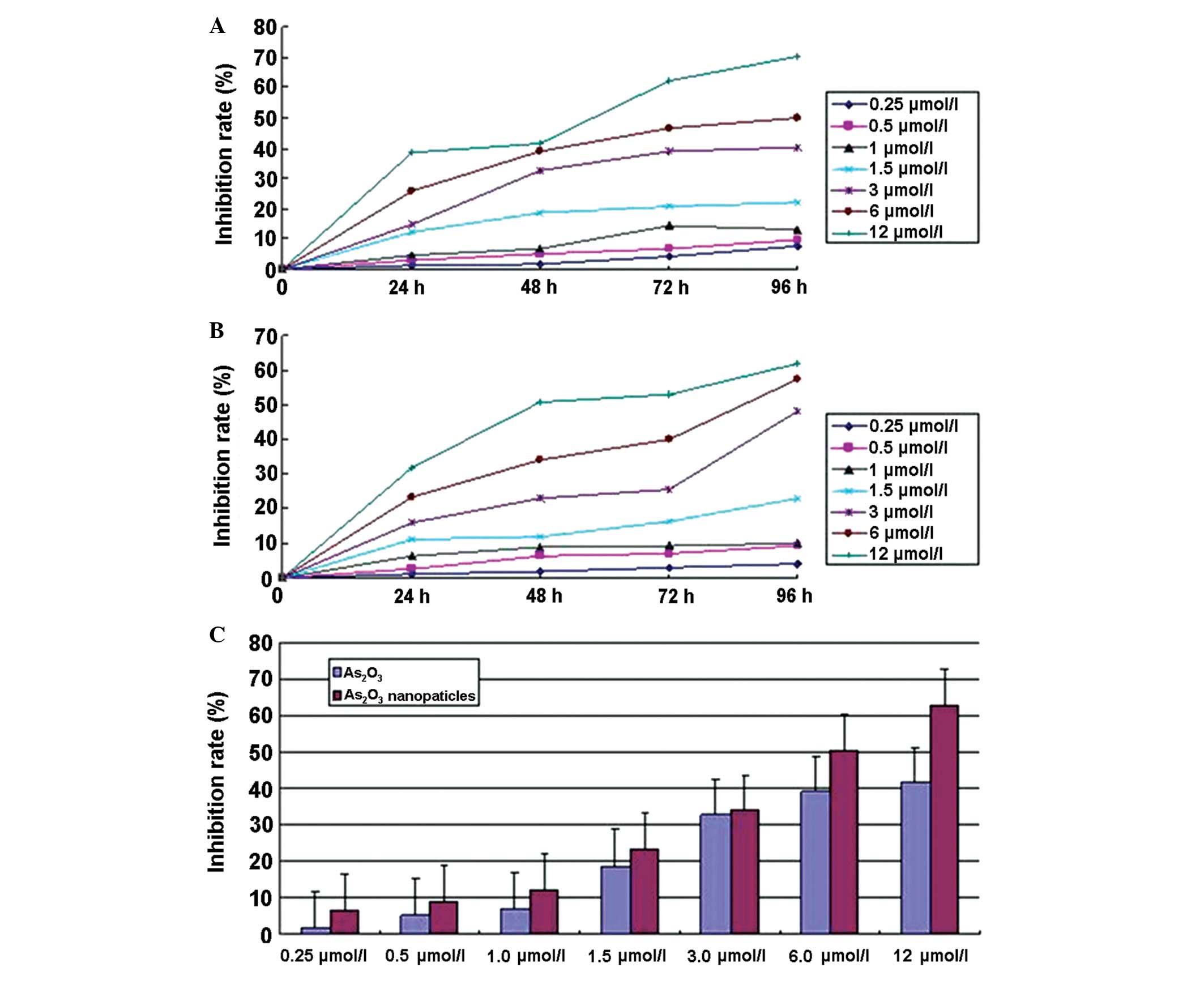
As2O3
nanoparticles inhibit NB4 cell growth
The results indicated that the
As2O3 nanoparticles were more effective than
the As2O3 solution in inhibiting NB4 cell
growth. At the same concentration and incubation time, the growth
rate of cells treated with As2O3
nanoparticles was significantly lower than that of cells treated
with the As2O3 solution. In both groups, the
inhibition rate was time- and dose-dependent (Fig. 4).
As2O3
nanoparticles induce NB4 cell apoptosis
As2O3 nanoparticles appeared
to be more effective than As2O3 solution in
inducing apoptosis of NB4 cells. Using the same concentration and
incubation time, the apoptosis level of cells treated with
As2O3 nanoparticles was significantly higher
than that of cells treated with the As2O3
solution (Fig. 5).
As2O3
nanoparticles downregulate Bcl-2 expression
The promotion of apoptosis by
As2O3 was further explored by examining the
expression level of the anti-apoptotic protein Bcl-2, which has
been implicated in several types of cancer (14). The results showed that, during
incubation at 3.0 µmol/l, As2O3 nanoparticles
caused a more significant reduction in Bcl-2 expression compared
with the As2O3 solution (Fig. 6).
Discussion
As2O3 is the primary active
component in arsenic. In Traditional Chinese Medicine, arsenic has
been shown to exhibit excellent efficacy in treating APL (15). As2O3 acts on
APL cells by promoting differentiation, as well as by inhibiting
growth and inducing apoptosis. Arsenic acid, however, is a highly
toxic substance and its clinical application is therefore limited
due to its severe side effects, which include serious heart
toxicity, cavity effusion, liver and kidney damage,
gastrointestinal adverse reactions and peripheral nervous infection
(16,17). It is therefore important to develop
new formulations of As2O3 with high
efficiency and low toxicity.
Nanotechnology is a technologically advanced field
that manipulates atoms and molecules on a spatial scale ranging
from 0.1 to 100 nm in order to serve specific functions. Changing
the traditional drug preparation technology by adopting modern
nanotechnology improves bioavailability by promoting drug
absorption by cells from the tissue space. Nanotechnology also
enables slow drug release, increasing drug concentrations in the
lesion site, reducing drug dosage and toxicity in non-targeted
sites and enhancing the curative effects. The sol-gel method is one
of the most common methods for preparing nanomaterials (18).
In the present study, As2O3
nanoparticles, measuring <10 nm and ~40 nm in diameter, were
successfully prepared using the sol-gel method. The results showed
that, compared with the As2O3 solution, the
As2O3 nanoparticles resulted in more
significant morphological changes in the NB4 cells (Fig. 3), exhibited stronger
growth-inhibition effects in the MTT assay (Fig. 4), induced apoptosis to a greater
extent (Fig. 5) and caused greater
downregulation of the anti-apoptotic protein Bcl-2 at high
concentrations (Fig. 6). These
findings were in agreement with a previous report that proposed
that As2O3 induced apoptosis mainly through
downregulating the expression of Bcl-2 (19).
The fact that the growth-inhibition and
apoptosis-induction functions of As2O3
nanoparticles are more effective than those of the traditional
As2O3 solution may be due to unique physical
and chemical properties, including higher chemical activities;
higher absorption and utilization; slow drug release, which helps
maintain effective drug concentrations in vivo; increased
ease of uptake by tumor cells and special pharmacological effects.
The surface of nanoparticles can be modified chemically and
biologically to generate nano-targeting drugs. In addition to the
aforementioned inherent advantages of nanoparticles, nano-targeting
drugs ensure targeted drug delivery, increasing curative effects
and reducing drug dosages to mitigate or avoid side effects
(20). The effectiveness of
nanomedicine is directly associated with the size of the
nanoparticles. A decrease in particle size causes an increase in
surface area and, correspondingly, an increase in the number of
surface atoms, leading to higher chemical activities. Small
particles, however, are more prone to aggregation, which increases
the total size of the particles and offsets the effects of
increased chemical activity; therefore, when preparing small
nanoparticles, it is necessary to adopt special methods of
preventing aggregation. Ultrasonic dispersion is a common and
effective method (21,22). In the present study,
As2O3 nanoparticles were prepared using the
sol-gel method as previously described (23–24).
Unlike in previous studies, smaller-sized
As2O3 nanoparticles (<10 nm) were prepared
for the present experiments. The findings showed that small
As2O3 nanoparticles (<10 nm) generate
significant antitumor effects, even at low concentrations (1.5
µmol/l), confirming that small As2O3
nanoparticles may have increased activity, thus requiring reduced
dosages for cancer treatment.
In the present study, the traditional preparation
technology was modified by employing modern nanotechnology, and
smaller As2O3 nanoparticles were successfully
prepared. The growth-inhibition and apoptosis-induction functions
of As2O3 nanoparticles were further
characterized, and the superiority of As2O3
nanoparticles over traditional As2O3
solutions in vitro was demonstrated. In conclusion,
As2O3 nanoparticles are a promising approach
for treating APL and the current data provide a theoretical and
experimental basis for applying nanomedicine in the clinical
treatment of acute leukemia.
Acknowledgements
The authors would like to thank Medjaden Bioscience
Limited for assisting in the preparation of this manuscript.
References
|
1
|
Hillestad LK: Acute promyelocytic
leukemia. Acta Med Scand. 159:189–194. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bernard J, Mathe G, Boulay J, Ceoard B and
Chome J: Acute promyelocytic leukemia: A study made on 20 cases.
Schweiz Med Wochenschr. 89:604–608. 1959.(In French). PubMed/NCBI
|
|
3
|
Bernard J, Weil M, Boiron M, Jacquillat C,
Flandrin G and Gemon MF: Acute promyelocytic leukemia: Results of
treatment by daunorubicin. Blood. 41:489–496. 1973.PubMed/NCBI
|
|
4
|
Ribeiro RC and Rego E: Management of APL
in developing countries: Epidemiology, challenges and opportunities
for international collaboration. Hematology Am Soc Hematol Educ
Program. 162–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang P, Wang SY, Hu LH, Shi FD, Qiu FG,
Hong ZZ, Han XY, Yang H, Song YZ, Liu YP, et al: Arsenic trioxide
treated 72 cases of acute promyelocytic leukemia. Zhong Hua Xue Ye
Xue Za Zhi. 17:58–60. 1996.(In Chinese).
|
|
6
|
Wang ZY: Ham-Wasserman lecture: Treatment
of acute leukemia by inducing differentiation and apoptosis.
Hematology Am Soc Hematol Educ Program. 1–13. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haverkamp W, Breithardt G, Camm AJ, Janse
MJ, Rosen MR, Antzelevitch C, Escande D, Franz M, Malik M, Moss A
and Shah R: The potential for QT prolongation and proarrhythmia by
non-antiarrhythmic drugs: Clinical and regulatory implications Eur
Heart. J. 21:1216–1231. 2000.
|
|
8
|
Unnikrishnan D, Dutcher JP, Garl S,
Varshneya N, Lucariello R and Wiernik PH: Cardiac monitoring of
patients receiving arsenic trioxide therapy. Br J Haematol.
124:610–617. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sinha R, Kim GJ, Nie S and Shin DM:
Nanotechnology in cancer thearpeutics: Bioconjugated nanoparticles
for drug delivery. Mol Cancer Ther. 5:1909–1917. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrari M: Cancer nanotechnology:
opportunities and challenges. Nat Rev Cancer. 5:161–171. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanna V, Pala N and Sechi M: Targeted
therapy using nanotechnology: focus on cancer. Int J Nanomedicine.
9:467–483. 2014.PubMed/NCBI
|
|
12
|
Song L: Preparation Method of
MaterialMaterials Introduction. Zhou DF: Chemical Industry Press;
Beijing: pp. 119–121. 2001
|
|
13
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mazel S, Burtrum D and Petrie HT:
Regulation of cell division cycle progression by bcl-2 expression:
a potential mechanism for inhibition of programmed cell death. J
Exp Med. 183:2219–2226. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Yang L and Qiao Z: An analysis of
the therapeutic effects and reactions in treating acute
promyelocytic leukemia with intravenous arsenic trioxide or
all-trans retinoic acid. Zhonghua Nei Ke Za Zhi. 38:113–115.
1999.(In Chinese). PubMed/NCBI
|
|
16
|
Liu L, Qin SK, Chen HY, Wang J, Chen H, Ma
J and Liu W: An experimental study on arsenic trioxide-selectively
induced human hepatocarcinoma cell lines apoptosis and its related
genes. Zhonghua Gan Zang Bing Za Zhi. 8:367–369. 2000.(In Chinese).
PubMed/NCBI
|
|
17
|
Huang SY, Chang CS, Tang JL, Tien HF, Kuo
TL, Huang SF, Yao YT, Chou WC, Chung CY, Wang CH, et al: Acute and
chronic arsenic poisoning associated with treatment of acute
promyelocytic leukemia. Br J Haematol. 103:1092–1095. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu X, Ma L and Hu N: Ailing No. 1 in
treating 62 cases of acute promyelocytic leukemia. Zhongguo Zhong
Xi Yi Jie He Za Zhi. 19:473–476. 1999.(In Chinese). PubMed/NCBI
|
|
19
|
Wang MD, Shin DM, Simons JW and Nie S:
Nanotechnology for targeted cancer therapy. Expert Rev Anticancer
Ther. 7:833–837. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mathews V, George B, Lakshmi KM,
Viswabandya A, Bajel A, Balasubramanian P, Shaji RV, Srivastava VM,
Srivastava A and Chandy M: Single-agent arsenic trioxide in the
treatment of newly diagnosed acute promyelocytic leukemia: Durable
remissions with minimal toxicity. Blood. 107:2627–2632. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang YQ, Zhang YQ and Hua RQ: Preparation
and properties of LLDPE/ Nano SiO2 composites. Zhong Guo
Su Liao. 17:252003.(In Chinese).
|
|
22
|
Zhao K, Chao YL and Yang Z: Development
and property study of zirconia toughened nano-composite alumina
ceramic powder for dental application. Zhonghua Kou Qiang Yi Xue Za
Zhi. 38:384–386. 2003.(In Chinese). PubMed/NCBI
|
|
23
|
Wang ZY, Zhang DS, Lu XL, Yan SY, Jin LQ
and Wan ML: Research of human liver cancer cell apoptosis induced
by As2O3 nanoparticles. Yi Xue Yan Jiu Sheng
Bao. 18:481–489. 2005.(In Chinese).
|
|
24
|
Wang Q, Wang ZY, Lin M, Zhang J, Zhao SS
and Zhang DS: Application of As2O3
nanoparticles in treatment on human lung cancer in vivo and
investigation of its anti-transfer mechanism. Na Mi Ji Shu He Jing
Mi Gong Cheng. 6:99–102. 2008.(In Chinese).
|