Introduction
Oral lichen planus (OLP) is a common lesion of the
oral mucosa. Its specific pathogenesis is unknown, although it is
considered to involve antigen-specific and antigen-nonspecific
mechanisms. The antigen-specific mechanism involves antigen
presentation by basal keratinocytes and antigen-specific
keratinocyte killing by CD8 cytotoxic cells. In the non-specific
mechanism, mast cell degranulation and matrix metalloproteinase
activation are involved. It has been reported that OLP shows
cancerous characteristics with a malignant transformation rate of
0.4–6.5% (1–3). Due to the abnormal expression of
certain oncogenes or anti-oncogenes in OLP, which serves as an
indication of its risk of progression to cancer, the World Health
Organization (WHO) has classified OLP as ‘potentially malignant
lesions’ (1,4). It has been demonstrated that the
carcinogenesis of OLP involves a combination of factors, which
induce disorders of epithelial cell proliferation and apoptosis,
and abnormalities of oncogenes, anti-oncogenes or certain signaling
pathways, resulting in the malignant development of OLP (5–7). In
recent years, the malignant potential of OLP has been
controversial. Certain researchers consider that OLP does not show
any higher malignancy rate than other types of benign damage of the
oral mucosa, and that the epithelial tissue of OLP is similar to
that of benign lesions but different from potentially malignant or
cancerous oral tissues (8). Three
tumor suppressor gene loci of OLP were found to be significantly
different from those of low-grade dysplasia, high-grade dysplasia
or oral squamous cell carcinoma (OSCC), but not different from
benign fibroma (8). Thus, scientific
assessment would be possible for the malignant risk of OLP if
specific biomarkers were discovered.
E-cadherin, a type-I cadherin, is a transmembrane
glycoprotein that mediates Ca2-dependent homotypic
cell-cell adhesion. E-cadherin is mainly expressed in the
epithelial cells of humans and animals, and is the first cadherin
expressed during mammalian development. E-cadherin is essential for
cell differentiation during embryonic development, and is involved
in tissue and organ development. It is vital to the maintenance of
the polarity of cells and cohesion of tissues, as well as to the
morphology of epithelial cell and tissue integrity. Therefore, the
E-cadherin-mediated junctions between cells play an important role
in tumorigenesis, since impairment of the junction will result in
increased cellular invasion and migration, facilitating the growth
and proliferation of tumor cells at metastatic positions to form
new lesions (9). Studies have shown
that E-cadherin mediates the non-adherent growth of a variety of
cells (10,11). There is also evidence that cleavage
fragments of E-cadherin are involved in cellular junction
destruction, cell migration, invasion and abnormal signal
activation, which indicates a promoting role of E-cadherin in
cancer progression. Studies have found the abnormal expression of
E-cadherin in a variety of malignant tumors of epithelial origin,
such as colorectal carcinoma, gastric carcinoma, esophageal
carcinoma, hepatoma, lung neoplasms, breast carcinoma, prostate
cancer and endometrial malignant tumors (12–14).
This reveals that E-cadherin might have a close association with
the pathological features and biological behavior of tumors.
E-cadherin not only plays an important role in cancer pathogenesis
and metastasis, but is also greatly involved in the development of
precancerous lesions, indicated by the fact that the abnormal
expression of E-cadherin has been observed in oral leukoplakia and
cervical intraepithelial neoplasia (15,16).
Therefore, it is suggested that E-cadherin might be a suitable
indicator for the early detection, diagnosis and prevention of
tumors.
The clinical evaluation of cancerous tissue is
primarily based on the histological appearance of epithelial
dysplasia in pathological sections at present. In recent years, the
application of biomarkers has contributed to objective evaluation
of the stage of OLP, and to the prediction of the potential risk of
malignant transformation (17).
Thus, the improvement of the specificity and reliability of
biomarkers remains the core task in this research field. The
discovery of a reliable biomarker that indicates the transformation
from OLP to OSCC will greatly improve the accurate and scientific
assessment of OLP. E-cadherin expression has been reported in OSCC
(18), but few studies have been
published concerning its expression in OLP. Therefore, whether
E-cadherin participates in cancerous changes to OLP remains to be
investigated.
The aim of the present study was to demonstrate the
expression of E-cadherin in OLP by immunochemistry, and to
elucidate the potential role of E-cadherin in the pathogenesis of
OLP. This may provide theoretical evidence to aid the early
diagnosis and clinical treatment of OLP.
Materials and methods
Patients and samples
In total, 52 OLP specimens were obtained from
patients admitted to the Second Hospital of Hebei Medical
University (Shijiazhuang, China) between 2005 to 2012. This study
was approved by the ethics committee of the Second Hospital of
Hebei Medical University (Shijiazhuang, China). Written informed
consent was obtained from the patients or their families. None of
the patients had received any treatment prior to sampling.
Following fixation in 10% formalin, the tissue specimens were
embedded in paraffin, stained with hematoxylin and eosin (H&E)
and analyzed by a pathologist to obtain a confirmed diagnosis. The
samples were obtained from a study group of 28 males and 24
females, with ages ranging from 38 to 73 years (mean age, 52.6
years). There were 41 normal specimens in the control group,
comprising specimens of oral mucosa tissue harvested from alveolar
bone cyst paraneoplastic plastic surgery that were processed and
analyzed by pathology. There were 22 males and 19 females in the
control group with age from 21 to 68 years (mean, 31.6 years).
Reagents
Rabbit anti-human monoclonal E-cadherin antibody
(BA0474) and biotin-labeled goat anti-rabbit IgG antibody (BA1003)
were acquired from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China). The streptavidin peroxidase (SP) immunohistology kit and
diaminobenzidine (DAB) staining kit were obtained from Hebei
Bio-High Technology Development Co., Ltd. (Shijiazhuang,
China).
Immunohistochemistry
All specimens were fixed in 10% formaldehyde,
embedded in paraffin, cut into three consecutive slices 5 µm in
thickness, and respectively subjected to H&E staining or
E-cadherin immunohistochemistry, or served as a negative
control.
Immunohistochemical SP staining was conducted
according to a published procedure (19), with rabbit anti-human monoclonal
E-cadherin antibody as primary antibody and biotin-labeled goat
anti-rabbit IgG as secondary antibody. The samples of normal oral
mucosa, known to be E-cadherin positive, were taken as the positive
control. A negative control was established using
phosphate-buffered saline (PBS) instead of the primary
antibody.
The immunohistochemical analysis was conducted as
follows: Sections were dewaxed in xylene and rehydrated through
graded concentrations of ethanol. The sections were then incubated
in 3% hydrogen peroxide in methanol for 25 min to block endogenous
peroxidase. Antigen retrieval was performed in a microwave at
92–98°C for 15 min. Sections were incubated with rabbit anti-human
monoclonal E-cadherin antibody (1:200) at 37°C for 30 min, followed
by washing with PBS for three times. Sections were incubated with
biotinylated goat anti-rabbit secondary antibodies (1:300) for 25
min at 37°C and then incubated with peroxidase-conjugated
streptavidin for 20 min at 37°C.
The peroxidase reaction was developed in PBS using
hydrogen peroxide as substrate and DAB as a chromogen. Sections
were counterstained with hematoxylin, dehydrated in graded alcohol,
and evaluated under a light microscope.
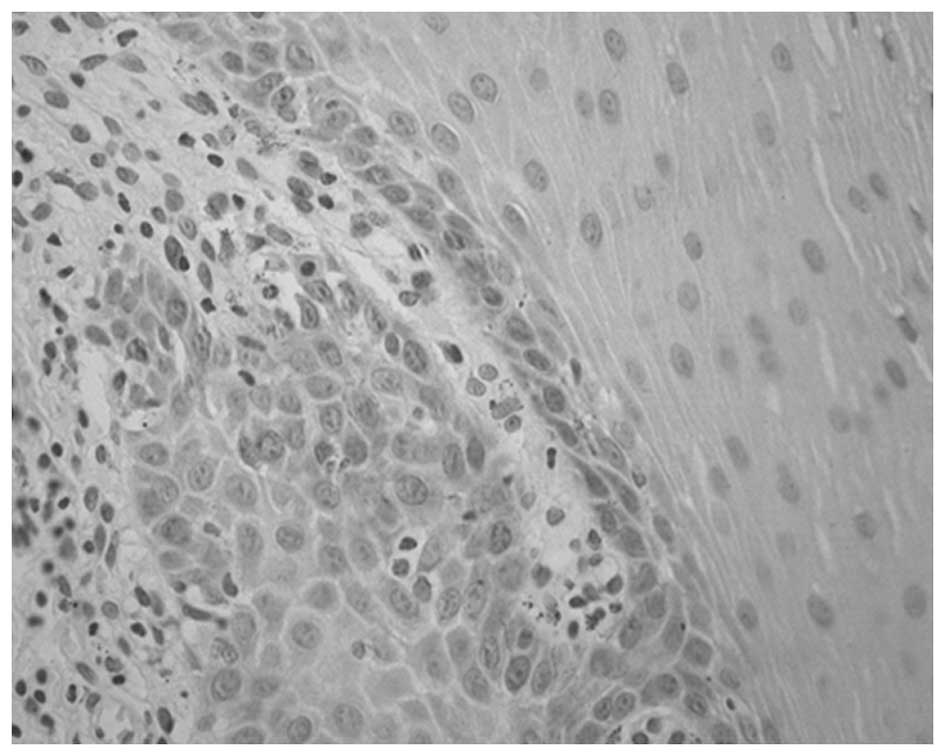
The staining distribution and intensity of
E-cadherin were observed following immunohistological staining. The
normal positive expression of E-cadherin was defined as the
presence of brownish or yellow staining of the cellular membrane,
while abnormal positive expression was defined as the presence of
lines or dots of yellow or brownish granules in the cytoplasm with
or without the same deposits on the cell membrane. The absence of
staining was rated as negative.
Statistical analysis
Data were analyzed using SPSS software, version 17.0
(SPSS, Inc., Chicago, IL, USA). The differences were analyzed by
χ2 test, with P<0.05 considered to indicate a
statistically significant difference.
Results
Expression of E-cadherin in normal
oral mucosa and OLP
Among the 41 specimens of normal oral mucosa, 39
showed normal positive expression and 2 exhibited abnormal positive
expression of E-cadherin, corresponding to an abnormal positive
rate of 4.88%. In the 52 OLP specimens, 25 presented normal
positive expression and 27 showed abnormal positive expression of
E-cadherin, corresponding to an abnormal positive rate of 51.92%.
There was a significant difference in abnormal positive rates
between the two groups (χ2, 23.64; P<0.05; Table I; Figs.
1 and 2).
 | Table I.Expression of E-cadherin in the normal
mucosa and oral lichen planus groups. |
Table I.
Expression of E-cadherin in the normal
mucosa and oral lichen planus groups.
| Tissue type | n | Normal positive | Abnormal
positive | Abnormal positive
rate, % | χ2 | P-value |
|---|
| Normal mucosa | 41 | 39 | 2 | 4.88 | 23.64 | <0.05 |
| Oral lichen
planus | 52 | 25 | 27 | 51.92 |
|
|
Association between the expression of
E-cadherin and clinicopathological parameters
No statistically significant difference was
identified between patients in the OLP group grouped according to
gender or age <50 or ≥50 years with regard to the abnormal
expression of E-cadherin (P>0.05), as shown in Table II.
 | Table II.Association between E-cadherin
expression and clinical characteristics in all patients with oral
lichen planus. |
Table II.
Association between E-cadherin
expression and clinical characteristics in all patients with oral
lichen planus.
| Variable | n | Normal positive | Abnormal
positive | Abnormal positive
rate, % | χ2 | P-value |
|---|
| Gender |
|
|
|
| 0.089 | >0.05 |
| Male | 28 | 14 | 14 | 50.00 |
|
|
|
Female | 24 | 11 | 13 | 54.17 |
|
|
| Age, years |
|
|
|
| 0.930 | >0.05 |
|
<50 | 18 | 7 | 11 | 61.11 |
|
|
| ≥50 | 34 | 18 | 16 | 47.06 |
|
|
Discussion
Oral lichen planus (OLP) is an autoimmune
inflammatory disease of the oral mucosa. The pathogenesis of OLP is
associated with chronic focal injury, drug stimulation, mental
stress, systemic diseases, and bacterial and viral infection
(20). OLP is a chronic disorder of
oral mucosal basal keratinocytes that is associated with a
comprised immune system, caused by exogenous or autologous antigens
and mediated by a cellular immune response. Chronic OLP lesions are
thought to be precancerous with the potential to become malignant.
OLP may transform into oral squamous cell carcinoma (OSCC) via a
series of complex processes. Carcinogenesis results from a
comprehensive network of components, and involves many factors such
as local immunodepression, viral infection, carcinogens and
cytokines. Oncogene activation, chromosomal mutation and
anti-oncogene abnormalities are required to occur before malignant
transformation eventually occurs. A previous study concerning the
mechanisms by which OLP becomes cancerous and the associated genes
has drawn widespread attention (21). Clarification of the mechanism would
be of great importance to the early prevention, diagnosis and
treatment of OLP with malignant potential.
E-cadherin, a calcium-dependent intercellular
adhesion molecule, is a member of the cadherin superfamily. Its
coding gene is located on chromosome 16q22.1, with its N terminus
located outside the cell while its C terminus is inside. E-cadherin
is an important cell adhesion molecule, which is involved in the
regulation of intercellular and cell-matrix adhesion, and the
maintenance of tissue morphology and integrity. E-cadherin-mediated
adhesion plays an important role in the maintenance of the polarity
of epithelial cells and the inhibition of cell migration (22,23). As
a vital tumor suppressor molecule, E-cadherin functions primarily
by enhancement of intercellular adhesion and by inhibition of
cellular proliferation. The abnormal gene expression of E-cadherin
is closely associated with the occurrence, development and
metastasis of tumors, and is strongly associated with the degree of
tumor differentiation, lymph node metastasis, distant metastasis
and recurrence rate, suggesting that the evaluation of abnormal
E-cadherin gene expression may be a vital indicator of tumor
progression and prognosis (24,25).
Studies on the expression of E-cadherin in a variety of tumors have
observed the presence of E-cadherin gene mutations and subsequent
functional changes, in the form of reductions or loss of E-cadherin
expression accompanied by cancer invasion and increased metastasis
(26–28). This indicates that the reduction or
loss of E-cadherin may be a notable change associated with
epithelial-mesenchymal transition. Extracellular signals activate
various transcription factors through diverse signal transduction
regulatory mechanisms, and these can then regulate the expression
of downstream proteins. As the core of this regulatory network,
E-cadherin plays an important role in the progression, invasion and
metastasis of tumors by endowing migrated tumor cells with an
epithelioid phenotype that results in further proliferation to form
a metastatic lesion (29–31). The present study is among the first
to report on whether E-cadherin plays a role in the occurrence,
development and progression of OSCC from OLP.
In the present study, the expression of E-cadherin
was detected in 41 normal oral mucosa specimens and 52 OLP
specimens. The results showed a significant difference between the
two groups, with significantly abnormal expression in the OLP
group. The loss of membranous expression of E-cadherin is involved
in the malignant transformation of OLP. Therefore, we consider that
OLP to be potentially malignant lesion. Oral leukoplakia is a
common precancerous lesion of the oral mucosa, as is OLP.
E-cadherin exhibits abnormal expression in oral leukoplakia
(32), which is comparable with the
abnormal expression in OLP observed in the present study. This
supports the association of E-cadherin with cancerous
potential.
In summary, the abnormal positive expression of
E-cadherin is significantly elevated in OLP patients, which
supports the malignant potential of OLP. The results of the present
study may help to clarify the canceration processes underlying OLP.
However, whether E-cadherin may be used as a determinate
observation index of OLP canceration required verification in
future studies.
References
|
1
|
Georgakopoulou EA, Achtari MD, Achtaris M,
Foukas PG and Kotsinas A: Oral lichen planus as a preneoplastic
inflammatory model. J Biomed Biotechnol. 2012:7596262012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bombeccari GP, Guzzi G, Tettamanti M,
Gianni AB, Baj A, Pallotti F and Spadari F: Oral lichen planus and
malignant transformation: A longitudinal cohort study. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 112:328–334. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen ZY, Liu W, Feng JQ, Zhou HW and Zhou
ZT: Squamous cell carcinoma development in previously diagnosed
oral lichen planus: De novo or transformation? Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 112:592–596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Warnakulasuriya S, Johnson NW and Van der
Waal I: Nomenclature and classification of potentially malignant
disorders of the oral mucosa. J Oral Pathol Med. 36:575–580. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van der Waal I: Potentially malignant
disorders of the oral and oropharyngeal mucosa; terminology,
classification and present concepts of management. Oral Oncol.
45:317–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Safadi RA, Al Jaber SZ, Hammad HM and
Hamasha AA: Oral lichen planus shows higher expressions of tumor
suppressor gene products of p53 and p21 compared to oral mucositis.
An immunohistochemical study. Arch Oral Biol. 55:454–461. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Georgakopoulou EA, Troupis TG, Troupis G
and Gorgoulis VG: Update of the cancer-associated molecular
mechanisms in oral lichen planus, a disease with possible
premalignant nature. J BUON. 16:613–616. 2011.PubMed/NCBI
|
|
8
|
Accurso BT, Warner BM, Knobloch TJ,
Weghorst CM, Shumway BS, Allen CM and Kalmar JR: Allelic imbalance
in oral lichen planus and assessment of its classification as a
premalignant condition. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 112:359–366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andrews JL, Kim AC and Hens JR: The role
and function of cadherins in the mammary gland. Breast Cancer Res.
14:2032012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodriguez FJ, LewisTuffin LJ and
Anastasiadis PZ: E-cadherin's dark side: Possible role in tumor
progression. Biochim Biophys Acta. 1826:23–31. 2012.PubMed/NCBI
|
|
11
|
Kang HG, Jenabi JM, Zhang J, Keshelava N,
Shimada H, May WA, Ng T, Reynolds CP, Triche TJ and Sorensen PH:
E-cadherin cell-cell adhesion in Ewing tumor cells mediates
suppression of anoikis through activation of the ErbB4 tyrosine
kinase. Cancer Res. 67:3094–3105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsalikidis C, Papachristou F, Pitiakoudis
M, Asimakopoulos B, Trypsianis G, Bolanaki E, Syrigos KN and
Simopoulos C: Soluble E-cadherin as a diagnostic and prognostic
marker in gastric carcinoma. Folia Med (Plovdiv). 55:26–32.
2013.PubMed/NCBI
|
|
13
|
Wójcik-Krowiranda K, Forma E, Zaczek A,
Bryś M, Anna MK and Bieńkiewicz A: Expression of E-cadherin and
beta1-integrin mRNA in endometrial cancer. Ginekol Pol. 84:910–914.
2013.(In Polish). View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hashiguchi M, Ueno S, Sakoda M, Iino S,
Hiwatashi K, Minami K, Ando K, Mataki Y, Maemura K, Shinchi H, et
al: Clinical implication of ZEB-1 and E-cadherin expression in
hepatocellular carcinoma (HCC). BMC Cancer. 13:5722013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Auvinen E, Carpen O, Korpela T, Ronty M,
Vaheri A and Tarkkanen J: Altered expression of ezrin, E-cadherin
and β-catenin in cervical neoplasia. Neoplasma. 60:56–61. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kyrodimou M, Andreadis D, Drougou A,
Amanatiadou EP, Angelis L, Barbatis C, Epivatianos A and
Vizirianakis IS: Desmoglein-3/γ-catenin and E-cadherin/β-catenin
differential expression in oral leukoplakia and squamous cell
carcinoma. Clin Oral Investig. 18:199–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Poomsawat S, Buajeeb W, Khovidhunkit SO
and Punyasingh J: Overexpression of cdk4 and p16 in oral lichen
planus supports the concept of premalignancy. J Oral Pathol Med.
40:294–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Supic G, Kozomara R, Jovic N, Zeljic K and
Magic Z: Prognostic significance of tumor-related genes
hypermethylation detected in cancer-free surgical margins of oral
squamous cell carcinomas. Oral Oncol. 47:702–708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soslow RA, Dannenberg AJ, Rush D, Woerner
BM, Khan KN, Masferrer J and Koki AT: Cox-2 is expressed in human
pulmonary, colonic and mammary tumors. Cancer. 89:2637–2645. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Regezi JA, Sciubba JJ and Jordan RCK: Oral
Pathology: Clinical Pathologic Correlations. 6th. Elsevier
Saunders; St. Louis, MO: 2012
|
|
21
|
Sun L, Feng J, Ma L, Liu W and Zhou Z:
CD133 expression in oral lichen planus correlated with the risk for
progression to oral squamous cell carcinoma. Ann Diagn Pathol.
17:486–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sleeman JP and Thiery JP: SnapShot: The
epithelial-mesenchymal transition. Cell. 145:162.e12011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wells A, Chao YL, Grahovac J, Wu Q and
Lauffenburger DA: Epithelial and mesenchymal phenotypic switchings
modulate cell motility in metastasis. Front Biosci (Landmark Ed).
16:815–837. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kolesnik AP, Shevchenko AI, Tumanskiĭ VA
and Evseev AV: Effect of the intercellular adhesion molecule
E-cadherin on the prognosis of non-small cell lung cancer. Arkh
Patol. 75:30–33. 2013.(In Russian). PubMed/NCBI
|
|
25
|
Horne HN, Sherman ME, GarciaClosas M,
Pharoah PD, Blows FM, Yang XR, Hewitt SM, Conway CM, Lissowska J,
Brinton LA, et al: Breast cancer susceptibility risk associations
and heterogeneity by E-cadherin tumor tissue expression. Breast
Cancer Res Treat. 143:181–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sekar P, Bharti JN, Nigam JS, Sharma A and
Soni PB: Evaluation of p53, HoxD10 and E-cadherin status in breast
cancer and correlation with histological grade and other prognostic
factors. J Oncol. 2014:7025272014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deep G, Jain AK, Ramteke A, Ting H,
Vijendra KC, Gangar SC, Agarwal C and Agarwal R: SNAI1 is critical
for the aggressiveness of prostate cancer cells with low
E-cadherin. Mol Cancer. 13:372014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakagawa H, Hikiba Y, Hirata Y,
FontBurgada J, Sakamoto K, Hayakawa Y, Taniguchi K, Umemura A,
Kinoshita H, Sakitani K, et al: Loss of liver E-cadherin induces
sclerosing cholangitis and promotes carcinogenesis. Proc Natl Acad
Sci USA. 111:1090–1095. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: Mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
30
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kalluri R: EMT: When epithelial cells
decide to become mesenchymal-like cells. J Clin Investig.
119:1417–1419. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
von Zeidler SV, de Souza Botelho T,
Mendonça EF and Batista AC: E-cadherin as a potential biomarker of
malignant transformation in oral leukoplakia: A retrospective
cohort study. BMC Cancer. 14:9722014. View Article : Google Scholar : PubMed/NCBI
|