Introduction
Bone fracture is a common injury, which may initiate
a series of biophysiological and pathological reactions. These
reactions may lead to fracture healing, but may additionally result
in tissue damage. A number of promising therapeutic approaches have
been developed, such as improvement of internal fixation devices
and the application of novel biological materials; however, delayed
healing or nonunion may occur in 5–10% of fractures, adding further
to patient morbidity and the expense of treatment (1). The improvement of patient morbidity and
reduction of costs is an incentive for the development of novel
therapies to enhance fracture healing (2).
Autophagy is the process of cellular
‘self-digestion’, and serves an essential role in energy and
nutrient regulation, in addition to the removal of damaged and
dysfunctional macromolecules and organelles (3,4). At the
cellular level, failure of autophagy results in the increased
expression of abnormal genes, and may lead to cell death (5). Potential consequences of autophagy
failure at the tissue and organismal level include
neurodegeneration, cardiomyopathies, abnormal skeletal development
and premature mortality (6–8). Microtubule-associated protein 2 light
chain 3 (LC3-II) is considered to be a primary marker of autophagy
(9).
The mammalian target of rapamycin (mTOR) complex is
a crucial suppressor of autophagy, functioning upstream of the
autophagy-related proteins, and is centrally regulated by multiple
upstream signaling pathways involving phosphatidylinositol-3-kinase
(PI-3K)/Akt and adenosine monophosphate-activated protein kinase
(10). Furthermore, imbalances in
the mTOR pathway are involved in cardiac hypertrophy, inflammatory
diseases, and diabetes, and pharmacological intervention of mTOR
has been proposed as a potential treatment for these conditions
(11). Rapamycin is a lipophilic
macrolide antibiotic that is used as an immunosuppressive drug in
solid organ transplantation, and is able to induce autophagy by
inhibiting ribosomal protein S6 (rpS6), a downstream target of mTOR
complex 1 (mTORC1) phosphorylation (12,13). In
addition, rapamycin treatment has been demonstrated to extend
lifespan in mice (14), and protects
against aging-associated pathologies of the brain and heart
(15–18). However, to the best of our knowledge
no prior studies have investigated the effects of autophagy on bone
fracture healing following an intervention affecting the mTOR
pathway.
Bone fractures may impair cellular homeostasis and
induce significant stress in bone cells, leading to the activation
of the autophagy pathway. Therefore, the aim of the present study
was to investigate the effects of the pharmacological enhancement
of autophagy on the process of experimental fracture healing in
rats.
Materials and methods
The study was performed in accordance with protocols
of the local governmental animal care committee and the
Institutional Animal Care and Use Committee at Xiamen University
(Zhangzhou, China). Every effort was made to minimize animal
suffering and to reduce the number of animals used.
Experimental groups and surgical
procedure
A total of 126 adult male Sprague-Dawley rats
weighing 250–270 g were obtained from Xiamen University. A total of
63 rats in the rapamycin group received a daily intraperitoneal
injection of rapamycin (1 mg/kg body weight/dose) from the day of
fracture until they were sacrificed at 2, 4 or 6 weeks after
fracture. Subsequently, the degree of fracture healing was analyzed
using radiological (n=15 each at 2, 4 and 6 weeks), hematoxylin and
eosin (H&E) staining (n=12), western blot analysis (n=12),
immunohistochemistry (n=12) and immunofluorescence (n=12) methods.
Rats in the vehicle-treated control group (n=63) received 0.4%
dimethyl sulfoxide (DMSO) in a total injection volume of 0.3 ml.
Among these animals, fracture healing was analyzed using
radiological (n=15 each at 2, 4 and 6 weeks), H&E staining
(n=12 each), western blot analysis (n=12 each),
immunohistochemistry (n=12 each) and immunofluorescence (n=12 each)
methods. For surgery, the rats were anesthetized by intraperitoneal
injection of ketamine (75 mg/kg) and xylazine (25 mg/kg), which was
provided by the Affiliated Southeast Hospital of Xiamen University.
The right femur of each animal was exposed and a wire saw was used
to make a middle transverse fracture, which was stabilized using a
1.0-mm diameter Kirschner wire as described previously (19). The resulting fracture was of type
A3.2, according to the Müller AO classification of fractures
(20). X-ray imaging (Multix TOP;
Siemens, Forchheim, Germany) was used to document the positions of
the implants.
X-ray radiography and micro-computed
tomography (microCT) imaging
Fractured limbs were observed at 2, 4 and 6 weeks by
performing posteroanterior X-ray radiography to record callus
formation. Subsequently, fractured limbs were dissected free of
soft tissues, and the intramedullary Kirschner wires were extracted
to facilitate scanning using a µCT 40 micro-CT device (Scanco
Medical AG, Brüttisellen, Switzerland) at 2,800 views, 5 frames per
view, 35 kV and 35 µA. Three-dimensional (3D) images were rendered,
and the areas of the transverse section and of void spots in each
transverse section image were evaluated using VGStudio MAX software
(Dürr, Bietigheim-Bissingen, Germany). The fraction of mineralized
callus was quantified by calculating the total void area (which
indicates the degree of residual non-mineralized tissue) as a
percentage of the total area of the transverse section image.
Histomorphometric analyses
Fractured limbs were harvested and fixed in 4%
formalin for 24 h, decalcified in 10% ethylenediaminetetraacetic
acid solution for 1 month, and embedded in paraffin for
histological analysis. Decalcified longitudinal sections were cut
to 4–5 µm, stained with H&E and the total number of osteoblasts
in each callus section was counted.
Immunohistochemistry
Sections from paraffin-embedded samples were
deparaffinized using the xylene substitute Pro-Par Clearant
(Anatech, Ltd., Battle Creek, MI, USA) and rehydrated in graded
ethanol and water. For antigen unmasking, sections in 10 mM sodium
citrate buffer (pH 6.0) were heated in a microwave oven at 80–85°C
for 2 min. Slides were then cooled at room temperature for 30 min.
After washing with phosphate-buffered saline (PBS), sections were
blocked with 5% serum for 30 min at room temperature. Polyclonal
anti-phospho-rpS6 primary antibody (1:100; Abcam, Cambridge, UK)
was applied and the sections were incubated overnight at 4°C. After
washing with PBS, sections were incubated with polyclonal
biotinylated goat anti-rabbit secondary antibody (1:100; Abcam) for
1 h at 37°C and incubated with Vectastain ABC-alkaline phosphatase
(AP) (Vector Laboratories, Inc., Burlingame, CA, USA) for 30 min.
Slides were washed 3 times with PBS, and sections were incubated
with AP substrate for 30 min.
Immunofluorescence
Paraffin-embedded samples were deparaffinized using
Pro-Par Clearant and rehydrated in graded ethanol and water. After
washing with PBS, sections were blocked with 5% serum for 30 min at
room temperature, then incubated with rabbit anti-LC3-II polyclonal
antibody (1:150; Abcam) overnight at 4°C. After washing with PBS,
sections were incubated with polyclonal Alexa Fluor 488 anti-rabbit
IgG secondary antibody (1:200; Abcam) for 1 h. Finally, slides were
washed and mounted with ProLong Gold Antifade Reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA).
Western blot analysis
Rats were anesthetized and the soft tissue covering
the diaphyseal part of the femora was removed prior to sacrifice.
The visible callus was resected and frozen in nitrogen. For
extraction of the whole-protein fraction, tissue samples were
homogenized in 250 µl radioimmunoprecipitation assay buffer,
containing 150 mM NaCl, 1% NP-40, 25 mM Tris-HCl (pH 7.6), 0.1%
sodium dodecyl sulfate polyacrylamide (SDS), 1% sodium deoxycholate
and a protease inhibitor cocktail (Abcam). Next, the samples were
incubated for 30 min on ice and centrifuged for 30 min at 16,000 ×
g. The supernatant was saved as a whole-protein fraction, and the
protein concentration was by Lowry assay. Subsequently, 60 mg
protein per lane was separated on 10% SDS gel and transferred to a
polyvinyldifluoride membrane (Thermo Scientific, Waltham, MA, USA).
After blocking with 5% skimmed milk solution for 1 h, membranes
were incubated for 2 h with 0.5 µg/ml rat monoclonal anti-VEGF
(Abcam) or 0.5 µg/ml anti-PCNA (Abcam) antibodies. Antibody-protein
complexes were visualized using chemiluminescence (ProLong Gold
Antifade Reagent) and photographs of the specific protein bands
were captured with a Fusion FX7 imaging system (Vilber Lourmat
Deutschland GmbH, Eberhardzell, Germany). Quantity One software
(Bio-Rad Laboratories GmbH, Munich, Germany) was used for the
analysis and quantification of the images. Glyceraldehyde
3-phosphate dehydrogenase was used as an internal control.
Statistical analysis
Results are presented as the mean ± standard
deviation. Comparisons between two groups were conducted with
t-tests. Statistical analysis was performed using SPSS version 20.0
(IBM, Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
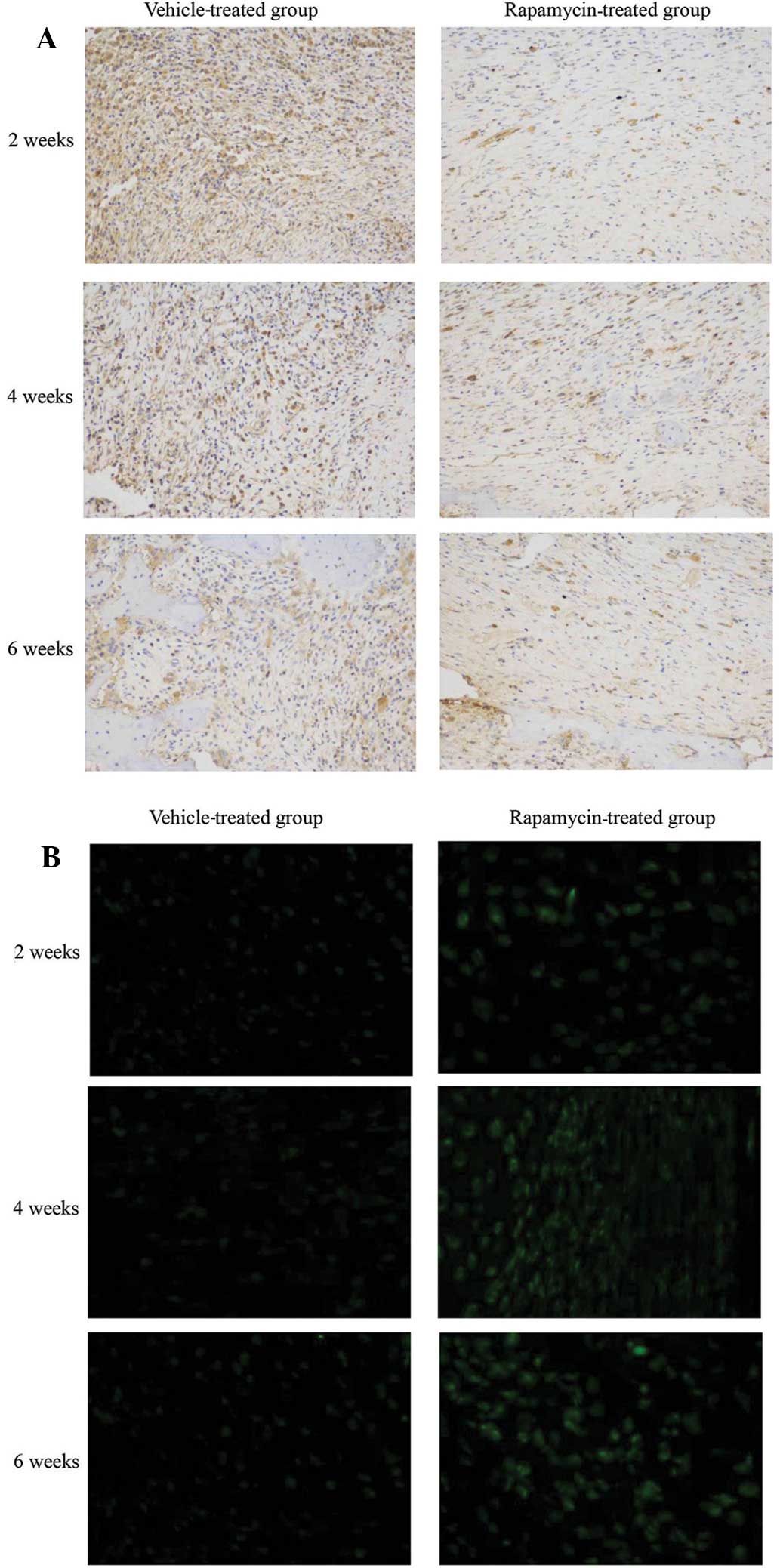
Systemic administration of rapamycin
modulates mTOR signaling and autophagy in a rat fracture model
The phosphorylation levels of rpS6, a downstream
target of mTORC1 (21), were
determined to evaluate the effect of rapamycin on the mTOR
signaling pathway in a rat femur fracture model. Rapamycin
treatment suppressed rpS6 phosphorylation in bone tissue cells in
the callus compared with that in vehicle-treated rats (Fig. 1A). In order to determine whether
autophagy is promoted as a result of mTOR inhibition by rapamycin,
bone tissue sections were stained with LC3-II antibody. An increase
in LC3-II expression was detected following rapamycin treatment.
This increase correlated with an increase in LC3-II puncta,
indicating a marked activation of autophagy in the bone tissue
(Fig. 1B). These results indicate
that mTOR signaling and autophagy were induced in bone tissue by
the intraperitoneal administration of rapamycin.
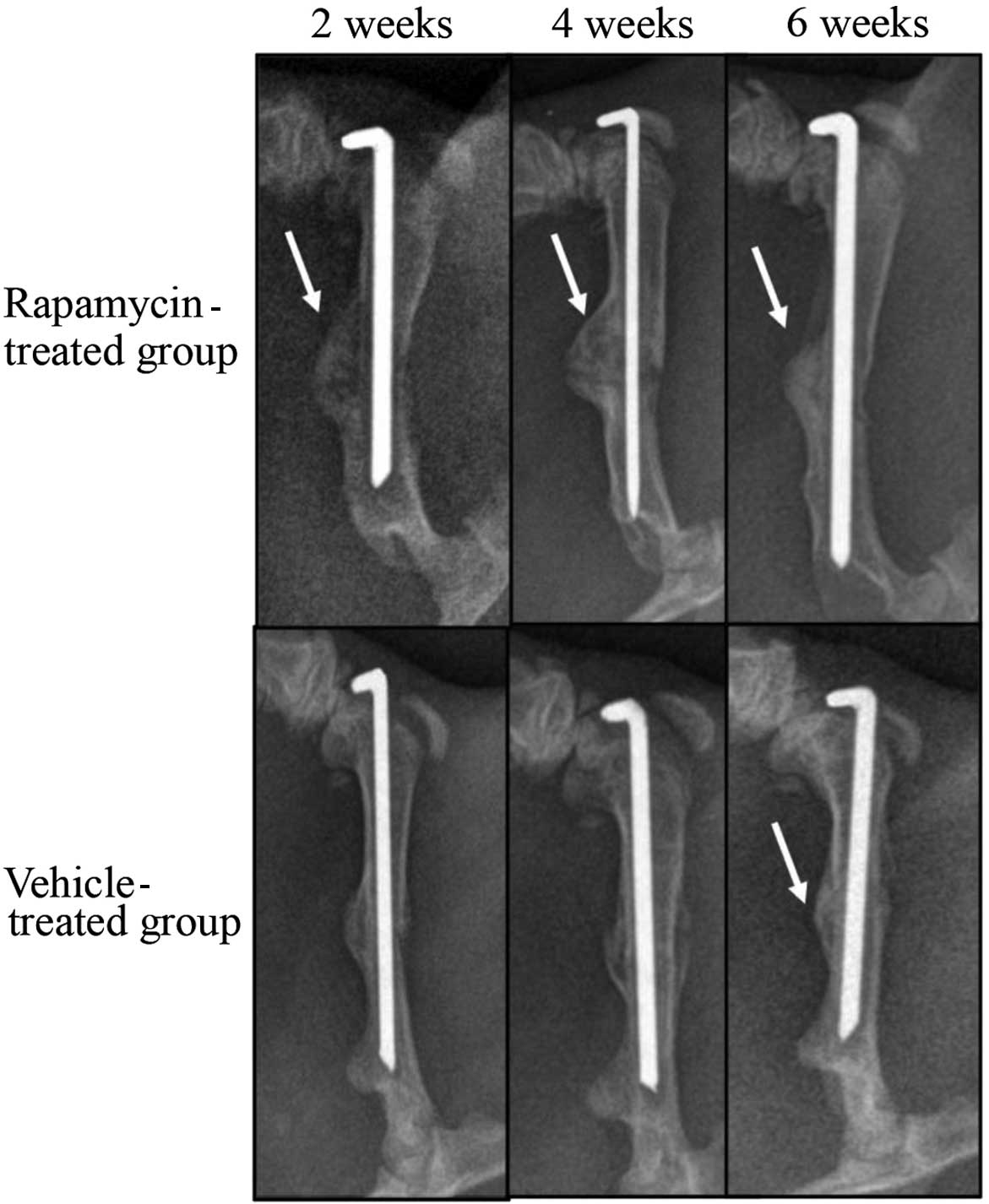
Rapamycin promotes callus formation
and remodeling
To investigate the role of mTOR in fracture repair,
radiographic analyses of the healing bones were conducted at 2, 4
and 6 weeks after fracture (Fig. 2).
The calluses from the rats in the vehicle-treated group were not
mineralized at 2 weeks after fracture. At 4 weeks, the calluses in
this group exhibited a higher mineral density. At 6 weeks
post-fracture, calluses from the vehicle-treated group appeared to
have been resorbed, as indicated by their reduced size compared
with that at week 4. In contrast with the vehicle-treated group,
fracture calluses from the rapamycin-treated group were mineralized
at 2 weeks post-fracture. By week 4, the calluses from the
rapamycin group were increased in size compared with those from the
vehicle-treated group. The calluses from the rapamycin group
closely resembled those from the vehicle-treated group at 6 weeks,
with resorption of the calluses; however, the mineral density was
increased in the rapamycin group compared with that in the
vehicle-treated group.
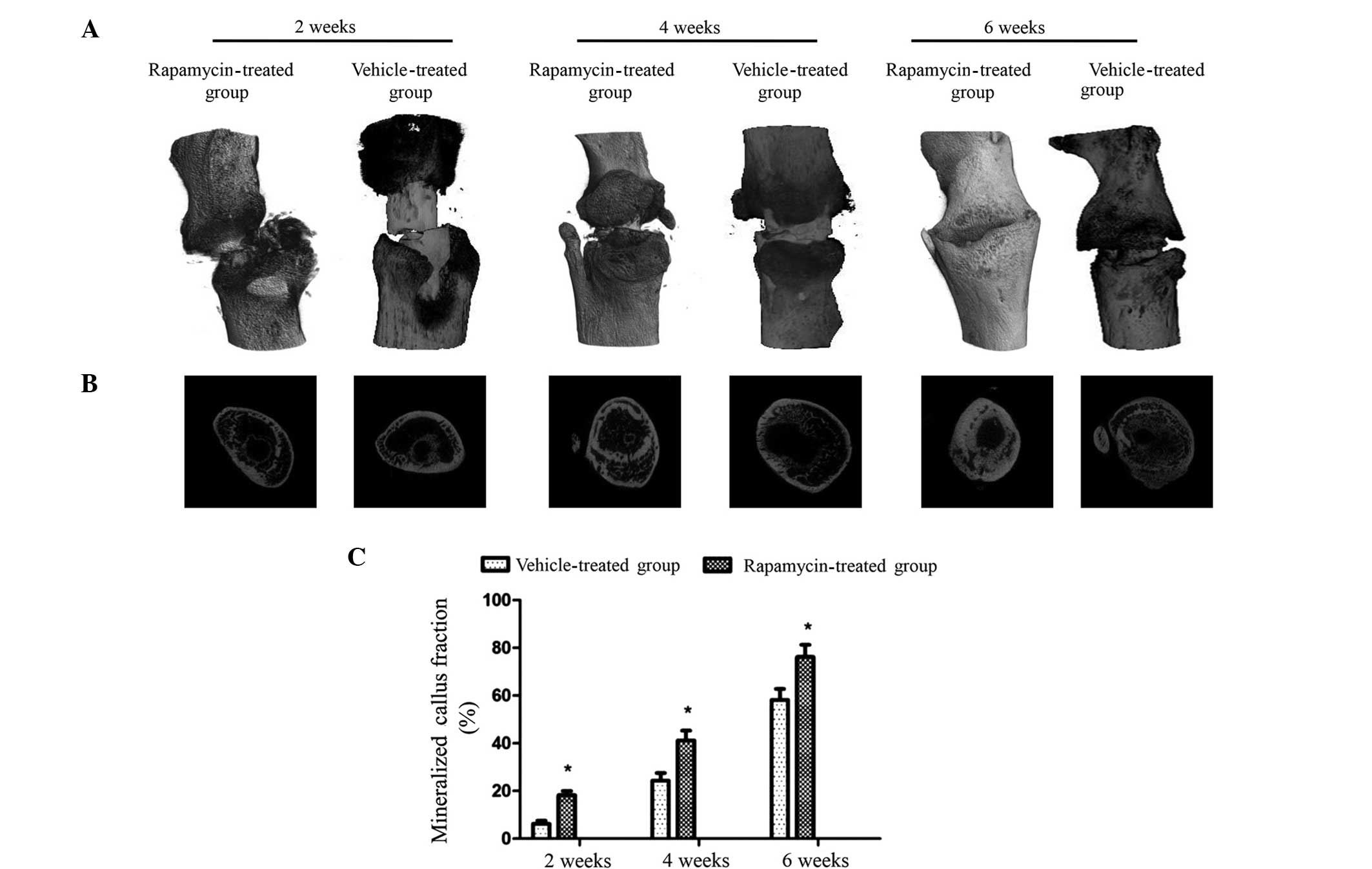
Micro-CT imaging was used to remodel the fracture
calluses and to quantify the degree of mineralization at 2, 4 and 6
weeks post-fracture (Fig. 3A). 3D
representations of the fracture showed that at 2, 4 and 6 weeks
post-fracture, the mineralized fracture calluses in the rapamycin
group were larger than those in the vehicle-treated group.
Transverse sections demonstrated that the rapamycin group exhibited
mineralized fracture calluses with a higher degree of
mineralization compared with those in the vehicle-treated group at
all three time points (Fig. 3B).
Quantitative analysis, which involved determining the total
percentage area of void regions in the transverse section image
indicated that the fraction of mineralized callus in the rapamycin
group rats was increased with time by 18.3±1.6, 41.2±4.1 and
76.2±5.1% at 2, 4 and 6 weeks post-fracture, respectively. These
values are significantly increased compared with those in the
vehicle-treated group at each time point (Fig. 3C). These data indicate that treatment
with rapamycin is able to enhance callus mineralization and promote
callus formation and remodeling in this rat fracture model.
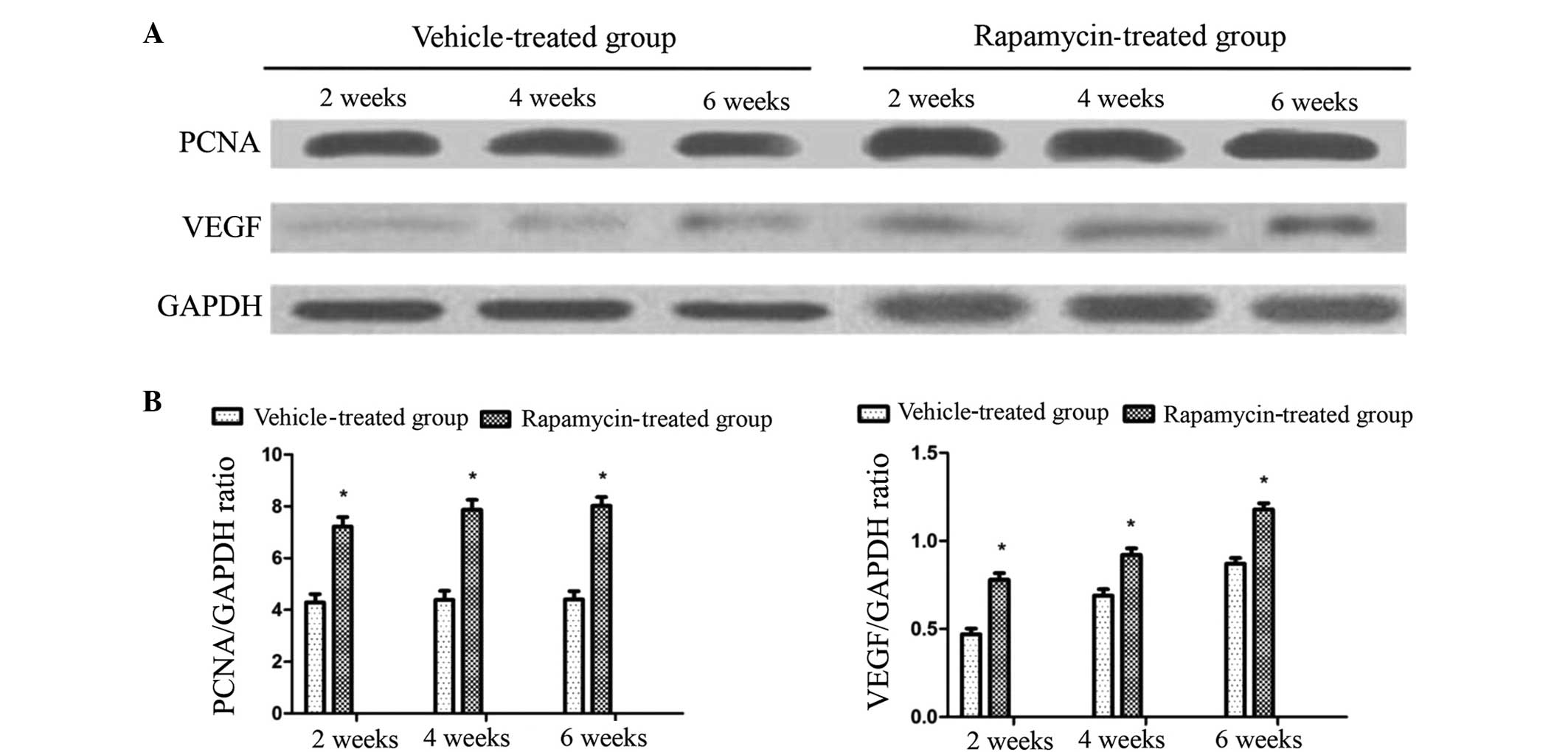
Rapamycin increases the number of
osteoblasts in the rat callus, and the expression levels of PCNA
and VEGF in cells
To investigate the mechanism of action of rapamycin
in the rats, a total of four sections were observed for each group
at each time point to analyze the osteoblast cell density in the
callus. It was observed that osteoblast formation occurred at a
greater extent in the rats of the rapamycin group at the three time
points post-fracture (Fig. 4A), and
the difference in osteoblast number between the two groups was
statistically significant (P<0.01; Fig. 4B).
Western blot analysis was performed to determine the
expression levels of PCNA and VEGF. PCNA is a crucial protein for
the proliferation of osteoblasts, and VEGF is known to be a crucial
molecule in the promotion of angiogenesis during fracture healing.
Increased expression levels of PCNA and VEGF were detected in the
rapamycin group rats compared with the vehicle-treated rats at each
time point. This increase was statistically significant (P<0.05;
Fig. 5). This finding indicates that
rapamycin contributes to osteoblast proliferation by promoting PCNA
and callus blood supply through the promotion of VEGF
expression.
Discussion
There is evidence to suggest that effective
autophagy exists within a narrow homeostatic range to regulate
protein homeostasis and cell survival (22). To a certain extent, autophagy affects
bone formation or loss (23,24). However, to the best of our knowledge
there are no previous studies regarding the effects of autophagy on
bone repair. On this basis, the aim of the present study was to
investigate whether the activation of autophagy affects early bone
fracture healing. Rapamycin was selected as an inhibitor of the
mTOR signaling pathway, which regulates the initiation of
autophagy. Rapamycin is a lipophilic macrolide antibiotic that is
used as an immunosuppressive drug and to induce autophagy in a
variety of cell types (12–18).
The results of the present study suggest that the
systemic administration of rapamycin inhibits the mTOR signaling
pathway in the rat callus, as indicated by the reduced
phosphorylation of rpS6, which integrates the processes of protein
translation with cell growth and proliferation (25). Treatment of cells with rapamycin
blocks S6 kinase 1 (S6K1) phosphorylation, and inhibits the
activation of S6K1 (26).
Furthermore, rapamycin-activated autophagy was indicated by
increased expression levels of LC3-II, the most specific
autophagosomal marker in the rat callus. These results confirm that
the systemic administration of rapamycin modulates mTOR signaling
and autophagy in a rat fracture model.
In the present study, treatment with rapamycin was
observed to induce a significant improvement in callus formation
and mineralization. In fracture healing, the fraction of
mineralized callus is an important parameter, which indicates the
presence of newly formed bone and the effect of bone formation or
remodeling. Surface calluses formed more rapidly in the rapamycin
group than in the vehicle-treated group. Furthermore,
mineralization in the fracture calluses of the rapamycin group was
significantly increased compared with that in the vehicle-treated
group at 2, 4 and 6 weeks post-fracture.
On the basis of the established efficacy of
rapamycin in the promotion of callus formation, the expression of
VEGF was investigated, as a key growth factor for the promotion of
endochondral ossification during secondary fracture healing. This
stage of fracture healing comprises: i) Osteocyte survival and cell
death; ii) degradation and calcification of the extracellular
matrix; and iii) the formation of new blood vessels and new bone
tissue (27). VEGF is considered to
exert a direct effect on osteoprogenitor cells by promoting
osteoblast differentiation and increasing the mineralization of
regenerated bone (28). Therefore,
the positive effect of rapamycin on fracture repair may be due to
the activation of VEGF expression in callus tissue, as suggested by
western blot analysis. The increased expression of VEGF may be
associated with altered cell proliferation, as indicated by the
elevated PCNA levels in the calluses of the rapamycin group. These
results are consistent with a previous study in which rapamycin
increased the cellular levels of PCNA (29).
Histological analyses showed that the number of
osteoblasts in the callus at different time points differed
significantly between the rapamycin and vehicle-treated group rats.
This difference may be associated with the increased levels of
PCNA; thus, it was hypothesized that the observed effects on
fracture healing, VEGF and PCNA expression, and osteoblast activity
were a result of the inhibition of mTOR by rapamycin. Rapamycin is
a specific inhibitor of mTOR, particularly for the mTORC1 protein,
and there is no evidence that it exerts off-target effects on
enzymes other than the mTOR kinase (30,31).
mTOR inhibition is known to induce autophagy; however, other
signaling pathways are affected directly or indirectly by mTOR
inhibition, such as the PI-3K/Akt signaling pathway (32). With regard to the role of autophagy
activation in the effects observed in the present study, it is
possible that rapamycin restores the suppressed autophagy, which a
previous study had observed in cartilage and osteoarthritis
(33), and as result, inhibits cell
death. The induction of cell death is a well-known effect of
defective autophagy (34).
Therefore, it appears that the preservation of osteoblasts in
calluses and the promotion of fracture reparation observed in the
present study are attributable to the activation of autophagy.
The promotion of VEGF and PCNA expression in the
rapamycin-treated rats may be associated with the increased levels
of autophagy and mTORC1 inhibition. Previous studies have observed
that decreased levels of autophagy and mTORC1 activation are
associated with aging-related bone loss and other pathologies
(35,36). Conversely, increased levels of
autophagy and a reduction in mTORC1 expression may lead to an
extension of lifespan (37,38). However, the increased VEGF and PCNA
expression levels observed in the rapamycin-treated rats in the
present study are inconsistent with those of a previous study by
Holstein et al (39). In this
study, Holstein et al observed that rapamycin initially
delayed fracture healing and reduced VEGF and PCNA expression. It
is plausible that this discrepancy is a result of the different
doses of rapamycin used in the two studies, or the different
animals. Further studies may be required to investigate the effect
of various concentrations of rapamycin on bone healing.
There are a number of limitations to the present
study. For example, the effect of rapamycin-induced autophagy on
the formation of osteoclasts and osteocytes was not investigated,
and the results of animal studies do not always correlate with
results in humans. However, the most notable limitation of the
present study is that rapamycin is a therapeutic immunosuppressant
drug, which is used widely to treat cancer and reduce organ
transplant rejection. The clinical feasibility of the application
of mTOR inhibitors to the treatment of fractures may be enhanced by
recent advances in the development of novel rapamycin analogs that
exhibit improved safety. Rapamycin analogs that are more
specifically targeted may activate autophagy with fewer
side-effects (40).
The effects of rapamycin on fracture healing have
been well documented by Holstein et al (41); however, these authors did not
investigate the phosphorylation of rpS6 and the activation of
LC3-II. To the best of our knowledge, the present study is the
first to investigate the therapeutic benefits of rapamycin-induced
autophagy in an animal model of fracture. Adult rats were subjected
to surgical femur fracture stabilization, which is a widely used
model. For further preclinical development of autophagy activators,
future studies may be required to investigate the effects of
autophagy on fracture healing in older animals, in order to more
realistically model the human condition.
In summary, rapamycin was used to induce the
activation of autophagy in rats and the efficacy of this
intervention on fracture healing was investigated. These results
indicate that the pharmacological inhibition of mTOR and the
induction of autophagy by rapamycin may be an effective therapeutic
approach for the promotion of fracture healing.
References
|
1
|
Einhorn T: Enhancement of
fracture-healing. J Bone Joint Surg Am. 77:940–956. 1995.PubMed/NCBI
|
|
2
|
O'Neill KR, Stutz CM, Mignemi NA, et al:
Micro-computed tomography assessment of the progression of fracture
healing in mice. Bone. 50:1357–1367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mathew R, Karp CM, Beaudoin B, et al:
Autophagy suppresses tumorigenesis through elimination of p62.
Cell. 137:1062–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hara T, Nakamura K, Matsui M, et al:
Suppression of basal autophagy in neural cells causes
neurodegenerative disease in mice. Nature. 441:885–889. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Komatsu M, Waguri S, Ueno T, et al:
Impairment of starvation-induced and constitutive autophagy in
Atg7-deficient mice. J Cell Biol. 169:425–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shibata M, Lu T, Furuya T, et al:
Regulation of intracellular accumulation of mutant Huntingtin by
Beclin 1. J Biol Chem. 281:14474–14485. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su JC, Tseng PH, Hsu CY, et al:
RFX1-dependent activation of SHP-1 induces autophagy by a novel
obatoclax derivative in hepatocellular carcinoma cells. Oncotarget.
5:4909–4919. 2014.PubMed/NCBI
|
|
10
|
Wullschleger S, Loewith R and Hall MN: TOR
signaling in growth and metabolism. Cell. 124:471–484. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dann SG, Selvaraj A and Thomas G: mTOR
Complex1-S6K1 signaling: At the crossroads of obesity, diabetes and
cancer. Trends Mol Med. 13:252–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shigemitsu K, Tsujishita Y, Hara K,
Nanahoshi M, Avruch J and Yonezawa K: Regulation of translational
effectors by amino acid and mammalian target of rapamycin signaling
pathways. Possible involvement of autophagy in cultured hepatoma
cells. J Biol Chem. 274:1058–1065. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sabers CJ, Martin MM, Brunn GJ, et al:
Isolation of a protein target of the FKBP12-rapamycin complex in
mammalian cells. J Biol Chem. 270:815–822. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sarkar S and Rubinsztein DC: Huntington's
disease: Degradation of mutant huntingtin by autophagy. FEBS J.
275:4263–4270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harrison DE, Strong R, Sharp ZD, et al:
Rapamycin fed late in life extends lifespan in genetically
heterogeneous mice. Nature. 460:392–395. 2009.PubMed/NCBI
|
|
16
|
Pan T, Rawal P, Wu Y, Xie W, Jankovic J
and Le W: Rapamycin protects against rotenone-induced apoptosis
through autophagy induction. Neuroscience. 164:541–551. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spilman P, Podlutskaya N, Hart MJ, et al:
Inhibition of mTOR by rapamycin abolishes cognitive deficits and
reduces amyloid-beta levels in a mouse model of Alzheimer's
disease. PLoS One. 5:e99792010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inuzuka Y, Okuda J, Kawashima T, et al:
Suppression of phosphoinositide 3-kinase prevents cardiac aging in
mice. Circulation. 120:1695–1703. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Holstein JH, Menger MD, Culemann U, Meier
C and Pohlemann T: Development of a locking femur nail for mice. J
Biomech. 40:215–219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Müller ME, Nazarian S, Koch P and
Schatzker J: The Comprehensive Classification of Fractures of Long
Bones. 1st edition. Springer; New York, NY: 1994
|
|
21
|
Ekim B, Magnuson B, AcostaJaquez HA,
Keller JA, Feener EP and Fingar DC: mTOR kinase domain
phosphorylation promotes mTORC1 signaling, cell growth and cell
cycle progression. Mol Cell Biol. 31:2787–2801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moscat J and Diaz-Meco MT: p62 at the
crossroads of autophagy, apoptosis and cancer. Cell. 137:1001–1004.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin NY, Stefanica A and Distler JH:
Autophagy: A key pathway of TNF-induced inflammatory bone loss.
Autophagy. 9:1253–1255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang L, Guo YF, Liu YZ, et al:
Pathway-based genome-wide association analysis identified the
importance of regulation-of-autophagy pathway for ultradistal
radius BMD. J Bone Miner Res. 25:1572–1580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
From growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guertin DA and Sabatini DM: The
pharmacology of mTOR inhibition. Sci Signal. 2:pe242009.PubMed/NCBI
|
|
27
|
Yu X, Guo Y, Kang Q and Luo C: Effects and
mechanisms of mechanical stress on secondary fracture healing.
Front Biosci (Landmark Ed). 18:1344–1348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Keramaris NC, Calori GM, Nikolaou VS,
Schemitsch EH and Giannoudis PV: Fracture vascularity and bone
healing: A systematic review of the role of VEGF. Injury. 39 (Suppl
2):S45–S57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morrow PW, Tung HY and Hemmings HC Jr:
Rapamycin causes activation of protein phosphatase-2A1 and nuclear
translocation of PCNA in CD4+ T cells. Biochem Biophys
Res Commun. 323:645–651. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kobayashi S, Kishimoto T, Kamata S, Otsuka
M, Miyazaki M and Ishikura H: Rapamycin, a specific inhibitor of
the mammalian target of rapamycin, suppresses lymphangiogenesis and
lymphatic metastasis. Cancer Sci. 98:726–733. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kawahara T, Asthana S and Kneteman NM:
m-TOR inhibitors: What role in liver transplantation? J Hepatol.
55:1441–1451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cirstea D, Hideshima T, Rodig S, et al:
Dual inhibition of Akt/mammalian target of rapamycin pathway by
nanoparticle albumin-bound-rapamycin and perifosine induces
antitumor activity in multiple myeloma. Mol Cancer Ther. 9:963–975.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Caramés B, Taniguchi N, Otsuki S, Blanco
FJ and Lotz M: Autophagy is a protective mechanism in normal
cartilage and its aging-related loss is linked with cell death and
osteoarthritis. Arthritis Rheum. 62:791–801. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wong E and Cuervo AM: Autophagy gone awry
in neurodegenerative diseases. Nat Neurosci. 13:805–811. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen K, Yang YH, Jiang SD and Jiang LS:
Decreased activity of osteocyte autophagy with aging may contribute
to the bone loss in senile population. Histochem Cell Biol.
142:285–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stanfel MN, Shamieh LS, Kaeberlein M and
Kennedy BK: The TOR pathway comes of age. Biochim Biophys Acta.
1790:1067–1074. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sciarretta S, Volpe M and Sadoshima J:
Mammalian target of rapamycin signaling in cardiac physiology and
disease. Circ Res. 114:549–564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mirzaei H and Longo VD: Acetyl-CoA
synthetase is a conserved regulator of autophagy and life span.
Cell Metab. 19:555–557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Holstein JH, Klein M, Garcia P, et al:
Rapamycin affects early fracture healing in mice. Br J Pharmacol.
154:1055–1062. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wagner R, Mollison KW, Liu L, et al:
Rapamycin analogs with reduced systemic exposure. Bioorg Med Chem
Lett. 15:5340–5343. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Holstein JH, Klein M, Garcia P, Histing T,
Culemann U, Pizanis A, Laschke MW, Scheuer C, Meier C, Schorr H, et
al: Rapamycin affects early fracture healing in mice. Br J
Pharmacol. 154:1055–1062. 2008. View Article : Google Scholar : PubMed/NCBI
|