Introduction
Collagen-induced arthritis (CIA) was first described
by Trentham et al (1) in both
outbred and inbred rat strains following immunization with type II
collagen (CII), the major constituent protein of articular
cartilage. CIA, an experimental model of autoimmune arthritis, has
been shown to exhibit similar histological, immunological and
clinical characteristics and genetic linkage to human rheumatoid
arthritis (RA) (1,2). CIA has therefore been widely used in
laboratories worldwide in studies focusing on the pathogenesis of
RA and the preclinical evaluation of novel therapeutics for the
disease (2,3).
The immune response to CII and subsequent
development of arthritis in rats is linked to the RT1 locus of the
rat major histocompatibility complex (4). The arthritogenic potency of CII from
different species used for immunization varies; in general, porcine
CII is the most potent, followed by bovine and chicken CII
(2). As described in numerous
studies from Eastern and Southern Asia, CIA is induced in rats
predominantly by immunizing the animals with either chicken or
bovine CII emulsified in complete Freund's adjuvant (CFA)
containing heat-killed Bacillus Calmette-Guérin in liquid paraffin
(5–8). The injection is administered in either
the subplantar tissues of one of the hind paws (5), at the base of the tail (7,8) or at
both the base of the tail and the back (6). These immunization protocols, however,
are associated with several problems, one of which is that CFA
alone can induce adjuvant arthritis (AIA) in rats (9,10). The
use of CFA for the preparation of CII emulsion could therefore
produce an immune response to antigens other than CII in the
immunized rats. In addition, the injection of the rat footpads with
a high concentration of the CII/CFA emulsion puts the injected paws
at risk of a severe infection from bacteria contained in the CFA,
which could eventually result in the failure of the experiments.
Furthermore, intradermal injection at multiple locations at once,
such as the base of the tail and the back of the rat, is not only
time-consuming and inconvenient to perform, but also requires
larger amounts of CII emulsion (0.5–1 ml) for the induction of
arthritis.
The aim of the present study was to determine a more
simple, specific and efficient method for the induction of CIA in
rats. Incomplete Freund's adjuvant (IFA) was used instead of CFA to
emulsify bovine CII, and the CII/IFA emulsion was intradermally
injected at the base of the tail, with a booster injection
administered on day 7 after primary immunization. Using this
immunization method, different strains of rats of both genders were
compared according to the susceptibility they exhibited to CIA.
Finally, the most successfully established CIA model was
characterized in terms of clinical, hematological,
histopathological and radiological features of human RA, in order
to provide scientific evidence for the reliability of the model and
for its specific use in studies on the etiopathogenetic mechanisms
of RA and for the development of novel anti-arthritic drugs.
Materials and methods
Preparation of collagen emulsion
A CII/IFA emulsion (1 mg/ml) was freshly prepared as
previously described (2,11). Briefly, an equal volume of 2 mg/ml
bovine CII in 0.05 M acetic acid and IFA (Chondrex, Inc., Redmond,
WA, USA) was thoroughly mixed in an ice water bath, using a
homogenizer, to produce a stable emulsion that would remain as a
solid clump and not dissipate in the water.
Development of CIA
Specific pathogen-free outbred male and female
Wistar, Wistar Furth and Sprague Dawley (SD) rats (Vital River
Laboratory Animal Technology Co., Ltd., Beijing, China), aged 6–7
weeks, were used. The rats were housed 4 per cage in rooms
maintained at 20±0.5°C with a 12-h light/dark cycle. The animals
had free access to food and water and were acclimated to their
surroundings for 1 week prior to the initiation of the
experiments.
The CIA model was induced in the rats as previously
described (11,12). CIA was induced in 6 rats per group to
define the most susceptible strain to CIA, and in 8 rats per group
to characterize the features of CIA in female Wistar rats. Briefly,
the rats were intradermally injected in two sites at the base of
the tail with a total of 0.1 ml CII/IFA emulsion. On day 7 after
primary immunization, a booster injection of 0.1 ml CII/IFA
emulsion was administered to all rats. All experimental protocols
regarding the animals and their care were approved by the
Institutional Animal Care and Use Committee of Hunan University of
Chinese Medicine (Changsha, China) and carried out in strict
accordance with the Guide for the Care and Use of Laboratory
Animals (National Institutes of Health, Bethesda, MD, USA).
Clinical evaluation of the development
of CIA
Arthritic signs in the paws of the rats,
characterized by edema and erythema, were inspected daily following
the primary CII/IFA injection, and the onset of arthritis was
monitored. To evaluate the incidence and severity of the arthritis,
lesions on all 4 paws of each rat (i.e. the arthritic signs) were
graded by two independent investigators using an arthritic scoring
system. Lesions on all four paws of each rat (i.e., the arthritic
signs) were graded from 0 to 4 according to the extent of edema and
erythema of the periarticular tissues; 16 was the potential maximum
of the combined arthritic scores per animal (Rosloniec, Cai). The
severity scores were defined as follows: 0, No evidence of erythema
and swelling; 1, erythema and mild swelling confined to the
mid-foot (tarsals) or ankle joint; 2, erythema and mild swelling
extending from the ankle to the foot; 3, erythema and moderate
swelling extending from the ankle to the metatarsal joints; and 4,
erythema and severe swelling encompass the ankle, foot and digits.
The hind paw volumes were measured every 3 days using a
plethysmometer chamber (UGO SLR, Comerio, Italy), beginning on day
9 after the primary immunization (11,12). In
addition, the body weight of the rats was monitored every 3
days.
Determination of erythrocyte
sedimentation rate (ESR)
Blood samples were collected from the veins of the
rat tails 0, 6, 12, 18, 24 and 30 days after the CII/IFA injection
for laboratory testing. The ESR was determined by a modified method
as previously described (13).
Radiological and histopathological
studies
Upon conclusion of the experiments, the rats were
sacrificed by diethyl ether asphyxiation. The hind paws were
radiographed and then histopathologically analyzed as previously
described (11,12).
Statistical analysis
All statistical analyses were performed using SPSS
software, version 20.0 (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard error of the mean. The differences
between the groups were analyzed using the Student's t-test. A
two-tailed P<0.05 was considered to indicate a statistically
significant difference.
Results
Susceptibility of different rat
strains to CIA
As shown in Table I,
neither male nor female SD rats were susceptible to the
immunization with bovine CII/IFA at the base of the tail, while
Wistar Furth rats were moderately responsive to CIA. By contrast,
female Wistar rats were greatly susceptible to CIA, developing
severe arthritis with an incidence of >83%.
 | Table I.Susceptibility of different rat
strains to collagen II-induced arthritis. |
Table I.
Susceptibility of different rat
strains to collagen II-induced arthritis.
| Strain | Gender |
Incidencea,
% | Onset
dayb, range (mean) | Severityc |
|---|
| Sprague Dawley | Male | 0.0 | N/A | N/A |
|
| Female | 0.0 | N/A | N/A |
| Wistar | Male | 0.0 | N/A | N/A |
|
| Female | 83.3 | 10–16 (13) | 7.6 |
| Wistar Furth | Male | 50.0 | 10–18 (14) | 2.5 |
|
| Female | 33.3 | 9–15
(12) | 1.7 |
Clinical progression of CIA in female
Wistar rats
Following immunization with the CII/IFA emulsion at
the base of the tail, the onset of arthritis occurred in female
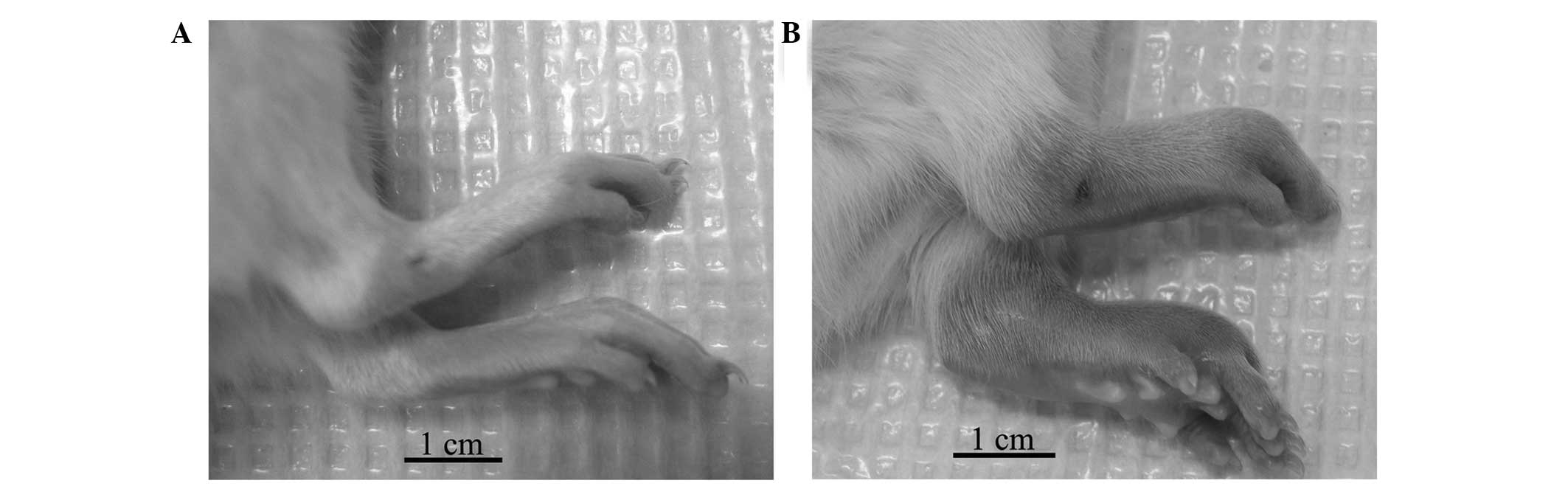
Wistar rats around days 10 to 16. In contrast to the control rats
(Fig. 1A), the CIA rats exhibited
conspicuous inflammatory lesions, characterized by edema and
erythema in the paws (Fig. 1B).
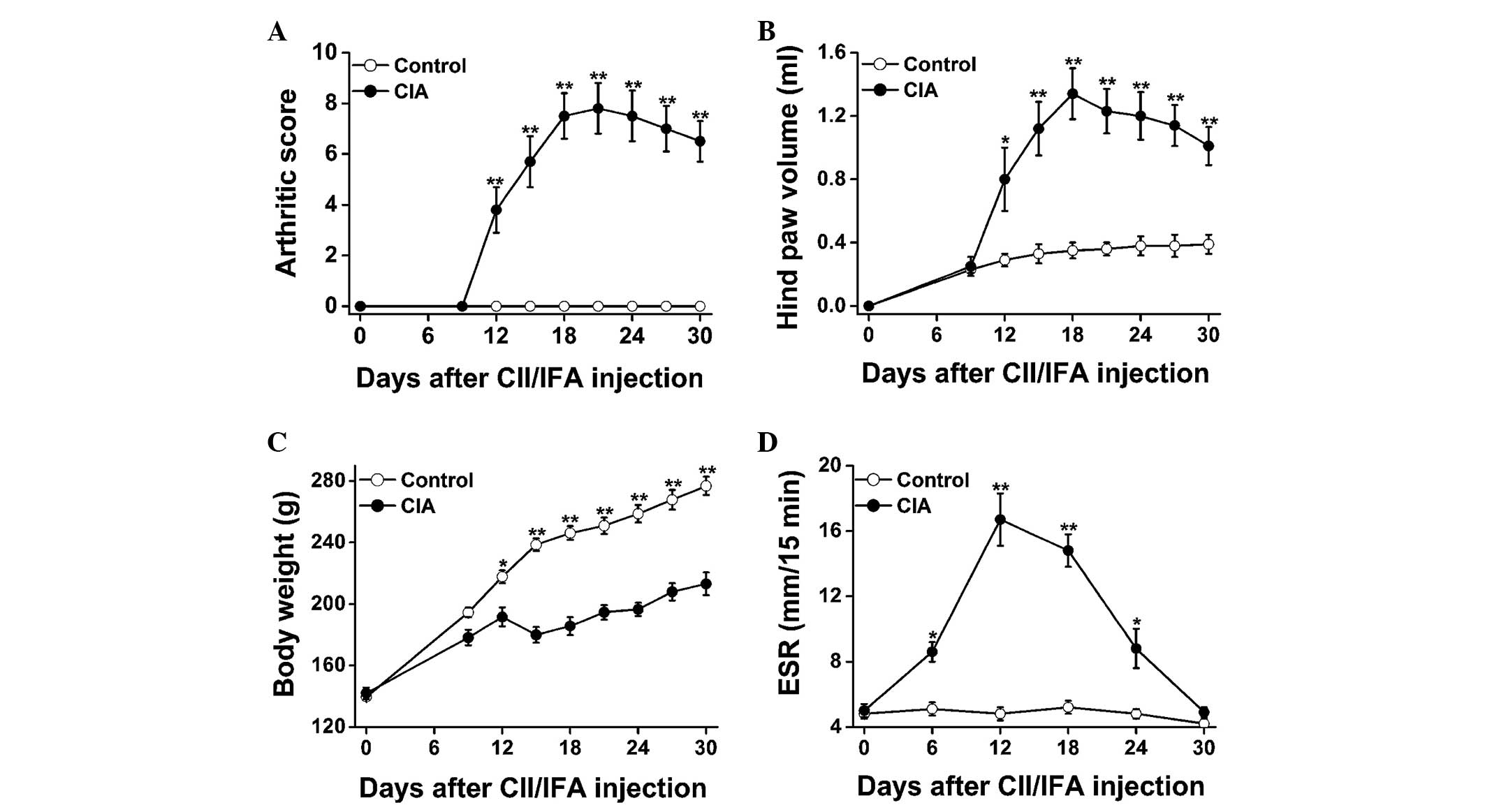
Fig. 2 shows the disease progression
of the CII-induced arthritic lesions in female Wistar rats. From
day 12 onwards, the arthritic score (Fig. 2A) and hind paw volume (Fig. 2B) were significantly increased in the
CIA rats as compared with those in the control rats; the parameters
reached a plateau on days 18–21 and decreased gradually thereafter.
Marked body weight loss was also observed in the CIA rats as
compared with the control rats (Fig.
2C). With regard to the disease progression, the ESR was
significantly elevated in the CIA versus control rats on days 6,
12, 18 and 24, peaking on day 12, and showed a sharp decrease from
then onwards (Fig. 2D).
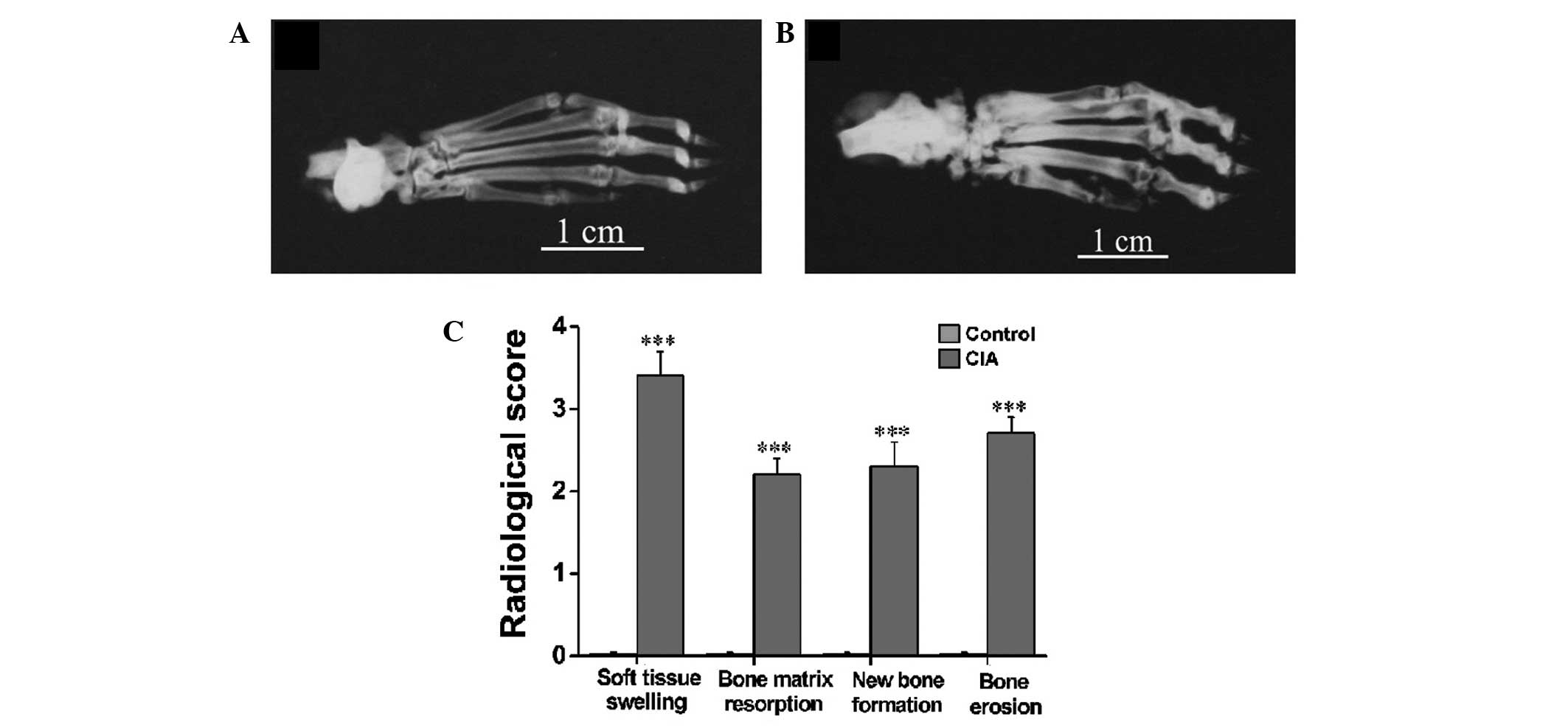
Radiological analysis of CIA in female
Wistar rats
Radiographs were taken on day 30 after immunization
(Fig. 3A and B) when no active
inflammatory signs were visible in the CIA rats. It was evident in
the representative radiographs that the CIA rats (Fig. 3B) exhibited severe soft tissue
swelling, a prominent decrease in bone density, notable destruction
of bony structures and ossifications not contiguous with normal
bone-line in the tarsal, metatarsal and interphalangeal regions as
compared with the control rats (Fig.
3A). The radiological scoring of soft tissue swelling, bone
matrix resorption, periosteal new bone formation and bone erosion
showed significant differences between the CIA and control rats
(Fig. 3C).
Histopathological analysis of CIA in
female Wistar rats
As noted in the representative hematoxylin and
eosin-stained sections of the ankle joints from the control
(Fig. 4A) and CIA (Fig. 4B) rats, a clear space between the
thin synovial membrane and the bones was found in the control rats
(Fig. 4A), while extensive synovial
proliferation, marked cellular infiltration and considerable pannus
formation were observed in the CIA rats (Fig. 4B). The CIA rats also exhibited a
marked loss of joint space and severe erosion of the bones and
cartilage (Fig. 4B). Fig. 4C illustrates statistically
significant differences in the histopathological scoring of
cellular infiltration, joint space narrowing, cartilage
destruction, bone erosion, synovial hyperplasia and pannus
formation between the CIA and control rats (P<0.001).
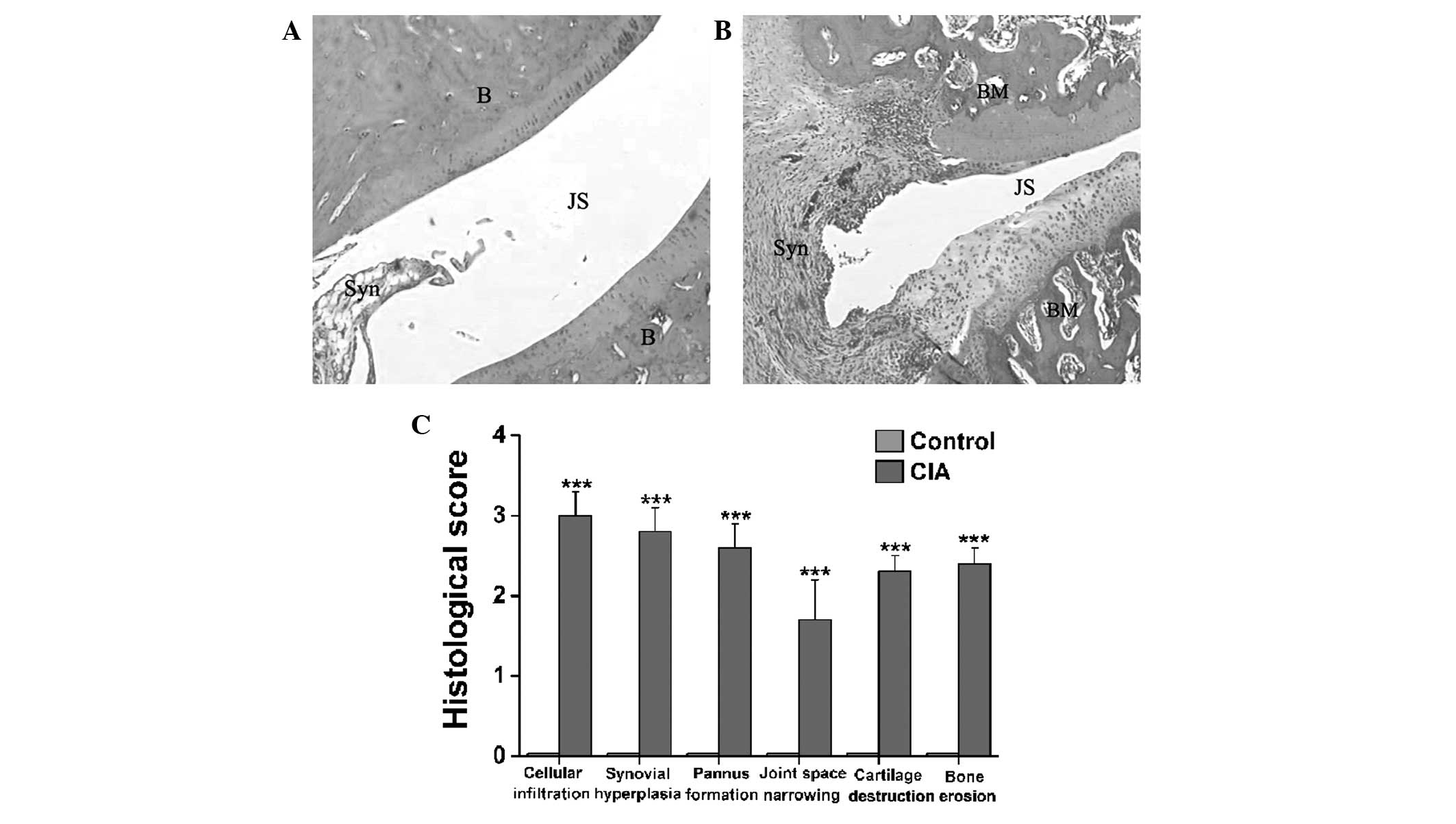 | Figure 4.Histopathological changes in the
tibiotarsal joints of female Wistar rats with CIA. (A and B)
Representative hematoxylin and eosin-stained sections
(magnification, ×40) revealing histopathological changes in the
tibiotarsal joints of the (A) control and (B) CIA model female
Wistar rats. (C) Significant differences in cellular infiltration,
synovial hyperplasia, pannus formation, joint space narrowing,
cartilage destruction and bone erosion in the synovial joints were
found between the CIA and control rats. Data are presented as the
mean ± standard error of the mean (n=8). ***P<0.001 versus
control rats. CIA, collagen-induced arthritis; B, bone; JS, joint
space; Syn, synovial membrane; BM, bone marrow. |
Discussion
CIA is an experimental model of autoimmune-mediated
arthritis that has been used worldwide in a wide variety of
studies, including studies focusing on the understanding of the
immunopathogenic mechanisms of arthritis, the analysis of genetic
susceptibility factors and the preclinical development of novel
therapeutic approaches. CIA can be induced in susceptible strains
of rodents (rats and mice) and non-human primates by CII
immunization (1,2,14). With
regard to the establishment of CIA in rats, which are generally
susceptible to AIA, the use of potent immunoadjuvants (e.g. CFA)
should be obviated. A more simple and efficient immunization
method, which requires smaller amounts of CII emulsion, could be
particularly advantageous for research purposes.
In the present study, the susceptibility of
different rat strains to immunization with bovine CII emulsified in
IFA at the base of the tail was in the following order: SD, Wistar
Furth and Wistar (from lowest to highest, respectively) (Table I). SD rats were relatively resistant
to CIA and Wistar Furth rats were exhibited a moderate response
(Table I). By contrast, female
Wistar rats were highly responsive to CIA and exhibited a high
incidence and severity of arthritis (Table I). Following primary and booster
immunization with a total of 0.2 ml CII/IFA emulsion at the base of
the tail, female Wistar rats developed pronounced arthritis with a
high incidence and a low variability in clinical signs (Figs. 1 and 2).
In association with the development of arthritis,
the ESR in the CIA rats was found to be markedly elevated between
days 6 and 24 after CII/IFA immunization, as compared with that in
the control rats (Fig. 2D). ESR is a
common laboratory test used to aid the definitive diagnosis of RA,
the assessment of the disease activity and its response to
therapeutics. Studies have reported that the ESR is affected by
increases in the plasma levels of acute-phase reactant proteins
(e.g. fibrinogen, α- and β-globulin); consequently, the ESR is
considered to reflect the acute-phase response to inflammatory
diseases (15,16). In this CIA model of female Wistar
rats, the ESR peaked on day 12 and underwent a sharp decrease
thereafter, which indicated a relatively early production and
stimulation of acute-phase proteins during the progression of the
disease (Fig. 2D).
RA is initially characterized by the presence of an
immune-mediated inflammatory response of the synovial membrane,
accompanied by diffuse infiltration of a variety of mononuclear
cells and the formation of new vessels. Chronic inflammation and
the abnormal proliferation of the synovial membrane ultimately form
a destructive pannus, which progressively invades and destroys the
cartilage and bone in joints over the course of the disease
(17,18). In patients with RA, distinct patterns
of bone loss are observed, such as juxta-articular osteopenia
adjacent to inflamed joints and the presence of focal erosion
within the subchondral bone and at the joint margins in areas of
direct pannus invasion (19,20). Bone remodeling also occurs in the
arthritic joints (21,22). In the present CIA model of female
Wistar rats, histological sections of the ankle joints revealed
diffuse cellular infiltration, extensive synovial proliferation,
the formation of erosive panni, notable narrowing of the joint
space and marked cartilage and bone erosion (Fig. 4). The radiological examination also
revealed distinct patterns of bone loss and erosion associated with
periosteal new bone formation in the tarsal, metatarsal and
interphalangeal regions (Fig. 3).
The combination of pronounced bone matrix resorption and
heterotopic new bone formation was strongly indicative of the
presence of a disordered pattern of bone remodeling in the CIA
rats. Thus, the findings in the female Wistar rat CIA model closely
resemble the disease characteristics of human RA.
In conclusion, a more specific, simple and efficient
method of establishing a CIA rat model delineating the development
of human RA has been determined in the present study. An
intradermal injection of only 200 µg bovine CII emulsified in IFA
at the base of the tail can successfully induce a pronounced
autoimmune-mediated polyarthritis in female Wistar rats. This CIA
rat model shows a high disease incidence and low variability in
clinical signs and closely resembles its human counterpart, RA.
This well-developed and well-characterized CIA model in female
Wistar rats could therefore be specifically and efficiently used to
study the pathological and immunological processes of RA, as well
as to test and develop novel anti-arthritic agents.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81373540), the Key
Science & Research Program of Hunan Department of Science &
Technology (grant no. 2012TF-1005), the Hunan Chinese Medicine
Science & Research Key Project (grant no. 201308) and the
Open-Ended Science Foundation of National Key Subject of
Traditional Chinese Medicine Diagnostics (grant no. 2013ZYZD10,
2014-8).
Abbreviations:
|
CIA
|
collagen-induced arthritis
|
|
CII
|
type II collagen
|
|
IFA
|
incomplete Freund's adjuvant
|
|
SD
|
Sprague Dawley
|
|
CFA
|
complete Freund's adjuvant
|
|
ESR
|
erythrocyte sedimentation rate
|
|
RA
|
rheumatoid arthritis
|
|
AIA
|
adjuvant-induced arthritis
|
References
|
1
|
Trentham DE, Townes AS and Kang AH:
Autoimmunity to type II collagen: An experimental model of
arthritis. J Exp Med. 146:857–868. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosloniec EF, Cremer M, Kang A and Myers
LK: Collagen-induced arthritis. Curr Protoc Immunol Chapter.
15:Unit 15.5. 2001. View Article : Google Scholar
|
|
3
|
Billingham ME: Models of arthritis and the
search for anti-arthritic drugs. Pharmacol Ther. 21:389–428. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Griffiths MM: Immunogenetics of
collagen-induced arthritis in rats. Int Rev Immunol. 4:1–15. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu L, Wei W and Zheng YQ: Effect and
mechanism of action of total glucosides of paeony on synoviocytes
from rats with collagen-induced arthritis. Yao Xue Xue Bao.
41:166–170. 2006.(In Chinese). PubMed/NCBI
|
|
6
|
Wang Y, Zhao HY, Liu MJ, et al:
Establishment of a rat model of rheumatoid arthritis with kidney
deficiency syndrome. Zhong Xi Yi Jie He Xue Bao. 9:973–982.
2011.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin CM, Gu J, Zhang Y, Shen LJ, Ma L, Ni
J, Wang ZQ and Wu W: Effect of UC-MSCs on inflammation and
thrombosis of the rats with collagen type II induced arthritis.
Zhonghua Xue Ye Xue Za Zhi. 33:215–219. 2012.(In Chinese).
PubMed/NCBI
|
|
8
|
Srivastava NK, Sharma S, Purusottam RN, et
al: Abnormal lipid metabolism in collagen-induced arthritis rat
model: In vitro, high resolution NMR spectroscopy based analysis.
Indian J Exp Biol. 52:673–682. 2014.PubMed/NCBI
|
|
9
|
Pearson CM: Experimental joint disease
observations on adjuvant-induced arthritis. J Chronic Dis.
16:863–874. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai X, Wong YF, Zhou H, et al:
Manipulation of the induction of adjuvant arthritis in Sprague
Dawley rats. Inflamm Res. 55:368–377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai X, Zhou H, Wong YF, et al: Suppression
of the onset and progression of collagen-induced arthritis in rats
by QFGJS, a preparation from an anti-arthritic Chinese herbal
formula. J Ethnopharmacol. 110:39–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou H, Wong YF, Wang J, Cai X and Liu L:
Sinomenine ameliorates arthritis via MMPs, TIMPs and cytokines in
rats. Biochem Biophys Res Commun. 376:352–357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen FY, Li X, Huang HY, et al: A simple,
rapid and accurate method for determination of erythrocyte
sedimentation rate using capillary tubes in experimental models of
rodents. Zhong Guo Yao Li Xue Tong Bao. 29:1762–1765. 2013.(In
Chinese).
|
|
14
|
Courtenay JS, Dallman MJ, Dayan AD, Martin
A and Mosedale B: Immunisation against heterologous type II
collagen induces arthritis in mice. Nature. 283:666–668. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh AS, Atam V, Yathish BE, Das L and
Koonwar S: Role of erythrocyte sedimentation rate in ischemic
stroke as an inflammatory marker of carotid atherosclerosis. J
Neurosci Rural Pract. 5:40–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Solberg BL and Olson RJ: Clinical utility
of the erythrocyte sedimentation rate: A case study. Clin Lab Sci.
27:72–77. 2014.PubMed/NCBI
|
|
17
|
McGonagle D, Conaghan PG, O'Connor P, et
al: The relationship between synovitis and bone changes in early
untreated rheumatoid arthritis: A controlled magnetic resonance
imaging study. Arthritis Rheum. 42:1706–1711. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goldring SR and Gravallese EM:
Pathogenesis of bone erosions in rheumatoid arthritis. Curr Opin
Rheumatol. 12:195–199. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gravallese EM and Goldring SR: Cellular
mechanisms and the role of cytokines in bone erosions in rheumatoid
arthritis. Arthritis Rheum. 43:2143–2151. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smolen JS and Steiner G: Therapeutic
strategies for rheumatoid arthritis. Nat Rev Drug Discov.
2:473–488. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deodhar AA and Woolf AD: Bone mass
measurement and bone metabolism in rheumatoid arthritis: A review.
Br J Rheumatol. 35:309–322. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fardellone P, Séjourné A, Paccou J and
Goëb V: Bone remodelling markers in rheumatoid arthritis. Mediators
Inflamm. 2014:4842802014. View Article : Google Scholar : PubMed/NCBI
|