Introduction
Osteoarthritis (OA) is a disease characterized by
degradation of articular cartilage, osteophyte formation,
subchondral sclerosis and synovial proliferation, which results in
the loss of joint function and disability (1). The loss of articular cartilage matrix
has been shown to involve the cleavage of native collagen by matrix
metalloproteinases (MMPs) (2).
MMP-13, also known as collagenase 3, is crucially involved in OA as
it preferentially degrades type II collagen (3). In previous studies, the expression of
MMP-13 was observed to be markedly increased in patients with OA
(2,4). Osteoporosis (OP) is a disorder
characterized by reduced bone mass, leading to diminished physical
bone strength and increased susceptibility to fracture (5). OA and OP are the most common
musculoskeletal disorders among elderly populations, and are
associated with estrogen deficiency (6–8).
Furthermore, previous studies have demonstrated that ovariectomy
may be used as an experimental intervention for treating
postmenopausal OA and OP (9–12).
Estrogen replacement therapy (ERT) is an
effectiveness treatment for the prevention of OA and OP (13). A number of large-scale
epidemiological studies have demonstrated a decreased incidence of
OA- and OP-induced hip fracture in patients who were administered
estrogen compared with untreated patients; however, long-term ERT
is associated with an increased risk of breast and uterine cancers,
in addition to cardiovascular and thromboembolic disease (14,15). In
response to these side-effects, studies have been conducted with
the aim of identifying alternative anti-osteoarthritic and
anti-osteoporotic approaches with comparable effectiveness to
estrogen.
Ultrasound (US) therapy is based on the application
of high frequency sound waves to the tissues of the body to induce
mechanical or thermal effects (16).
In in vivo studies, US has been demonstrated to improve
mechanical strength and bone mineral density (BMD) (17,18) and
to repair joint cartilage in animal models of cartilage injury
(19). In in vitro studies,
US therapy has been shown to effectively suppress osteoclast
formation (20), enhance osteoblast
formation and function (21),
stimulate the synthesis of extracellular matrix (ECM) proteins in
cartilage, and alter chondrocyte maturation and endochondral bone
formation (22,23). A systematic review of clinical
studies indicated that US is efficacious for decreasing pain and
may improve physical function in patients with knee OA (24). However, contrary results have been
also reported (25). Although a
number of studies have indicated the utility of ultrasound and
estrogen in the treatment of OA and OP (14–19), it
is not clear which therapeutic strategy is more effective in
improving serum estrogen levels, BMD, bone biomechanical strength,
cartilage structure and MMP-13 expression.
The aim of the present study was to investigate the
efficacy of US and ERT in the prevention and treatment of
ovariectomized (OVX) rabbits. Efficacy was assessed by evaluating a
number of parameters, including serum estrogen level, BMD, bone
biomechanical function and cartilage structure, in addition to
MMP-13 transcription and translation. In addition, the present
study aimed to determine whether US treatment induces equivalent
therapeutic effects compared with estrogen treatment for
postmenopausal OA and OP in OVX rabbits.
Materials and methods
Laboratory animals
A total of 28 virgin, 5-month-old, New Zealand white
(NZW) rabbits, weighing 2.5–3 kg, were purchased from the
Experimental Animal Center of West China Hospital, Sichuan
University (SYXK-Sichuan-2009-045; Sichuan, China). The rabbits
were allocated at random into four groups (n=7 per group): Normal
control with no treatment (control group); ovariectomized control
(OVX group); ovariectomy with ultrasound therapy (US group); and
ovariectomy with exogenous estrogen replacement therapy (ERT
group). A total of 7 rabbits from each group were tested in the
BMD, bone biomechanics, histological analysis, polymerase chain
reaction (PCR) and western blot analysis.
Animals were housed in individual
cages with a 12:12-h light-dark cycle at 20–26°C, and received a
standard laboratory diet and drinking water
All rabbits were subjected to the bilateral
ovariectomy, with the exception of the control group. All animal
care and experimental procedure were approved by the Institutional
Animal Care Center of Sichuan University and Ethics Committee on
Research Animal of People's Republic of China.
Bilateral ovariectomy
Bilateral ovariectomy was performed by anesthetizing
rabbits with 5% chloral hydrate (3 ml/kg) and placing the animal in
a supine position on a rabbit fixation board (Hepu Co. Ltd,
Tianjin, China) as previously described (26). The abdominal region of the
anesthetized rabbit was aseptically prepared and a 5-cm incision
was made on the middle abdomen of the rabbit. The ovarian artery
and vein were ligated and the bilateral ovaries were then excised.
Following irrigation with sterile saline solution, the wounds were
closed in layers. Rabbits received appropriate postoperative care
and were allowed freedom of activity within their individual
cages.
Treatment
US therapy was performed using a commercial,
clinically-approved proprietary SonicMaster ES-2 ultrasound device
(Ultrasonic apparatus ES-2, OG Giken Co., Ltd., Okayama, Japan). At
8 weeks after the ovariectomy, US with an intensity of 300
mW/cm2 and a frequency of 1 MHz was administered to the
US group for 10 min/day over 10 days (27). Bilateral legs were shaved and the
rabbits were fixed on a rabbit fixation board in a supine position.
US transducers were attached to the medial aspect of the femur and
knee, with a moving speed of 1 cm/sec manually. Standard ultrasonic
coupling gel was applied between the transducer head and the limb
to minimize reflection and attenuation of the US energy.
Concurrently, rabbits in the ERT group were orally treated with
conjugated estrogens tablets (Pfizer, New York, USA) at 0.625
mg/kg/day for 10 days (28,29). The OVX and control groups remained
untreated. All rabbits were anesthetized by 5% chloral hydrate (3
ml/kg) and sacrificed at week 10 by air embolism.
Serum estradiol assay
Blood samples were collected from each experimental
animal at baseline, week 8 and week 10 to assess the serum levels
of 17β-estradiol. A ~2-ml serum sample was extracted from each
blood sample by centrifugation at 2,000 × g for 20 min, and stored
at −80°C until required for analysis. Estradiol levels were
detected using an enzyme-linked immunosorbent assay (ELISA),
following the manufacturer's instructions (R&D Systems, Inc.,
Minneapolis, MN, USA).
BMD testing
The femur from the rabbit was separated and stored
at −20°C for BMD and biomechanical testing. The average BMD of the
entire femur was detected with dual-energy X-ray absorptiometry
(DEXA) using a GE Lunar iDXA Bone Densitometer (GE Healthcare Life
Sciences, Fairfield, CT, USA). An individual femur was immersed in
2-cm deep deionized water, which accurately simulated the bone
surrounding with soft tissues observed in the clinical setting
(30). The neck and head of the
femur was oriented perpendicularly to the DEXA beams, which limited
the rotational effects that can alter DEXA measurements (31). Each femur was positioned at the same
place to ensure consistency of results in the scanning process. The
data were collected and analyzed by the small animal research
software (Lunar iDAXTM, version 13.6, GE provided by GE Healthcare
Life Sciences (Madison, WI, USA).
Bone biomechanics
Bone strength was determined by three-point bending
testing of the femur, using an Instron 8874 axial-torsion fatigue
testing system (Instron Co., Boston, TH, USA), which included a
three-point bending apparatus. Femurs were placed on supports and
were bent until the 40% maximum load was reached by lowering a
centrally placed blade (1-mm width) at a constant crosshead speed
of 10 mm/min. Maximum load (N) was for each femur was recorded by a
computer interfaced with the Instron analyzer and using
WaveMatrixTM, Version V6.7, Instron Co. Ltd (Boston, TH, USA)
software.
Histological analysis
The distal femur of the rabbit knee was separated
and fixed in 10% buffered formalin. Following decalcification, the
samples were sequentially dehydrated in alcohol and embedded in
paraffin blocks. The samples were sectioned at a thickness of 5 µm
for histopathological classification. Sections were stained with
hematoxylin and eosin (H&E). Modified Mankin scoring (32) was conducted to grade the
histopathological classification of articular cartilage for OA
(Table I). Scoring was performed by
two independent observers, blind to the origin of the sections.
Digital images were captured using an optical microscope (Eclipse
80i, Nikon, Tokyo, Japan) at a magnification of x40.
 | Table I.Modified Mankin scoring system. |
Table I.
Modified Mankin scoring system.
| Category | Score |
|---|
| Structure |
|
|
Normal | 0 |
| Surface
irregularities | 1 |
| Pannus
and surface irregularities | 2 |
| Clefts
to transitional zone | 3 |
| Clefts
to radial zone | 4 |
| Clefts
to calcified zone | 5 |
|
Complete disorganization | 6 |
| Cells |
|
|
Normal | 0 |
| Diffuse
hypercellularity | 1 |
|
Cloning | 2 |
|
Hypocellularity | 3 |
| Safranin O
staining |
|
|
Normal | 0 |
| Slight
reduction | 1 |
|
Moderate reduction | 2 |
| Severe
reduction | 3 |
| No dye
noted | 4 |
| Tidemark
integrity |
|
|
Intact | 0 |
| Crossed
by blood vessels | 1 |
| Total score |
|
|
Minimal | 0 |
|
Maximal | 14 |
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Cartilage tissue was obtained from the femoral
condyle and the tibial plateau of each rabbit and immediately
stored in liquid nitrogen for RT-qPCR analysis and western blot
analysis. Total RNA was isolated from the cartilage using TRIzol
reagent (Takara Holdings Inc., Kyoto, Japan). A 0.5-µg total RNA
sample from each specimen was used for cDNA synthesis and RNA
amplification using one-step RT-qPCR, following the instruction of
PrimeScript One Step RT-PCR kit (Takara Holdings Inc.). The PCR
procedure was programmed to proceed as follows: 50°C, 30 min, 94°C,
2 min, 1 cycle; 94°C, 30 sec, 54°C [melting temperature (Tm) of
MMP-13]/49°C (Tm of GAPDH), 30 sec, 72°C, 30 sec, 30 cycles; 72°C,
5 min, 1 cycle; and 16°C, 20 min.
Each experiment was repeated at least three times.
The sequences of reaction products were confirmed by agarose gel
electrophoresis, and the negative control reactions run in the
absence of reverse transcription did not generate any products. The
mRNA expression levels of MMP-13 were determined relative to those
of GAPDH. The specific sequences of the PCR primers for MMP-13 and
GAPDH and the expected PCR product lengths are listed in Table II.
 | Table II.Primer sequences and PCR product
lengths. |
Table II.
Primer sequences and PCR product
lengths.
| Primer | Sequence 5′-3′
direction | NCBI Accession | PCR product length
(bp) |
|---|
| MMP-13 | F
GTTATCTGAACTGGATT | AF059201 | 147 |
|
| R
TCTAGGGTAGTCTTGGTC |
|
|
| GAPDH | F
GGTCGGAGTGAACGGATTT | NM_001082253 | 226 |
|
| R
CTCGCTCCTGGAAGATGG |
|
|
Western blot analysis
The cartilage was lysed at 4°C in
radioimmunoprecipitation assay buffer containing protease inhibitor
cocktail, 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride and 2 mM
dithiothreitol. The lysate concentration was measured in duplicate
using the Bradford assay (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Samples were separated by SDS-PAGE and electro-transferred to
PVDF membranes. Non-specific binding was blocked by 5% non-fat milk
in Tris-buffered saline containing 0.05% Tween 20 for 1 h.
Membranes were incubated with primary antibodies (rabbit monoclonal
anti-MMP-13, 1:1,000, Wuhan Boster Biological Technology, Ltd.,
Wuhan, China; rabbit monoclonal anti-GAPDH, 1:1,000, Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C overnight to
detect the specific protein expression. Membranes were subsequently
incubated with the anti-rabbit horseradish peroxidase
(HRP)-conjugated secondary antibody (Wuhan Boster Biological
Technology, Ltd.) in 5% blocking buffer for 1 h at room
temperature. Next, streptavidin-HRP was applied in 5% blocking
buffer for 30–60 min at room temperature, followed by incubation
with BeyoECL Plus reagents A and B (BeyoECL Plus, Beyotime
Institute of Biotechnology, Shanghai, China) for 2 min prior to
visualization using an HRP-driven chemiluminescence reaction
(Beyotime Institute of Biotechnology) and exposure to
autoradiographic film in the dark room. The expression levels of
MMP-13 were normalized against GAPDH.
Statistical analysis
Results are expressed as the mean ± standard
deviation. Statistical analyses were conducted using SPSS, version
20.0 (IBM SPSS, Armonk, NY, USA). One-way analysis of variance
followed by least significant difference correlation and
χ2 test were used to compare differences among all
groups. The Kruskal-Wallis test was used if the homogeneity of the
variance test showed heterogeneity. P<0.05 was considered to
indicate a statistically significant difference.
Results
Serum estradiol assay
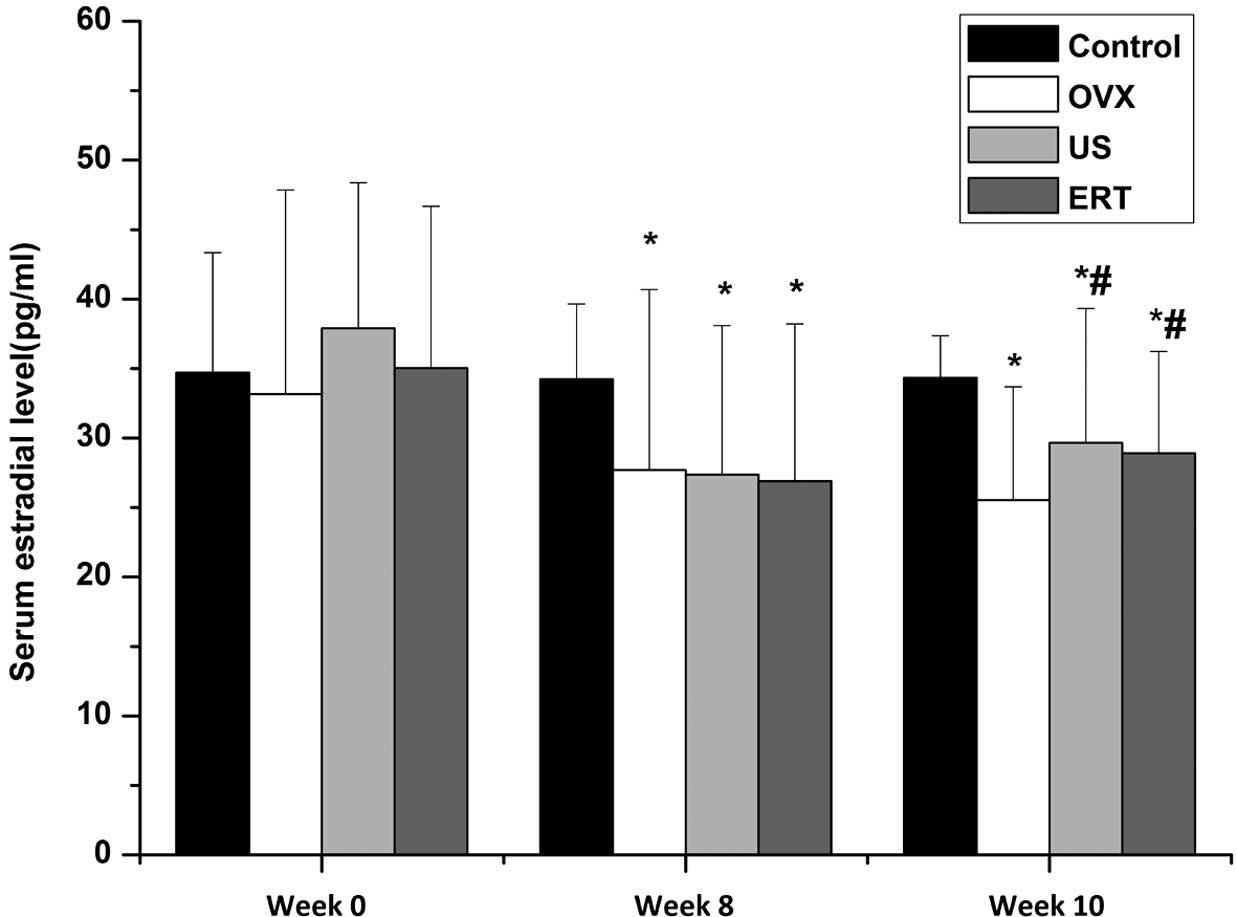
The serum 17β-estradiol levels among the groups at
various time points are shown in Fig.
1. No statistically significant difference was observed among
the 17β-estradiol levels of the groups prior to ovariectomy at week
0, indicating that the baseline estradiol levels in all groups were
comparable. After the ovariectomy at week 8, the serum estradiol
level was significantly reduced in rabbits that underwent an
ovariectomy compared with the control group, indicating that the
model of ovariectomy was established successfully. At week 10, at 2
weeeks after the start of the intervention, the levels of serum
estradiol in the US and ERT groups were significantly higher
compared with those in the OVX group. No statistically significant
difference was observed between the 17β-estradiol levels in the US
and ERT groups. Although the estradiol level was increased
following the US and ERT treatment of the OVX rabbits, it remained
significantly reduced compared with that in the control group
(Fig. 1).
BMD
As shown in Fig. 2,
there were no statistically significant differences in the femur
BMD of the US group compared with the control group 2 weeks after
the start of treatment; however, the BMD in the ERT group was
significantly reduced compared with that in the control group. The
US and ERT groups exhibited significantly higher BMD compared with
the OVX group (P<0.001 and 0.027, respectively; Fig. 2).
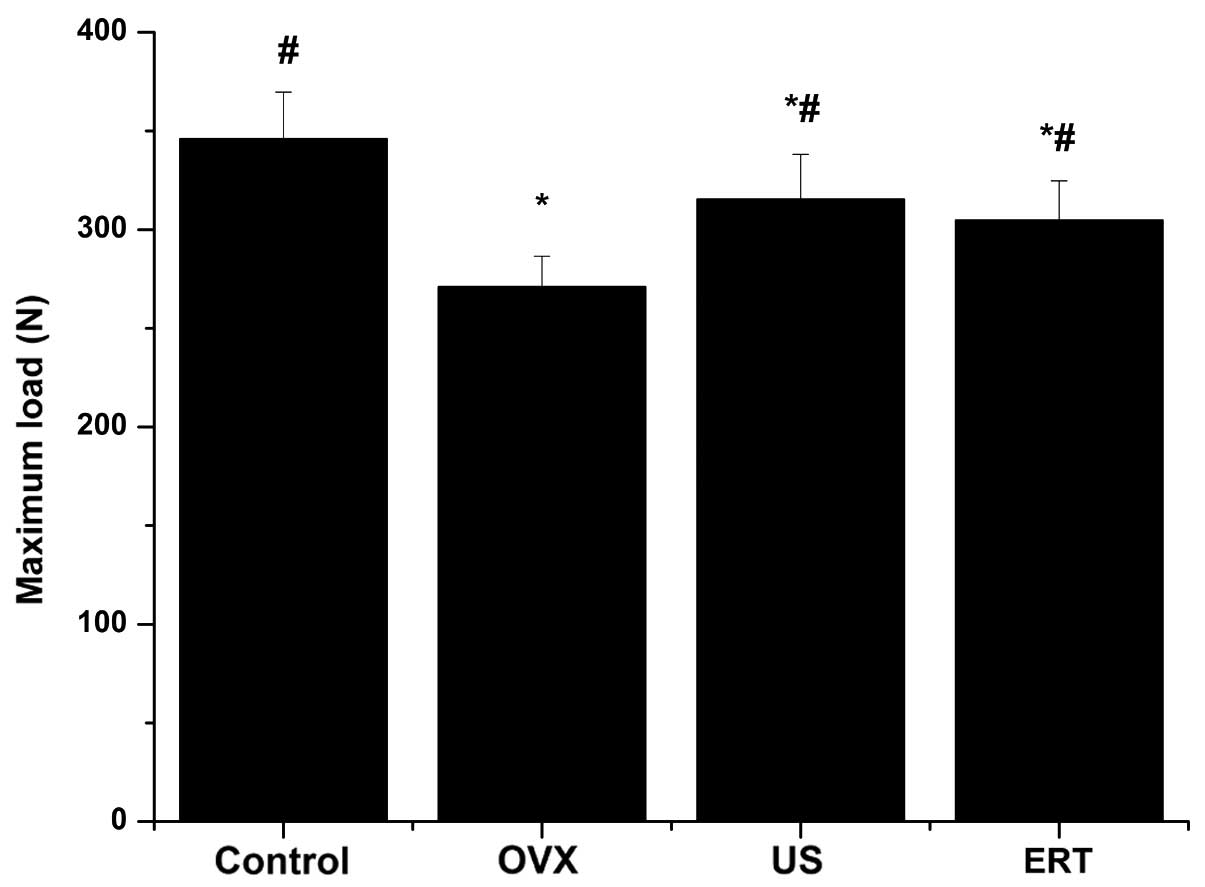
Bone biomechanical strength
testing
The maximum load of the femurs in the OVX, US and
ERT groups were statistically reduced compared with the control
group, as determined by the three-point bending test; however, the
maximum load was significantly higher following the intervention
with US and ERT compared with the OVX group (Fig. 3).
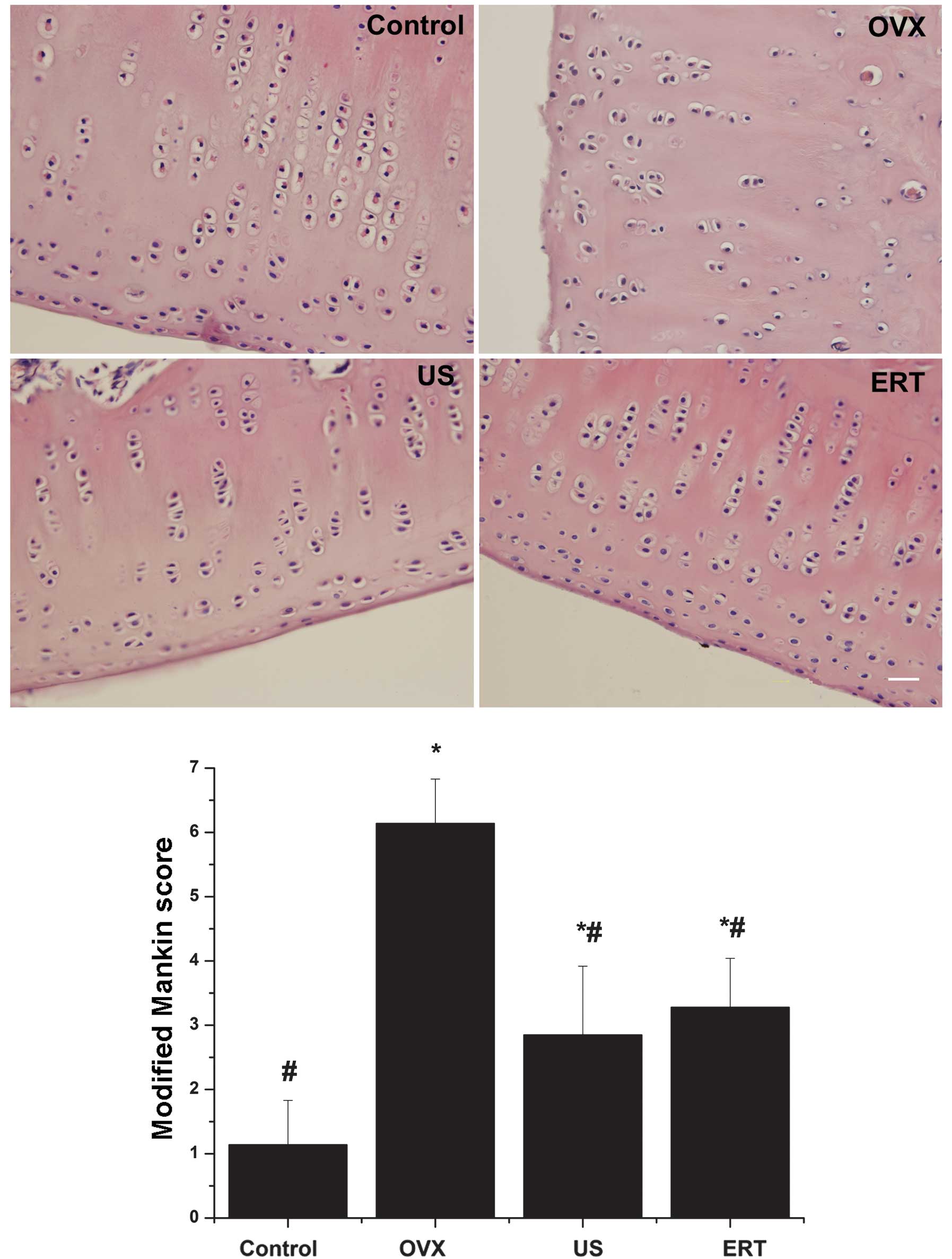
Modified Mankin score
In the OVX group, the cartilage surface exhibited
erosion and a limited amount of pannus. The chondrocytes were
organized irregularly; hypercellularity and clusters were detected
in the middle layer of the cartilage. Severe degradation of the ECM
and tidemark crossed by blood vessels were observed. The mean
modified Mankin score was assessed to be 6.14. However, markedly
smoother surfaces and mild degeneration of chondrocytes and the ECM
were observed in the US and ERT groups compared with the OVX group.
Furthermore, the mean modified Mankin scores of the US and ERT
groups (2.85 and 3.28 points, respectively) were significantly
reduced compared with the score of the OVX group, but significantly
higher compared with that of the control group. In the control
group, healthy cartilage with a smooth surface, normal chondrocyte
arrangement and ECM were detected. According to the modified Mankin
score system, the control group received the lowest score of 1.14
points (Fig. 4).
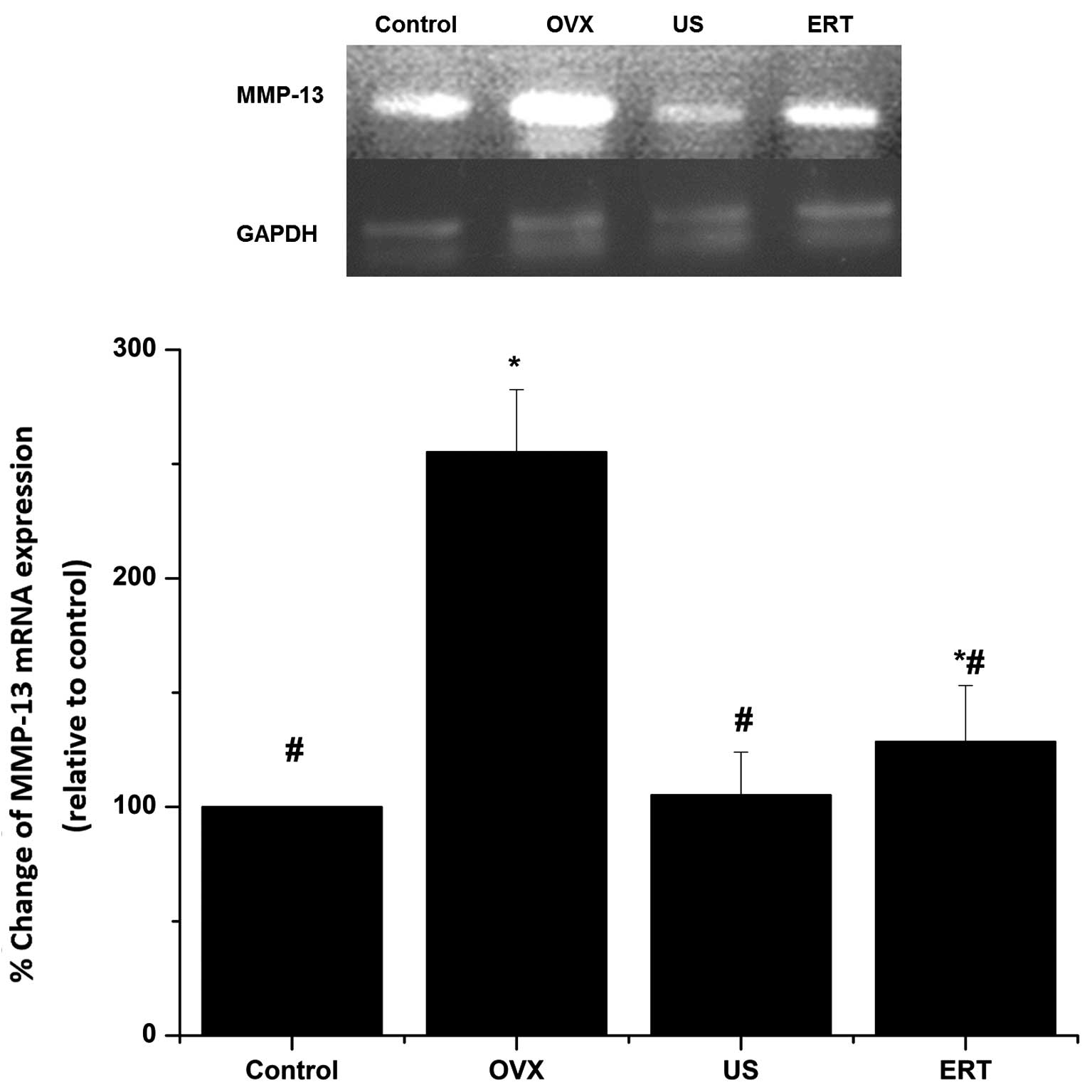
RT-qPCR
The mRNA expression of MMP-13 in the cartilage
relative to that of GAPDH was assessed using RT-qPCR. As shown in
Fig. 5, the expression levels of
GAPDH mRNA exhibited no difference among the four groups. The mRNA
expression levels of MMP-13 in the OVX group were markedly higher
compared with those in the control group. US treatment led to a
significant reduction in MMP-13 mRNA expression compared with the
OVX group, but did not statistically differ compared with the
control group. The expression levels of MMP-13 mRNA in the ERT
group were significantly lower than those in the OVX group, but
remained higher compared with those in the control group (Fig. 5).
Western blot analysis
MMP-13 protein expression relative to that of GAPDH
was detected by western blot analysis. As shown in Fig. 6, there was no difference in GAPDH
expression among the four groups. MMP-13 expression increased
notably in the OVX group compared with the control group. MMP-13
protein expression in US group was significantly reduced compared
with that in the OVX group but did not statistically differ from
that in the control group. The expression levels of MMP-13 protein
in the ERT group were significantly reduced in comparison with
those in the OVX group but higher than the control group levels.
The differences of MMP-13 protein expression among the four groups
were in accordance with the differences in mRNA expression level
(Fig. 6).
Discussion
A marked increase has been observed in the incidence
of OA and OP among postmenopausal women (33). The manner in which bone loss occurs
in postmenopausal OP is determined by the differences in skeletal
metabolism and architecture. Furthermore, estrogen deficiency
accelerates cartilage turnover by increasing the surface erosion of
the cartilage and the expression of MMP-13 (34), leading to the pathological
manifestations associated with OA. In the present study, the OVX
and control groups indicate that ovariectomy in rabbits
significantly decreased the level of serum estrogen, reduced BMD
and bone biomechanical function, eroded the cartilage surface, and
increased the mRNA and protein expression levels of MMP-13,
indicating that reduced levels of estradiol are associated with the
development of OA and OP.
In the present study, the levels of serum estradiol
were significantly increased in the US group compared with those in
the OVX group, and the effect was comparable to that of estrogen
treatment. However, the biological mechanisms underlying the
effects of US on the estradiol level remain unclear. It may be
hypothesized that US is able to increase estradiol levels in OVX
rabbits by regulating the endocrine and immune systems. Thus,
further studies are required to investigate the possible signaling
pathways involved in the US-mediated regulation of estradiol
level.
The results of the present study demonstrate that
the BMD and maximum femur load were increased following 2 weeks of
US therapy or estrogen treatment. At least 20 million women in
developed countries are estimated to be currently receiving hormone
replacement therapy (HRT) to prevent postmenopausal OP (35). A meta-analysis based on trials
measuring BMD indicated that the use of HRT is associated with a
reduction of non-vertebral fractures, in particular in women that
receive HRT prior to reaching the age of 60 years (36). Bourrin et al reported that 5
months of treatment with selective estrogen receptor modulator
therapy (SERM) increased tibia BMD and strength, but failed to
correct cancellous bone architecture (37). However, studies reported as early as
1949 first suggested that US might stimulate osteogenesis (38). The biophysics of US are categorized
as thermal or non-thermal effects. The ability of US to stimulate
changes in tissues and cells may be due to an increase in
temperature and associated energy absorption, which may affect
specific enzymes, including MMPs (39). Furthermore, differences observed in
tissues and cells following US treatment may be associated with
non-thermal processes such as acoustic streaming and caviation
(40). A previous study suggested
that US therapy may induce an increase in protein synthesis and
directly modulate cell membrane permeability, which may increase
micromechanical blood pressure leading to accelerated osteogenesis
(41). A number of studies have
reported an increase in BMD and mechanical strength and stiffness
within the initial 7–38 days of US treatment of rabbit tibia
(18,42–44).
Furthermore, low-intensity ultrasound stimulation in OVX mice is
able to activate new bone formation and maintain bone structure,
preventing estrogen deficiency-induced bone loss (45). The data presented in the present
study suggest that 2 weeks of US and ERT treatment increased the
BMD and maximum bone load in OVX rabbits, which is consistent with
the results of previous studies.
The results also demonstrated that lesions of the
knee joint cartilage were attenuated by the US and estrogen
therapy. Estrogen usage in postmenopausal women has been considered
as a potential treatment for OA (46). Estrogen exerts its effects on target
tissues by binding to the activating estrogen receptor (ER).
Estrogen may affect OA formation via MMP expression. Type II
collagen is the primary collagen present in articular cartilage,
and MMP-13 degrades type II collagen. It has previously been
reported that 17β-estradiol promotes the synthesis of type II
collagen, in addition to other proteins such as insulin in
articular chondrocytes (47). Lee
et al demonstrated that 17β-estradiol inhibits the
expression of MMP-13 in chondrocytes (48). Lu et al reported that estrogen
deficiency may result in increased expression of MMP-13 (49). US has been applied in a number of
in vitro and in vivo studies to certify its ability
to increase the self-repair capacity of cartilage with OA (17–20).
Molecular studies have shown increased chondrocyte differentiation
as a result of increased aggrecan expression, proteoglycan
synthesis and upregulation of chondroitin sulfate release following
US treatment (23). The results of
the present study demonstrate improved cartilage structure and lost
cellular matrix straining following US treatment. Furthermore, the
results of the RT-qPCR and western blot analysis indicated an
overall reduction in the transcription and translation of MMP-13,
indicating that US is able to attenuate type II collagen digestion
by reducing the transcription and translation of MMP-13. Similar
results were obtained by Gurkan et al, who demonstrated that
MMP-13 expression was decreased as a result of US stimulation
(50). In addition, Naito et
al reported that low-intensity pulsed ultrasound (LIPUS)
increased the articular cartilage type II collagen in a rat
osteoarthritis model (51). However,
a negative result was obtained by Park et al, who observed
unchanged MMP-13 mRNA levels following LIPUS treatment (52). In the present study, US and estrogen
therapy appeared to mitigate cartilage degradation by inhibiting
the transcription and translation of MMP-13.
Although ERT may be applied to preserve bone
structure and articular cartilage structural integrity in OVX
rabbits, a major drawback of long-term ERT is an increased risk of
breast cancer (53), endometrial
cancer (54) and ovarian cancer
(55). US therapy has fewer
side-effects, and there is evidence that US therapy has a positive
effect on bone and cartilage regeneration (18,20,23). US
treatment may be applicable to the treatment of women with
postmenopausal OA and OP. Whether different intensities,
frequencies and treatment times of US result in different effects
on serum estrogen level, BMD, bone biomechanical strength and
MMP-13 expression requires further investigated. Further studies
are also required to determine the molecular mechanisms underlying
the effects of US treatment on postmenopausal OA and OP.
In conclusion, the results of the present study
demonstrate that within 8 weeks of undergoing ovariectomy, rabbits
exhibited reductions in serum estradiol levels, BMD and bone
biomechanical strength, and increased transcription and expression
of MMP-13, which may be associated with alterations in articular
cartilage structure. Following 2 weeks of US and estrogen therapy
post-ovariectomy, the serum estradiol levels had reduced, BMD and
bone biomechanical function were protected, and the degradation of
articular cartilage in OVX rabbits had slowed. These effects may
have been mediated by the downregulation of MMP-13 gene expression
and translation. Furthermore, by comparing the responses of bone
and cartilage to US and estradiol therapy, it is concluded that US
was superior to ERT as a therapy as fewer side-effects are caused
by US treatment.
Acknowledgements
The authors thank Dr Cao Xueqing for her assistance
with the hematoxylin and eosin staining.
References
|
1
|
Abramson SB and Attur M: Developments in
the scientific understanding of osteoarthritis. Arthritis Res Ther.
11:2272009. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tetlow LC, Adlam DJ and Woolley DE: Matrix
metalloproteinase and proinflammatory cytokine production by
chondrocytes of human osteoarthritic cartilage: Associations with
degenerative changes. Arthritis Rheum. 44:585–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Knäuper V, López-Otin C, Smith B, Knight G
and Murphy G: Biochemical characterization of human collagenase-3.
J Biol Chem. 271:1544–1550. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poole AR, Kobayashi M, Yasuda T, Laverty
S, Mwale F, Kojima T, Sakai T, Wahl C, El-Maadawy S, Webb G, et al:
Type II collagen degradation and its regulation in articular
cartilage in osteoarthritis. Ann Rheum Dis. 61 (Suppl 2):ii78–ii81.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun SS, Ma HL, Liu CL, Huang CH, Cheng CK
and Wei HW: Difference in femoral head and neck material properties
between osteoarthritis and osteoporosis. Clin Biomech (Bristol,
Avon). 23 (Suppl 1):S39–S47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cooley HM, Stankovich J and Jones G: The
association between hormonal and reproductive factors and hand
osteoarthritis. Maturitas. 45:257–265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wluka AE, Cicuttini FM and Spector TD:
Menopause, oestrogens and arthritis. Maturitas. 35:183–199. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ivey JL and Baylink DJ: Postmenopausal
osteoporosis : Proposed roles of defective coupling and estrogen
deficiency. Metab Bone Dis Relat Res. 3:3–7. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Høegh-Andersen P, Tankó LB, Andersen TL,
Lundberg CV, Mo JA, Heegaard AM, Delaissé JM and Christgau S:
Ovariectomized rats as a model of postmenopausal osteoarthritis:
Validation and application. Arthritis Res Ther. 6:R169–R180. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai G, Wang S, Li J, Liu C and Liu Q: The
validity of osteoarthritis model induced by bilateral ovariectomy
in guinea pig. J Huazhong Univ Sci Technolog Med Sci. 26:716–719.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Turner R, Maran A, Lotinun S, Hefferan T,
Evans GL, Zhang M and Sibonga JD: Animal models for osteoporosis.
Rev Endocr Metab Disord. 2:117–127. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalu DN: The ovariectomized rat model of
postmenopausal bone loss. Bone Miner. 15:175–191. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
RomanBlas JA, Castañeda S, Largo R and
Herrero-Beaumont G: Osteoarthritis associated with estrogen
deficiency. Arthritis Res Ther. 11:2412009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grady D, Rubin SM, Petitti DB, Fox CS,
Black D, Ettinger B, Ernster VL and Cummings SR: Hormone therapy to
prevent disease and prolong life in postmenopausal women. Ann
Intern Med. 117:1016–1037. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gallagher JC and Sai AJ: Molecular biology
of bone remodeling: Implications for new therapeutic targets for
osteoporosis. Maturitas. 65:301–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baker KG, Robertson VJ and Duck FA: A
review of therapeutic ultrasound: Biophysical effects. Phys Ther.
81:1351–1358. 2001.PubMed/NCBI
|
|
17
|
Tsumaki N, Kakiuchi M, Sasaki J, Ochi T
and Yoshikawa H: Low-intensity pulsed ultrasound accelerates
maturation of callus in patients treated with opening-wedge high
tibial osteotomy by hemicallotasis. J Bone Joint Surg Am.
86-A:2399–2405. 2004.PubMed/NCBI
|
|
18
|
Shimazaki A, Inui K, Azuma Y, Nishimura N
and Yamano Y: Low-intensity pulsed ultrasound accelerates bone
maturation in distraction osteogenesis in rabbits. J Bone Joint
Surg Br. 82:1077–1082. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cook SD, Salkeld SL, Patron LP, Doughty ES
and Jones DG: The effect of low-intensity pulsed ultrasound on
autologous osteochondral plugs in a canine model. Am J Sports Med.
36:1733–1741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Perry MJ, Parry LK, Burton VJ, Gheduzzi S,
Beresford JN, Humphrey VF and Skerry TM: Ultrasound mimics the
effect of mechanical loading on bone formation in vivo on
rat ulnae. Med Eng Phys. 31:42–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Unsworth J, Kaneez S, Harris S, Ridgway J,
Fenwick S, Chenery D and Harrison A: Pulsed low intensity
ultrasound enhances mineralisation in preosteoblast cells.
Ultrasound Med Biol. 33:1468–1474. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishikori T, Ochi M, Uchio Y, Maniwa S,
Kataoka H, Kawasaki K, Katsube K and Kuriwaka M: Effects of
low-intensity pulsed ultrasound on proliferation and chondroitin
sulfate synthesis of cultured chondrocytes embedded in
Atelocollagen gel. J Biomed Mater Res. 59:201–206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Parvizi J, Wu CC, Lewallen DG, Greenleaf
JF and Bolander ME: Low-intensity ultrasound stimulates
proteoglycan synthesis in rat chondrocytes by increasing aggrecan
gene expression. J Orthop Res. 17:488–494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Loyola-Sánchez A, Richardson J and
MacIntyre NJ: Efficacy of ultrasound therapy for the management of
knee osteoarthritis: A systematic review with meta-analysis.
Osteoarthritis Cartilage. 18:1117–1126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Warden SJ, Bennell KL, Forwood MR,
McMeeken JM and Wark JD: Skeletal effects of low-intensity pulsed
ultrasound on the ovariectomized rodent. Ultrasound Med Biol.
27:989–998. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Castañeda S, Largo R, Calvo E,
Rodríguez-Salvanés F, Marcos ME, Díaz-Curiel M and Herrero-Beaumont
G: Bone mineral measurements of subchondral and trabecular bone in
healthy and osteoporotic rabbits. Skeletal Radiol. 35:34–41. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng D, Luo Q, Lin H, Zhang J and He C:
The effect of therapeutic ultrasound to apoptosis of chondrocyte
and caspase-3 and caspase-8 expression in rabbit surgery-induced
model of knee osteoarthritis. Rheumatol Int. 32:3771–3777. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen J, Huang LQ, Xia QJ and He C-Q:
Effects of pulsed electromagnetic fields on the mRNA expression of
CAII and RANK in ovariectomized rats. Rheumatol Int. 32:1527–1532.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li S, Luo Q, Huang L, Hu Y, Xia Q and He
C: Effects of pulsed electromagnetic fields on cartilage apoptosis
signalling pathways in ovariectomised rats. Int Orthop.
35:1875–1882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mazess R, Collick B, Trempe J, Barden H
and Hanson J: Performance evaluation of a dual energy x-ray bone
densitometer. Calcif Tissue Int. 44:2281989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cohen B and Rushton N: Accuracy of DEXA
measurement of bone mineral density after total hip arthroplasty. J
Bone Joint Surg Br. 77:4791995.PubMed/NCBI
|
|
32
|
Mankin H, Dorfman H, Lippiello L and
Zarins A: Biochemical and metabolic abnormalities in articular
cartilage from osteo-arthritic human hips. II. Correlation of
morphology with biochemical and metabolic data. J Bone Joint Surg
Am. 53:523–537. 1971.PubMed/NCBI
|
|
33
|
Kanis JA: Estrogens, the menopause and
osteoporosis. Bone. 19 (Suppl 5):185S–190S. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu T, Achari Y, Rattner JB and Hart DA:
Evidence that estrogen receptor enhances MMP-13 promoter activity
in HIG-82 cells and that this enhancement can be influenced by
ligands and involves specific promoter sites. Biochem Cell Biol.
85:326–336. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dören M: Estrogen therapy for prevention
and treatment of osteoporosis. Maturitas. 43 (Suppl):S53–S56. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Torgerson DJ and Bell-Syer SE: Hormone
replacement therapy and prevention of nonvertebral fractures: A
meta-analysis of randomized trials. JAMA. 285:2891–2897. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bourrin S, Ammann P, Bonjour JP and
Rizzoli R: Recovery of proximal tibia bone mineral density and
strength, but not cancellous bone architecture, after long-term
bisphosphonate or selective estrogen receptor modulator therapy in
aged rats. Bone. 30:195–200. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Buchtala V: Present state of ultrasound
therapy. Dia Med. 22:2944–2950. 1950.PubMed/NCBI
|
|
39
|
Claes L and Willie B: The enhancement of
bone regeneration by ultrasound. Prog Biophys Mol Biol. 93:384–398.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chang WH, Sun JS, Chang SP and Lin JC:
Study of thermal effects of ultrasound stimulation on fracture
healing. Bioelectromagnetics. 23:256–263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dyson M: Non-thermal cellular effects of
ultrasound. Br J Cancer. Suppl:165–171. 1982.
|
|
42
|
Sakurakichi K, Tsuchiya H, Uehara K,
Yamashiro T, Tomita K and Azuma Y: Effects of timing of
low-intensity pulsed ultrasound on distraction osteogenesis. J
Orthop Res. 22:395–403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Uglow MG, Peat RA, Hile MS, Bilston LE,
Smith EJ and Little DG: Low-intensity ultrasound stimulation in
distraction osteogenesis in rabbits. Clin Orthop Relat Res.
303–312. 2003.PubMed/NCBI
|
|
44
|
Tis JE, Meffert RH, Inoue N, McCarthy EF,
Machen MS, McHale KA and Chao EY: The effect of low intensity
pulsed ultrasound applied to rabbit tibiae during the consolidation
phase of distraction osteogenesis. J Orthop Res. 20:793–800. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lim D, Ko CY, Seo DH, Woo DG, Kim JM, Chun
KJ and Kim HS: Low-intensity ultrasound stimulation prevents
osteoporotic bone loss in young adult ovariectomized mice. J Orthop
Res. 29:116–125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang KGA, Saris DBF, Dhert WJA and Verbout
AJ: Osteoarthritis of the knee: Current treatment options and
future directions. Curr Orthop. 18:311–320. 2004. View Article : Google Scholar
|
|
47
|
Claassen H, Schluter M, Schunke M and Kurz
B: Influence of 17beta-estradiol and insulin on type II collagen
and protein synthesis of articular chondrocytes. Bone. 39:310–317.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee YJ, Lee EB, Kwon YJ, Lee JJ, Cho WS,
Kim HA and Song YW: Effect of estrogen on the expression of matrix
metalloproteinase (MMP)-1, MMP-3 and MMP-13 and tissue inhibitor of
metalloproternase-1 in osteoarthritis chondrocytes. Rheumatol Int.
23:282–288. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lu T, Achari Y, Sciore P and Hart DA:
Estrogen receptor alpha regulates matrix metalloproteinase-13
promoter activity primarily through the AP-1 transcriptional
regulatory site. Biochim Biophys Acta. 1762:719–731. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gurkan I, Ranganathan A, Yang X, Horton WE
Jr, Todman M, Huckle J, Pleshko N and Spencer RG: Modification of
osteoarthritis in the guinea pig with pulsed low-intensity
ultrasound treatment. Osteoarthritis Cartilage. 18:724–733. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Naito K, Watari T, Muta T, Furuhata A,
Iwase H, Igarashi M, Kurosawa H, Nagaoka I and Kaneko K:
Low-intensity pulsed ultrasound (LIPUS) increases the articular
cartilage type II collagen in a rat osteoarthritis model. J Orthop
Res. 28:361–369. 2009.
|
|
52
|
Park K, Hoffmeister B, Han DK and Hasty K:
Therapeutic ultrasound effects on interleukin-1beta stimulated
cartilage construct in vitro. Ultrasound Med Biol.
33:286–295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Collaborative Group on Hormonal Factors in
Breast Cancer, . Breast cancer and hormone replacement therapy:
Collaborative reanalysis of data from 51 epidemiological studies of
52,705 women with breast cancer and 108,411 women without breast
cancer. Lancet. 350:1047–1059. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Grady D, Gebretsadik T, Kerlikowske K,
Ernster V and Petitti D: Hormone replacement therapy and
endometrial cancer risk: A meta-analysis. Obstet Gynecol.
85:304–313. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rodriguez C, Patel AV, Calle EE, Jacob EJ
and Thun MJ: Estrogen replacement therapy and ovarian cancer
mortality in a large prospective study of US women. JAMA.
285:1460–1465. 2001. View Article : Google Scholar : PubMed/NCBI
|