Introduction
Oligodendroglioma is the third most common type of
intracranial glioma and originates from neuroepithelial cells,
accounting for 2–5% of primary brain tumors and 4–15% of gliomas
(1). The World Health Organization
(WHO) classification system separates oligodendrogliomas
histopathologically into low-grade (WHO II, 77%) and high-grade or
anaplastic (WHO III, 23%) tumor categories. Anaplastic
oligoastrocytomas with necrosis are classified as glioblastomas
(WHO IV) (2). The median survival
times of patients with WHO II and WHO III oligodendrogliomas are
9.8 and 3.9 years, respectively (1,2), and 6.3
and 2.8 years, respectively, if mixed with astrocytes (3,4).
Surgical excision and postoperative adjuvant radiotherapy is the
traditional therapy for oligodendroglioma; however, studies have
observed that, among intracranial tumors, anaplastic
oligodendrogliomas are particularly sensitive to chemotherapy, and
the prognosis of patients treated with chemotherapy is more
favorable than that of patients treated with radiotherapy (5–7). The
preoperative differential diagnosis is, therefore, particularly
important for therapeutic decisions and determining the prognosis
of the patient. Magnetic resonance imaging (MRI) is widely
discussed in the literature and employed in clinical practice
(6); however, it may produce unclear
results that can hinder a definitive diagnosis of
oligodendroglioma. The present study describes a case of
oligodendroglioma in which the results of the MRI examination were
unclear, and pathological analysis was subsequently used to confirm
a diagnosis of atypical anaplastic oligodendroglioma.
Case report
A 34-year-old man who suffered from headache and
right upper-extremity weakness for 2 months was referred to The
Second Affiliated Hospital of Dalian Medical University (Dalian,
China) for medical care. Informed consent was obtained from the
patients family. The patient exhibited no impairment of
consciousness, hearing, vision or sensory perception. Physical
examinations revealed that the patient exhibited decreased hearing
in his left ear, upper-extremity numbness and muscle strength
decline. Axial computed tomography (CT) examination of the brain
showed a large, high-density mass with calcification in the right
parietal-temporal-occipital area, and the top of the mass pressed
against the parieto-occipital subdural matter (Fig. 1A). An MRI scan revealed a
mushroom-shaped mass, which was divided by the Sylvian fissure. The
mass exhibited heterogeneous hypointensity under T1-weighted
imaging (T1WI) and hyperintensity under T2WI and fluid-attenuated
inversion recovery imaging, which was caused by brain parenchyma
deformation, with obvious peritumoral edema (Fig. 1B–D). The mass was heterogeneously
enhanced following the intravenous administration of gadolinium,
with prominent feeding arteries. In addition, the boundary of the
tumor was enhanced, which appeared as blurriness under T2WI
(Fig. 1E). The frontal view of the
internal carotid artery, obtained using digital subtraction
angiography (DSA), did not show an obvious mass with large
arteriovenous shunts or a vascular nidus resembling a true
arteriovenous malformation (AVM); however, the right middle
cerebral artery and the draining vein were thickened. Based on the
presurgical evaluation, it was suggested that the patient had a WHO
grade II or III meningioma or AVM, as the radiological
manifestations were unclear. Due to the patient's impaired function
and the results of the radiological examination, a surgical
resection was performed. During surgery, the partial dura mater was
pushed outward by the tumor with high tension. Notably, soft,
gray-red, cystic, highly liquid tissue with high-transmittance,
with a jelly-like appearance, was expelled instead of adhering to
the dura mater when cut radially. As expected, the insidious tumor
growth boundaries were clear on the surrounding normal brain, which
was covered by slightly yellowish particles on the membrane;
however, the results of the pathological examination of the frozen
section revealed an anaplastic glioma. The residual tumor, which
was gelatinous and pinkish-gray in color, was subsequently
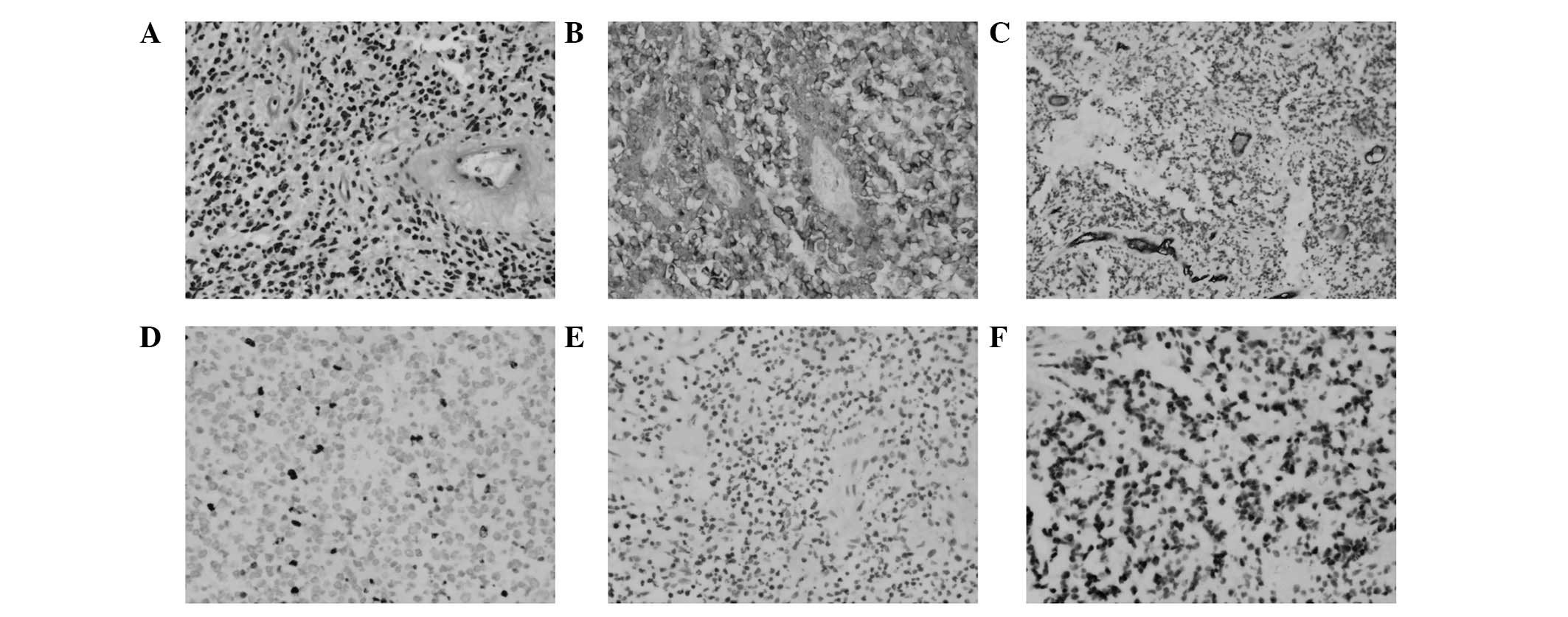
separated in order to achieve subtotal excision. The immunostaining
results (Fig. 2) were positive for
glial fibrillary acidic protein (Fig.
2B); cluster of differentiation (CD) 34, which indicated the
presence of blood vessel proliferation (Fig. 2C); and oligo-2, which is closely
associated with oligodendrogliomas (Fig.
2F), but negative for NeuN (Fig.
2E). The Ki-67 index was 10%, which indicated a high risk of
recurrence (Fig. 2D). Accordingly,
the postsurgical assessment confirmed that the mass was an
anaplastic oligodendroglioma (WHO III), and the postoperative
treatment proceeded with adjuvant radiation and chemotherapy.
Radiographic follow-up evaluation after 6 months revealed no
indications of tumor recurrence.
Discussion
The present case was notable due to the unclear
radiological manifestations, which increased the difficulty of
presurgical diagnosis. The rare growth pattern and morphology of
the oligodendroglioma was also notable.
Oligodendrogliomas typically appear as hypodense
(57–70%) or isodense images under CT examination; however, the
present case had a tendency towards a hyperdense appearance. It is
established that intratumoral calcification is typical for
meningioma; however, in cases of oligodendroglioma the incidence
has been reported to be 50–90% (8,9). The
shape of the calcification can be categorized as coarse, punctate
or linear. The uneven edges of the calcified lesion manifest fairly
discrete margins under CT imaging (2,8). Thus,
in the present case the calcification complicated the differential
diagnosis. Additionally, the tumor was closely but non-aggressively
associated with the calvaria, and the boundary was relatively
clear. It was therefore not possible to differentiate
oligodendroglioma from meningioma based upon CT alone.
Under MRI, the most prominent feature of the present
tumor was the aforementioned mushroom-shape, which penetrated the
cerebral cortex and arachnoid to the subdural space. The region
near the posterior horn of the lateral ventricle represented the
stalk of the mushroom. The cap of the mushroom-shaped tumor grew
along the subdural space and connected with the endocranium to form
a wide base resembling a meningioma under MRI. This type of growth
pattern resembled two lesions at different sections. The stalk part
exhibited invasive growth; conversely, the tumor capsule forming
the cap of the mushroom, which was confirmed during surgery, was
obvious.
There are numerous forms of tumor enhancement,
including nodular and ring. Previous studies have suggested that a
number of anaplastic oligodendrogliomas cannot be enhanced;
however, a few low-grade (WHO II) tumors have been observed to be
enhanced (10,11). The absence of the dural tail sign and
uneven enhancement under enhanced MRI suggest that the possibility
of meningioma is limited.
Peritumoral edema is considered to be a key
indicator of high-grade intracranial tumors; however,
oligodendrogliomas are rarely associated with peritumoral vasogenic
edema. Peritumoral edema cannot, therefore, be used as an indicator
of tumor grading (12). In the
present study, peritumoral edema was evident, but the pattern of
edema was distinct from the finger-like pattern of edema associated
with gliomas; therefore the evidence for a glioma was also
insufficient.
Oligodendrogliomas are closely associated with AVMs,
both in terms of histopathology and radiology (13). In a previous study, a patient was
diagnosed with AVM and received embolization, yet developed a
glioma 10 years later at the same site. Certain lesions appear to
be oligodendrogliomas rich in vessels during preoperative
diagnosis, but are subsequently pathologically confirmed as AVM
through immunohistochemistry (14,15).
Vascular endothelial growth factor (VEGF) receptor, Ki-67 and CD34
have been associated with abnormal tumor angiogenesis, and the
overexpression of VEGF, angiogenesis (contrast enhancement or
endothelial hyperplasia) and absence of seizures are considered
high-risk factors for poor prognosis (13). In the present study, the thick
vascular enhancement on MRI was verified as a vein using DSA; this
vein was before the normal venous drainage on the hemisphere
surface from the sagittal view. Furthermore, the diameter was
~2-fold that of the normal venous drainage (Fig. 1D and F). Therefore, it may be useful
to conduct DSA for cases in which MRI is unable to differentiate
between oligodendroglioma and AVM.
In conclusion, the present study has described a
type of mushroom-shaped anaplastic oligodendroglioma in the
parietal-temporal-occipital region. This mushroom-shaped anaplastic
oligodendroglioma, albeit extremely rare, is a potential source of
misdiagnosis for meningioma and AVM.
Acknowledgements
The authors thank Mr. Hang Yin for his assistance in
the collection of materials.
References
|
1
|
Engelhard HH, Stelea A and Mundt A:
Oligodendroglioma and anaplastic oligodendroglioma: Clinical
features, treatment, and prognosis. Surg Neurol. 60:443–456. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
HoangXuan K, Capelle L, Kujas M,
Taillibert S, Duffau H, Lejeune J, Polivka M, Crinière E, Marie Y,
Mokhtari K, et al: Temozolomide as initial treatment for adults
with low-grade oligodendrogliomas or oligoastrocytomas and
correlation with chromosome 1p deletions. J Clin Oncol.
22:3133–3138. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shaw EG, Scheithauer BW, O'Fallon JR and
Davis DH: Mixed oligoastrocytomas: A survival and prognostic factor
analysis. Neurosurgery. 34:577–582. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perry JR: Oligodendrogliomas: Clinical and
genetic correlations. Curr Opin Neurol. 14:705–710. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jenkinson MD, du Plessis DG, Smith TS,
Joyce KA, Warnke PC and Walker C: Histological growth patterns and
genotype in oligodendroglial tumours: Correlation with MRI
features. Brain. 129:1884–1891. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mason WP and Cairncross JG: Invited
article: The expanding impact of molecular biology on the diagnosis
and treatment of gliomas. Neurology. 71:365–373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee YY and Van Tassel P: Intracranial
oligodendrogliomas: Imaging findings in 35 untreated cases. AJR Am
J Roentgenol. 152:361–369. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dolinskas CA and Simeone FA: CT
characteristics of intraventricular oligodendrogliomas. AJNR Am J
Neuroradiol. 8:1077–1082. 1987.PubMed/NCBI
|
|
10
|
Ginsberg LE, Fuller GN, Hashmi M, Leeds NE
and Schomer DF: The significance of lack of MR contrast enhancement
of supratentorial brain tumors in adults: Histopathological
evaluation of a series. Surg Neurol. 49:436–440. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
White ML, Zhang Y, Kirby P and Ryken TC:
Can tumor contrast enhancement be used as a criterion for
differentiating tumor grades of oligodendrogliomas? AJNR Am J
Neuroradiol. 26:784–790. 2005.PubMed/NCBI
|
|
12
|
Spampinato MV, Smith JK, Kwock L, Ewend M,
Grimme JD, Camacho DL and Castillo M: Cerebral blood volume
measurements and proton MR spectroscopy in grading of
oligodendroglial tumors. AJR Am J Roentgenol. 188:204–212. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Quon H, Hasbini A, Cougnard J, Djafari L,
Lacroix C and Abdulkarim B: Assessment of tumor angiogenesis as a
prognostic factor of survival in patients with oligodendroglioma. J
Neurooncol. 96:277–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McKinney JS, Steineke T, Nochlin D and
Brisman JL: De novo formation of large arteriovenous shunting and a
vascular nidus mimicking an arteriovenous malformation within an
anaplastic oligodendroglioma: Treatment with embolization and
resection. J Neurosurg. 109:1098–1102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gmeiner M, Sonnberger M, Wurm G and Weis
S: Glioblastoma with the appearance of arteriovenous malformation:
Pitfalls in diagnosis. Clin Neurol Neurosurg. 115:501–506. 2013.
View Article : Google Scholar : PubMed/NCBI
|