Introduction
Triple-negative breast cancer (TNBC), which
comprises 15–20% of all breast cancers, has a low survival rate due
to drug resistance. It is imperative for new therapeutic targets to
be identified in order to improve the outlook for these patients.
Epithelial to mesenchymal transition (EMT) plays a critical role in
drug resistance (1). EMT is a
process by which epithelial cells lose their cell polarity and
cell-cell adhesion, and gain migratory and invasive properties. The
process also controls chemoresistance and immune escape (2).
Tissue transglutaminase (TG2) belongs to the family
of transglutaminase enzymes that are active in the presence of
Ca2+, and catalyze Ca2+-dependent protein
crosslinking via the formation of amide bonds (3). It has been reported that TG2 induces
EMT in MCF10A and MCF12A mammary epithelial cells (4). Docetaxel (TXT) is one of the most
effective chemotherapy drugs and TXT chemotherapy is widely used to
treat breast cancer.
When the clinical and biological significance of TG2
was examined in ovarian cancer (5),
knockdown of the TG2 gene (TGM2) of HeyA8 cells with small
interfering (si)RNA strongly promoted TXT-induced cell death. Mouse
model studies indicate that TG2 is a potential therapeutic target
for chemo-resistant ovarian cancer (5). The combination of TGM2-siRNA and TXT
with chitosan hydrogel facilitated a greater inhibition of cancer
growth compared with that achieved using TXT and chitosan hydrogel
(92 vs. 55% reduction; P<0.001) (6).
Drug resistance poses a major challenge to the
treatment of breast cancer and until now little has been known
about the role that TG2 plays in breast cancer. It may be
speculated that TG2 is involved in EMT and TXT-induced cell death
in breast cancer. To better understand the effect of TG2 on drug
resistance, the role of TG2 in EMT and drug resistance in breast
cancer was examined in the present study. Breast cancer cells were
modified by TGM2-specific RNA interference (RNA)i and the effect of
TG2 on the sensitivity of breast cancer to TXT was investigated
in vitro and in xenograft tumor models in nude mice.
Materials and methods
Cell line and materials
MDA-MB-231 TNBC cells were purchased from the
Shanghai Institute of Biochemistry and Cell Biology, Chinese
Academy of Sciences (Shanghai, China) and cultured in L-15 medium
(WISENT, Inc., Nanjing, China) supplemented with 10% fetal bovine
serum (FBS) and 1% penicillin-streptomycin (10,000 U/ml penicillin
and 10 mg/ml streptomycin) at 37°C in a humidified atmosphere
(CO2 was not present).
An antisense lentiviral (LV) RNAi vector targeting
the TGM2 gene with short hairpin (sh)RNA (TGM2-shRNA-LV) was
designed, synthesized and stably transfected into MDA-MB-231 cells,
which subsequently expressed low levels of TG2. The targeting
sequence 5′-GCA GTG ACT TTG ACG TCT T-3′ was designed to target the
TGM2 gene (GenBank accession No. NM_004613), and was cloned into
the lentiviral vector GV115 (Shanghai GeneChem Co. Ltd., Shanghai,
China). The specificity was confirmed by a BLAST search of the
GenBank database (http://www.ncbi.nlm.nih.gov/genbank/). A green
fluorescent protein lentiviral vector containing a non-effective
(scrambled) shRNA cassette served as a negative control for gene
downregulation.
TXT was purchased from Jiangsu Hengrui Medicine Co.
Ltd. (Lianyungang, China). The MDA-MB-231 cells were divided into
the RNAi (TGM2-shRNA) and NC (scrambled shRNA) groups and the
expression levels of TG2, E-cadherin, vimentin and Bcl-2 in the
cells were examined via western blotting.
Western blot analysis
Cultured cells were washed and harvested in a lysis
solution containing SDS and NP-40 (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China). The proteins were separated
by SDS-PAGE and transferred to a polyvinylidene difluoride membrane
by electroblotting, and then blocked with 2.5% non-fat milk in
Tris-buffered saline with Tween 20 for 2 h at room temperature. The
membranes were then probed with the relevant mouse monoclonal
antibodies overnight at 4°C: Anti-TG2 (CUB7402), anti-E-cadherin
(HECD-1), anti-Bcl-2 (Bcl2/100), anti-GAPDH (9484; Abcam,
Cambridge, MA, USA) and anti-vimentin (RV202; Santa Cruz
Biotechnology, Inc., La Jolla, CA, USA), which were diluted
according to the manufacturer's recommendations. Washing steps were
performed using 0.1% Tris-buffered saline and Tween (5 min × 3).
Secondary antibodies were diluted 1:2,000 and incubated for 2 h at
room temperature. Immunoreactive proteins were detected via western
blot enhanced chemiluminescence (ECL Plus; Beijing Solarbio Science
& Technology Co., Ltd.). Quantitative densitometry of the
electrophoretic bands images was conducted using Image J software
(National Institutes of Health, Bethesda, MD, USA). Intensity
levels of the target protein were then normalized against those of
GAPDH, the house keeping protein.
Detection of cell proliferation by MTT
assay
Cells were passed through no. 400 stainless steel
meshes and inoculated into 96-well plates at 1.5×103
cells/well. The cells were divided into four groups, two of which
were treated with 3.7 µg/ml TXT. These were the NC, RNAi, TXT (NC +
TXT) and RNAi + TXT groups. Cells were cultured at 37°C for 24, 48
or 72 h, following which MTT was added. After another 4 h of
culturing, the complete medium containing the drug and the
unconverted MTT was removed, 200 µl dimethyl sulfoxide was added to
each well, and the absorbance of each well was read at 570 nm using
an ELISA microplate reader (ST-360; KHB, Shanghai, China). The
growth inhibition rate of the tumor cells was then calculated using
the following formula: Inhibition rate (%) = [1-optical density
(OD) drug exposure/OD control] × 100. The effective anticancer
activity was regarded as sensitive. All experiments were repeated
four times under the same conditions.
Detection of cell apoptosis by flow
cytometry
The transfected cells were seeded into 25 ml flasks.
They were treated with TXT at a concentration of 3.7 µg/ml (the
average peak plasma concentration) when they covered 80% of the
flask, or were untreated. Following 48 h of treatment, the cells
were washed with phosphate-buffered saline (PBS), digested with
trypsin, pelleted by centrifugation at 300 × g for 10 min,
resuspended in an EP tube with PBS, and fixed and permeabilized
following the addition of 2 ml ice-cold 70% ethanol. The cells were
washed three times in PBS and Annexin V/propidium iodide (ROTRN
Shanghai Biological Technology Co., Ltd., Shanghai, China) was
added. The cells were stored at 4°C, allowed to stand at room
temperature in the dark for 3 min, and then filtered through
400-mesh filter traps, prior to analysis of cell apoptosis using a
BD FACSCanto II flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA).
Animals and xenograft tumor model
study
Animals
The animal experiments were approved by the Ethics
Committee of Guangxi Medical University (Nanning, China). Female
Balb/c nude mice were purchased from the Medical Laboratory Animal
Center of Guangxi Medical University and maintained at the animal
laboratory under specific pathogen-free conditions. Six-week old
female nude mice, each weighing 20–25 g, were injected with cells
into a fat pad of mammary gland. Mice were housed in an animal
room, with water and food freely available. The mice (n=24) were
randomly divided into four experimental groups (n=6 per group).
Xenograft tumor model study
The antitumor effect of combination therapy with
TGM2-shRNA and TXT on human breast cancer xenografts derived from
MDA-MB-231 cells was investigated. To evaluate the antitumor
effects of TGM2-shRNA and TXT in vivo, 5×106
MDA-MB-231 cells transfected with scrambled shRNA or TGM2-shRNA
vector (in 100 µl PBS, pH 7.3) were injected subcutaneously and
once daily for 3 days into the right thoracic mammary fat pad of
the 6-week-old female nude mice. Following the injection, the mice
were monitored daily for tumor volume and survival. After 2 weeks,
all 24 animals showed tumor growth, and PBS or TXT (10 mg/kg) was
injected intraperitoneally on day 1 of every week. The tumor volume
was measured by vemier caliper every 3 days, and calculated
according to the formula: Tumor volume = π/6 × (width ×
length2), where width and length are the shortest and
the longest diameters of the tumor, respectively. The measurements
for the MDA-MB-231 xenografts were continued for 3 weeks.
Examination of TG2 expression within tumors
Immunohistochemical analysis was carried out to
explore whether TGM2-shRNA effectively downregulated the expression
of TG2 in vivo. Xenografted tumors from sacrificed nude mice
were collected and fixed in 10% formalin for immunohistochemical
analysis after weighing and measuring.
The analysis of the TG2 expression was conducted
using the TG2 mouse monoclonal Ab. The total staining of TG2 was
evaluated according to the percentage and intensity of cells with
TG2 cytoplasm staining.
Statistical analysis
Data were analyzed with SPSS software (version 17.0;
SPSS Inc., Chicago, IL, USA). Student's t-test was used for
quantitative results. The χ2 and Fisher's exact tests
were used to compare the expression of TG2 within tumors grown in
nude mice. Values of P<0.05 were considered to indicate a
statistically significant difference.
Results
Morphological appraisal of breast
cancer cells
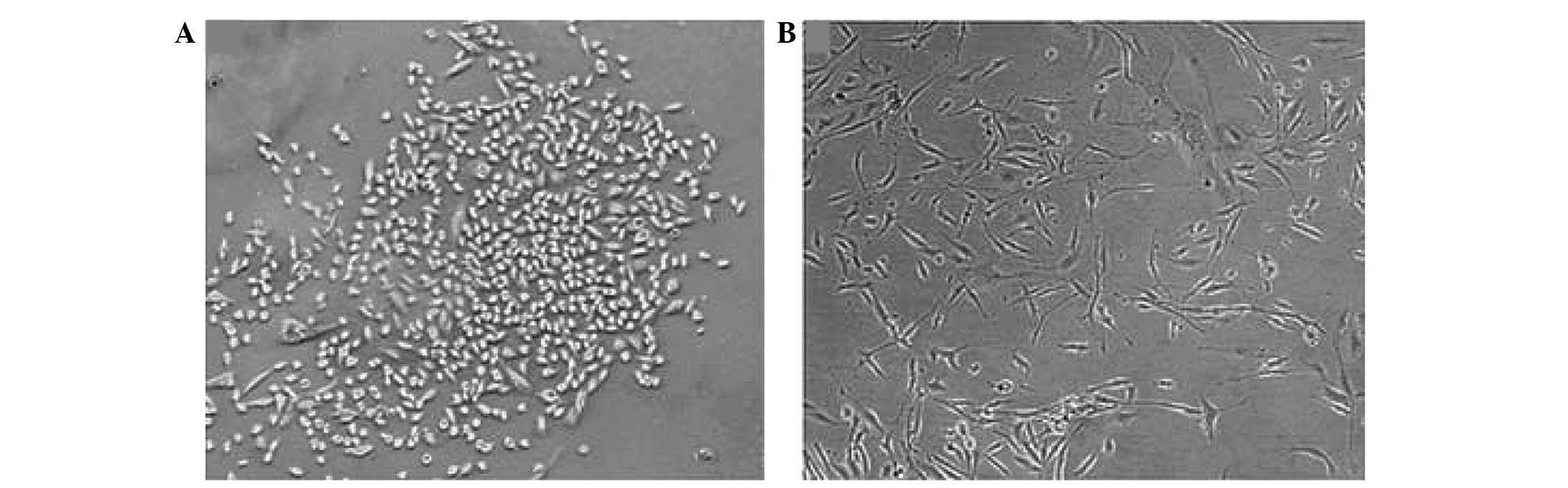
Following gene silencing of TGM2, the MDA-MB-231
cells showed changes in morphology. The MDA-MB-231 cells in the NC
group remained elongated and dispersed whereas the cells in the
TGM2-shRNA group were rounded with cobblestone epithelial
morphology, and were organized in compact structures (Fig. 1). These data suggest that an increase
in the expression of TG2 is associated with a mesenchymal
morphology (7).
Expression of TG2, E-cadherin,
vimentin and Bcl-2 in MDA-MB-231 cells
To analyze the biological consequences of reduced
TG2 expression in MDA-MB-231 cells, the expression of endogenous
TG2 in MDA-MB-231 cells was inhibited by RNAi and the expression of
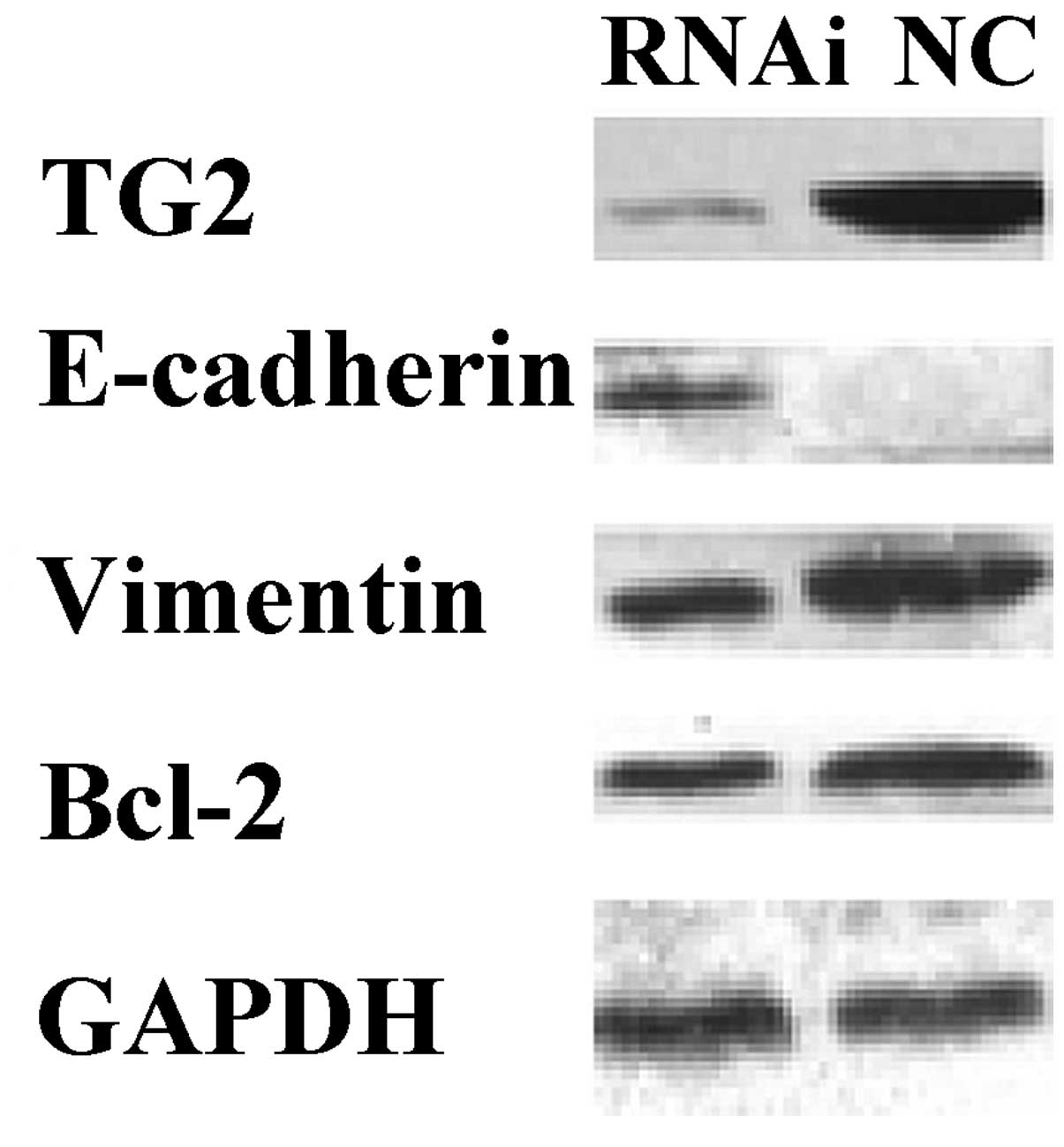
TG2, E-cadherin, vimentin and Bcl-2 was detected. Western blot
analysis demonstrated that once MDA-MB-231 cells had been stably
transfected with TGM2-shRNA for 96 h, the expression levels of TG2,
vimentin and Bcl-2 were significantly downregulated and the
expression level of E-cadherin was significantly upregulated,
compared with the levels in the NC group (P<0.05; Fig. 2).
Proliferation of breast cancer
cells
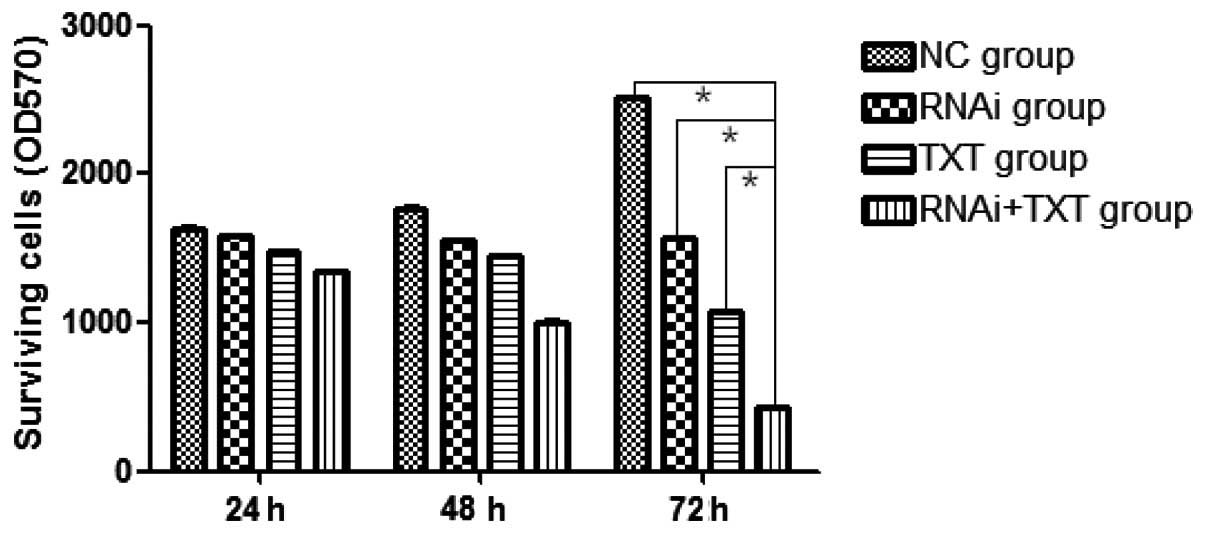
The transfected MDA-MB-231 cells were cultured in
the presence or absence of TXT for 24, 48 or 72 h, and the results
showed that the proliferation of the breast cancer cells was
strongly inhibited in the RNAi + TXT group, with the difference
between the RNAi + TXT group and the other groups being
statistically significant at 72 h (P<0.05). In addition, the
inhibitory level increased in a time-dependent manner (Fig. 3).
Apoptosis rate of MDA-MB-231
cells
Following treatment with TXT for 48 h, the apoptosis
of MDA-MB-231 cells was significantly promoted in the RNAi + TXT
group (27.93±1.1%) compared with the TXT group (12.39±0.67%)
(P<0.05). RNAi (6.82±0.12%) and TXT alone also promoted the
apoptosis of breast cancer cells, however, the effect of RNAi + TXT
was the strongest (Fig. 4).
Downregulation of TG2 improved the anticancer effect of TXT.
Knockdown of TG2 with shRNA promoted TXT-induced
cell death. The combination therapy of TGM2-shRNA with TXT was
observed to be more effective than treatment with TXT or RNAi alone
or no treatment. This indicates that there are synergistic effects
between TGM2-shRNA and TXT, and that the delivery of TGM2-shRNA
augmented the TXT-induced apoptosis.
Anticancer effect of combined
treatment with TGM2-shRNA and TXT on MDA-MB-231 xenografts
Expression of TG2 within tumors was detected
Immunohistochemical detection of TG2 expression was
performed in tissue samples from the xenograft tumors. TG2 was
strongly expressed in xenograft tumors from the TXT and NC groups
while it was significantly downregulated within tumors from the
RNAi and RNAi + TXT groups, which demonstrated that TGM2-shRNA
effectively downregulated the expression of TG2 in vivo
(P<0.05; Fig. 5).
Average tumor volume
Tumor volume is used to assess the response to
antitumor therapy in animal studies. The antitumor effects of the
combined treatment with shRNA and TXT were analyzed in a nude mouse
model in the present study.
Tumor volume measurements indicated that tumor
xenograft growth was suppressed by TGM2-shRNA and TXT. In the RNAi
+ TXT group the tumor volume remained low, whereas tumors of the
TXT group increased in volume. The administration of TXT alone
inhibited tumor growth by ~28% and the administration of TGM2-shRNA
alone inhibited tumor growth by ~23% compared with that in the
control group, while the combination of TGM2-shRNA and TXT
exhibited stronger antitumor effects than either treatment alone,
suppressing tumor growth by ~74% (Fig.
6).
In the RNAi + TXT group, the strongest antitumor
effect was observed. The data showed that the combination of
TGM2-shRNA and TXT significantly enhanced antitumor activity in
MDA-MB-231 xenografts, compared with TXT monotherapy, consistent
with the results of the in vitro study. TGM2-shRNA
effectively modulated the chemosensitivity of breast cancer to
TXT.
Discussion
EMT is characterized by the acquisition of a
fibroblast-like cell morphology, the dissolution of tight
junctions, cell scattering, the loss of epithelial markers
including E-cadherin, and the gain of mesenchymal markers such as
vimentin (8,9). MET is the reverse process of EMT, and
is a process by which motile mesenchymal cells are converted to
polarized epithelial cells. Mesenchymal cells have the ability,
which true epithelia do not, to invade and migrate as individual
cells through the extracellular matrix constructed by epithelial
sheets and by mesenchymal cells themselves (10). As shown in Fig. 1, cells transfected with TGM2-shRNA
were rounded with cobblestone epithelial morphology, and organized
in tight structures that adopted features of epithelial cells.
These characteristics induce adhesion, restrict motility, promote
intercellular communication and are frequently identified as
well-differentiated (11). Silencing
of TGM2 with shRNA changed the expression of EMT markers, such as
E-cadherin and vimentin.
The data presented in the present study suggest that
the changes observed in TG2-expressing cells were associated with a
mesenchymal phenotype. Downregulation of TG2 induces changes
associated with EMT, for example, in morphology and EMT-relevant
markers, which control chemoresistance (2).
When MDA-MB-231 cells were stably transfected with
TGM2-shRNA, the expression of Bcl-2 was significantly
downregulated. Bcl-2 is generally regarded as an important
anti-apoptotic protein and thus falls into the category of an
oncogene. Upregulation of Bcl-2 is common in breast carcinoma
(12). TXT-induced apoptosis is
linked to the inactivation of Bcl-2 (13). Bcl-2 confers resistance to apoptosis,
and prevents TXT-induced apoptosis, thereby reducing the
effectiveness of chemotherapy (14,15). The
level of TG2 is associated with the expression of Bcl-2 (16): Silencing TGM2 downregulates the level
of Bcl-2 (17). The compound
rotterlin regulates downstream proteins, including Bcl-2 and
nuclear factor-κB, through the TG2/protein kinase C δ axis
(18). Thus, silencing TGM2 may
promote apoptosis and increase the chemosensitivity of MDA-MB-231
cells to TXT by downregulating Bcl-2.
The MTT proliferation assay is broadly used to study
the induction and inhibition of cell proliferation, and drug
resistance in an in vitro model (19). The transfected MDA-MB-231 cells were
treated with TXT and the proliferation inhibition rate of the cells
was measured by MTT assay. In the RNAi + TXT group, the
proliferation of MDA-MB-231 cells was significantly inhibited
compared with that in the control group (P<0.05), and the level
of inhibition increased in a time-dependent manner. Thus, the
silencing of TGM2 in breast cancer was found to be associated with
decreased tumor cell growth, and improved the anticancer effect of
the chemotherapy drug TXT.
The apoptosis of MDA-MB-231 cells was significantly
promoted in the RNAi + TXT group compared with the control group.
These results suggest that the knockdown of TGM2 in breast cancer
is associated with an increased antitumor effect of chemotherapy.
Downregulation of TG2 expression promoted TXT-induced cell death.
The combination therapy of TGM2-shRNA with TXT was more effective
than TXT monotherapy. Hwang et al (5) reported that the combination of TG2
siRNA with TXT in ovarian cancer had a greater efficacy that
control siRNA with TXT.
In the present study, treatment with TGM2-shRNA
upregulated E-cadherin expression, and downregulated vimentin and
Bcl-2 expression in MDA-MB-231 cells. In addition, it significantly
increased the TXT-induced inhibition of proliferation and the
apoptosis of TNBC cells in vitro. The tumor inhibition rate
of the TXT group reached ~38%, compared with that of the combined
treatment group. The percentage of tumor inhibition was calculated
according to the formula [1 − (R/N)] × 100, where R and N represent
the mean tumor volumes of the RNAi and NC groups, respectively.
Compared with treatment with TXT or RNAi alone, tumor progression
was delayed in the RNAi + TXT group. The combination of TGM2-shRNA
with TXT demonstrated significant antitumor activity in
vivo. These results indicate that the TXT-sensitizing effect of
TGM2-knockdown on mammary cancer cells in vitro also took
place in vivo. Combination treatment with TGM2-shRNA and TXT
appears to be effective against TNBC since TGM2 silencing was found
to be effective for the inhibition of cancer cell proliferation in
two models of TXT-resistant TNBC.
Numerous lines of evidence supported the role of TG2
overexpression in the pathogenesis and poor clinical outcome of
breast cancer. Assi et al (20) reported that patients with stromal TG2
accumulation had significantly decreased disease-free survival
(DFS; mean DFS, 110 months) compared with patients with low TG2
expression (mean DFS, 130 months). On the basis of these
observations, it may be hypothesized that high TG2 expression
levels correlate with poor clinical outcome in patients with breast
cancer, which leads to a decreased sensitivity to TXT chemotherapy.
Downregulation of TG2 induces changes associated with MET, promotes
apoptosis and increases the chemosensitivity of breast cancer.
In conclusion, the current data indicate the
important roles of TG2 in the promotion of EMT and drug resistance.
Downregulation of TG2 appears to reverse EMT and increase the
chemosensitivity of breast cancer to TXT. However, clinical trials
are required to clarify whether TG2 expression may be helpful in
predicting the treatment efficacy of TXT in patients with breast
cancer.
Acknowledgements
The present study was supported by the Innovation
Project of Guangxi Graduate Education of China in 2014 (no.
YCBZ2014030).
References
|
1
|
Sarkar FH, Li Y, Wang Z and Kong D:
Pancreatic cancer stem cells and EMT in drug resistance and
metastasis. Minerva Chir. 64:489–500. 2009.PubMed/NCBI
|
|
2
|
Huang RY, Wong MK, Tan TZ, Kuay KT, Ng AH,
Chung VY, Chu YS, Matsumura N, Lai HC, Lee YF, et al: An EMT
spectrum defines an anoikis-resistant and spheroidogenic
intermediate mesenchymal state that is sensitive to e-cadherin
restoration by a src-kinase inhibitor, saracatinib (AZD0530). Cell
Death Dis. 4:e9152013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yakubov B, Chen L, Belkin AM, Zhang S,
Chelladurai B, Zhang ZY and Matei D: Small molecule inhibitors
target the tissue transglutaminase and fibronectin interaction.
PLoS One. 9:e892852014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumar A, Xu J, Brady S, Gao H, Yu D,
Reuben J and Mehta K: Tissue transglutaminase promotes drug
resistance and invasion by inducing mesenchymal transition in
mammary epithelial cells. PLoS One. 5:e133902010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hwang JY, Mangala LS, Fok JY, Lin YG,
Merritt WM, Spannuth WA, Nick AM, Fiterman DJ, VivasMejia PE,
Deavers MT, et al: Clinical and biological significance of tissue
transglutaminase in ovarian carcinoma. Cancer Res. 68:5849–5858.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han HD, Mora EM, Roh JW, Nishimura M, Lee
SJ, Stone RL, BarEli M, LopezBerestein G and Sood AK: Chitosan
hydrogel for localized gene silencing. Cancer Biol Ther.
11:839–845. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shao M, Cao L, Shen C, Satpathy M,
Chelladurai B, Bigsby RM, Nakshatri H and Matei D:
Epithelial-to-mesenchymal transition and ovarian tumor progression
induced by tissue transglutaminase. Cancer Res. 69:9192–9201. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nelson CM, Khauv D, Bissell MJ and Radisky
DC: Change in cell shape is required for matrix
metalloproteinase-induced epithelial-mesenchymal transition of
mammary epithelial cells. J Cell Biochem. 105:25–33. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie L, Law BK, Chytil AM, Brown KA, Aakre
ME and Moses HL: Activation of the Erk pathway is required for
TGF-beta1-induced EMT in vitro. Neoplasia. 6:603–610. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oakes SR, Vaillant F, Lim E, Lee L,
Breslin K, Feleppa F, Deb S, Ritchie ME, Takano E, Ward T, et al:
Sensitization of BCL-2-expressing breast tumors to chemotherapy by
the BH3 mimetic ABT-737. Proc Natl Acad Sci USA. 109:2766–2771.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nehmé A, Varadarajan P, Sellakumar G,
Gerhold M, Niedner H, Zhang Q, Lin X and Christen RD: Modulation of
docetaxel-induced apoptosis and cell cycle arrest by
all-trans retinoic acid in prostate cancer cells. Br J
Cancer. 84:1571–1576. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Emi M, Kim R, Tanabe K, Uchida Y and Toge
T: Targeted therapy against Bcl-2-related proteins in breast cancer
cells. Breast Cancer Res. 7:R940–R952. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mhaidat NM, Wang Y, Kiejda KA, Zhang XD
and Hersey P: Docetaxel-induced apoptosis in melanoma cells is
dependent on activation of caspase-2. Mol Cancer Ther. 6:752–761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho SY, Jeong EM, Lee JH, Kim HJ, Lim J,
Kim CW, Shin DM, Jeon JH, Choi K and Kim IG: Doxorubicin induces
the persistent activation of intracellular transglutaminase 2 that
protects from cell death. Mol Cells. 33:235–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SJ, Kim KH, Ahn ER, Yoo BC and Kim SY:
Depletion of cathepsin D by transglutaminase 2 through protein
cross-linking promotes cell survival. Amino Acids. 44:73–80. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maioli E, Torricelli C and Valacchi G:
Rottlerin and cancer: Novel evidence and mechanisms.
ScientificWorldJournal. 2012:3508262012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Meerloo J, Kaspers GJ and Cloos J:
Cell sensitivity assays: The MTT assay. Methods Mol Biol.
731:237–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Assi J, Srivastava G, Matta A, Chang MC,
Walfish PG and Ralhan R: Transglutaminase 2 overexpression in tumor
stroma identifies invasive ductal carcinomas of breast at high risk
of recurrence. PLoS One. 8:e744372013. View Article : Google Scholar : PubMed/NCBI
|