Introduction
Hyperpigmentation is commonly observed in humans
following skin damage and during the healing response; it includes
post-inflammatory hyperpigmentation after burns, wounds or laser
surgery. Hyperpigmentation is a major concern for the majority of
people it affects as it induces significant cosmetic problems that
may affect quality of life. However, the treatment of
hyperpigmentation remains a challenge and the results are
discouraging. Recently, a number of studies have focused on pigment
cells and wound healing (1–4); however, knowledge of skin
repigmentation following cutaneous injury remains sparse.
The role of angiotensin II (Ang II) in the control
of systemic blood pressure and volume homeostasis is well known and
has been extensively studied (5,6).
Recently, it was suggested that Ang II is also involved in the
healing of skin wounds. It has been proposed that exogenously
administered Ang II is a potent accelerator of cutaneous wound
repair (7,8). These reports indicated that the topical
administration of Ang II accelerated skin wound healing by
stimulating dermal repair, including angiogenesis and epidermal
repair, in vivo. Furthermore, studies have demonstrated that
Ang II is angiogenic and modulates the proliferation, growth factor
production and chemotaxis of numerous cell types (8–10).
Steckelings et al (11)
reported that human skin expresses Ang II type 1 (AT1) and type 2
(AT2) receptors, and that AT1 and AT2 receptor expression is
markedly enhanced within the epidermal and dermal areas of scar
tissue (12). A previous study
reported that inhibiting the AT1 receptor limited murine melanoma
growth (13). In addition,
Steckelings et al (11)
detected the expression of AT1 mRNA in cultured primary
melanocytes, suggesting its possible function in melanocytes.
Pigment production is complex and controlled by
numerous extrinsic and intrinsic factors involved in melanin
synthesis and melanocyte transport. Melanogenesis is controlled by
complex regulatory mechanisms. The genes encoding tyrosinase (TYR)
and TYR-related proteins contain common transcription initiation
sites, particularly microphthalmia-associated transcription factor
(MITF) binding sites. MITF serves a crucial function in the
transcriptional regulation of melanogenesis (14). The intracellular signal transduction
pathways of cyclic adenosine monophosphate (cAMP), protein kinase C
(PKC) and nitrogen oxide (NO) are involved in the regulation of
melanogenesis (15). A number of
previous studies have indicated that cAMP and PKC are involved in
key signal transduction pathways that participate in the regulation
of melanogenesis (16–18). In the cardiovascular system, Ang II
shares certain subcellular pathways with the following pathways,
widely interacting with them: AT1, PKC, and
Na+/H+ exchange (NHE) (19), endothelin-1 (ET-1) and NO (20) and cAMP (21). The present study was based on the
hypothesis that Ang II may be able to modulate melanocyte
melanogenesis via different pathways. To clarify this issue, the
effects of Ang II on human melanocyte melanogenesis were
investigated in order to elucidate the possible mechanisms
involved.
Materials and methods
Compounds and drugs
Mouse polyclonal antibodies against MITF (sc-56725)
and TYR (sc-20035), and horseradish peroxidase (HRP)-conjugated
goat anti-rabbit or anti-mouse antibodies (sc-2005) were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). A
protein quantification kit and agarose were purchased from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA). PCR Master Mix was
purchased from Promega Corporation (Madison, WI, USA).
Phosphate-buffered saline (PBS), M254 medium and human melanocyte
growth supplements were purchased from Cascade Biologics, Inc.
(Mansfield, UK). Fetal bovine serum (FBS) and an RNeasy Mini kit
were from Qiagen, Inc. (Valencia, CA, USA). Ang II, H-89 (PKA
inhibitor) and Ro-32-0432 (PKC inhibitor) were from EMD Millipore
(Billerica, MA, USA). L-3,4-dihydroxyphenylalanine (L-DOPA), EDTA,
glycine, sodium dodecyl sulfate (SDS) and Tris were purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Melanocyte culture
The present study was ethically approved by the
General Hospital of Beijing Military Region of PLA. Primary
melanocyte cultures were established as follows: Normal melanocytes
were isolated from the epidermis of human foreskins. Skin grafts
were cut into 5×5 mm pieces and incubated with trypsin-EDTA (0.25%
trypsin, 0.02% EDTA) at 4°C overnight. Trypsin activity was
required to separate the epidermis from the dermis, via
intercellular detachment. The next day, trypsin activity was
neutralized by adding FBS in a 1:1 ratio and replacing it with PBS
solution. The epidermis was separated from the dermis using sterile
forceps. The specimens were pipetted thoroughly to separate the
cells and form cell-rich suspensions. The solid waste tissue was
removed and the suspension was centrifuged at 1,000 × g for 5 min.
Then, melanocytes were selectively grown in M254 medium containing
human melanocyte growth supplements. The cell number was adjusted
to 2.5×104 cells/cm2. Cultures were
maintained at 37°C in a humidified 95% air and 5% CO2
atmosphere. The medium was changed at 2–3-day intervals, and the
cultures were routinely examined for contamination and cell
outgrowth. Cells were split at confluence using 5-min trypsin
treatment at room temperature. Subculturing was conducted once a
week and experiments were carried out from passages II–IV.
Treatment with Ang II alone or in
combination with H-89 or Ro-32-0432
Confluent cells were plated at subconfluent
densities (2×105 cells) in 6-well plates and grown for 4
days until confluent. Then, they were treated with different
concentrations (0.01, 0.1, 1, 10 and 100 nM) of Ang II for 24 h. In
some experiments, the cells were incubated with 1 µM H-89 or
Ro-32-0432 for 60 min prior to stimulation with 100 nM Ang II. The
culture medium was removed and the cells were washed twice with
PBS. Then, fresh assay medium supplemented with 0.1% FBS was added
for 24 h. Subsequently, the melanin content assay was performed,
and TYR activity and melanin content were determined. Cell
homogenates and supernatants were collected for RNA extraction
using the RNeasy Mini kit and for protein quantification using the
kit from Bio-Rad Laboratories, Inc. by a procedure based on the
Bradford method.
TYR activity assay
Melanocytes were treated with Ang II for the
indicated durations, and then washed with ice-cold 1X PBS. Lysis
buffer, containing 150 µl 1% Triton X-100 in 0.1 M phosphate
buffer, was added to each 6-well plate. Cells were scraped and
transferred to a 1.5-ml tube, lysed using 3–5 freeze-thaw cycles in
liquid nitrogen and centrifuged at 15,000 × g for 5–10 min at 4°C.
Samples (300–500 µg/80 µl) were transferred to a new 96-well plate
on ice. L-DOPA (20 µl, 5 mM) was added to each well, the plate was
incubated at 37°C for 1 h and the absorbance was measured at 475 nm
using a DU-70 spectrophotometer (Beckman Coulter, Brea, CA,
USA).
Melanin content assay
Melanin content was determined using a standard
procedure (22). Cells were lysed
with 200 µl 1 N NaOH and pipetted repeatedly to homogenize them.
Then, the cell extract was transferred to 96-well plates. Relative
melanin content was determined by absorbance at 405 nm using a
Synergy H1MF enzyme-linked immunosorbent assay plate reader
(BioTek, Winooski, VT, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA extraction and reverse transcriptase
reaction were performed as described previously (23). The MITF and TYR mRNA expression
levels were evaluated using RT-qPCR. Total RNA was extracted at the
indicated time points using a TRIzol kit (Invitrogen Life
Technologies, Carlsbad, CA, USA) and reverse-transcribed using an
RT kit (Toyobo Co., Ltd., Osaka, Japan). Semi-quantitative PCR was
performed using PCR Master Mix and primers for MITF and TYR;
Table I lists the primer sequences
and amplicon size. PCR was performed using a touchdown protocol as
previously described (24). Briefly,
touchdown PCR was performed using the following program: 1 cycle at
94°C for 2 min, 12 cycles at 92°C for 20 sec, 68°C for 30 sec and
70°C for 45 sec with a reduction of 1°C per cycle, and 22 cycles at
92°C for 20 sec, 55°C for 30 sec and 70°C for 45 sec. PCR products
were separated by electrophoresis on 2% agarose gels and visualized
with ethidium bromide staining. PCR band intensity was expressed as
the relative intensity against β-actin.
 | Table I.Primers used in reverse
transcription-quantitative polymase chain reaction. |
Table I.
Primers used in reverse
transcription-quantitative polymase chain reaction.
| Primer | Sequence (5′-3′) | Size (bp) |
|---|
| MITF | F:
CACAACCTGATTGAACGAAG |
|
|
| R:
GTGGATGGAATAAGGGAAAG | 284 |
| TYR | F:
ACGATGTGGACGAGTGT |
|
|
| R:
CAGAGGCAGGTGAAGGT | 133 |
| β-actin | F:
ATCATGTTTGAGACCTTCAACA |
|
|
| R:
CATCTCTTGCTCGAAGTCCA | 318 |
|
Western blot analysis
MITF and TYR protein expression was evaluated using
western blot analysis. Isolated human foreskin sheets were
homogenized using a pestle in lysis buffer and centrifuged for 30
min at 15,000 × g at 4°C. The supernatant was collected and protein
concentration determined using the protein quantification kit,
based on the bicinchoninic acid method. Proteins (20 µg) were
denatured with SDS sample buffer, boiled for 5 min and separated in
10–12% polyacrylamide gels (Novex; Life Technologies, Grand Island,
NY, USA). Following electrophoresis, proteins were transferred in
1X transfer buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, 20%
methanol, pH 8.4) to a 0.45-µm Immobilon-P polyvinylidene
difluoride membrane (EMD Millipore) in a Mini PROTEAN II Transfer
Cell (Bio-Rad Laboratories, Inc.) set at a constant voltage of 120
mV for 2 h. Membranes were blocked in 5% non-fat dry milk in
Tris-buffered saline (TBS) solution for ≥1 h at room temperature.
Blots were incubated overnight at 4°C with polyclonal mouse
anti-MITF (1:1,1000) or anti-TYR (1:1,000) antibodies. Membranes
were washed three times with TBS containing 1% Triton X-100
(TBS-T), incubated with HRP-conjugated goat anti-mouse antibodies
(1:2,000) for 2 h at room temperature, then washed 4 times with
TBS-T. Immunoreactive bands were visualized by exposing membrane
blots to a chemiluminescent solution and the proteins were
visualized on X-ray film using an enhanced chemiluminescence
western blot detection system (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Three independent experiments were performed in
triplicate.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 14.0 (SPSS Inc., Chicago, IL, USA). Data are
presented as the mean ± standard error. Statistical analysis
between groups was performed using analysis of variance. P<0.05
was considered to indicate a statistically significant
difference.
Results
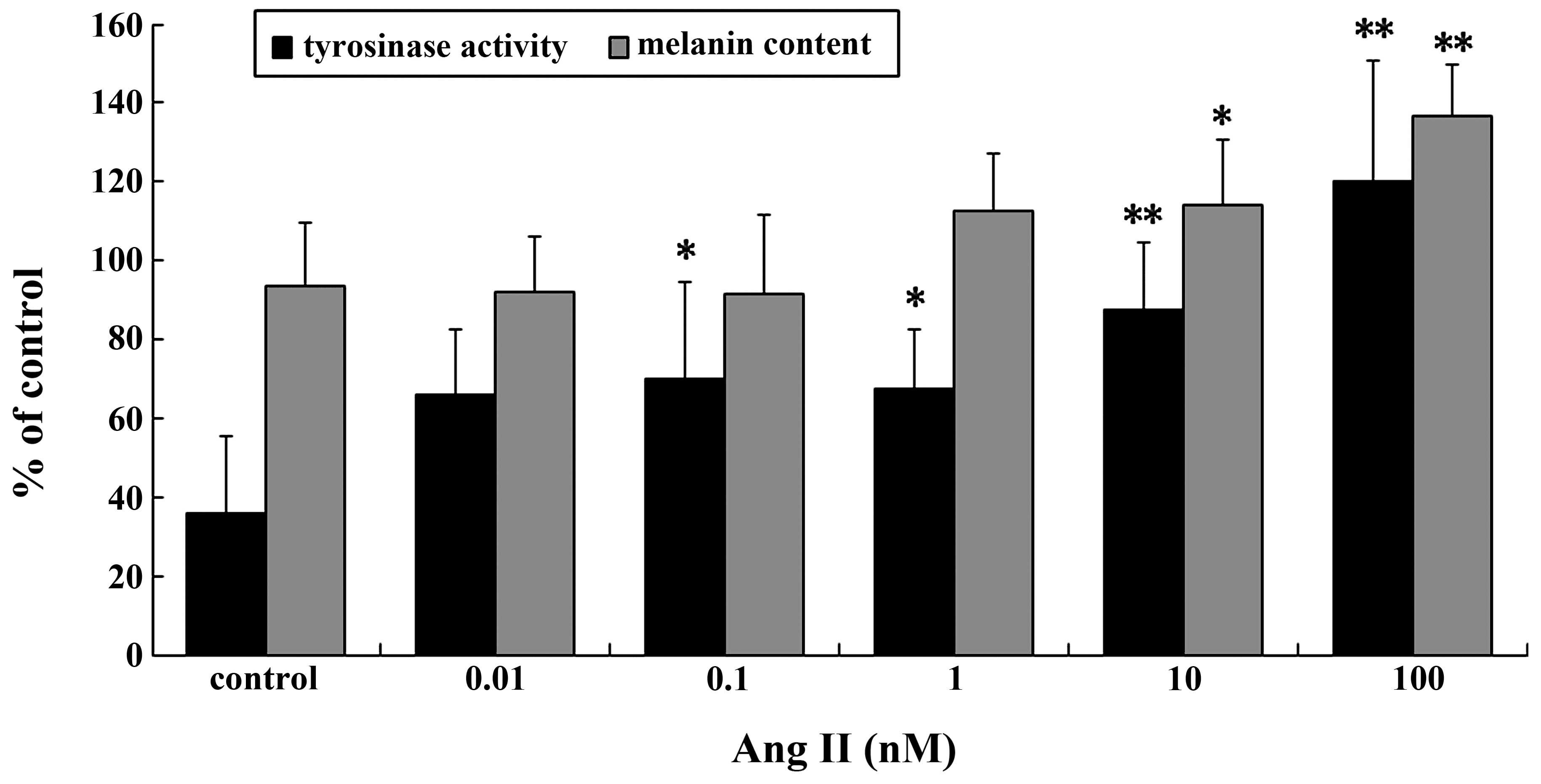
Ang II regulates TYR activity and
melanin content
TYR activity and melanin content increased
significantly in a dose-dependent manner following treatment with
Ang II (Fig. 1). Significant
increases in TYR activity and melanin content were observed in the
cells incubated with 0.1–100 nM Ang II for 24 h. The maximal
increases were achieved with 100 nM Ang II (P<0.01).
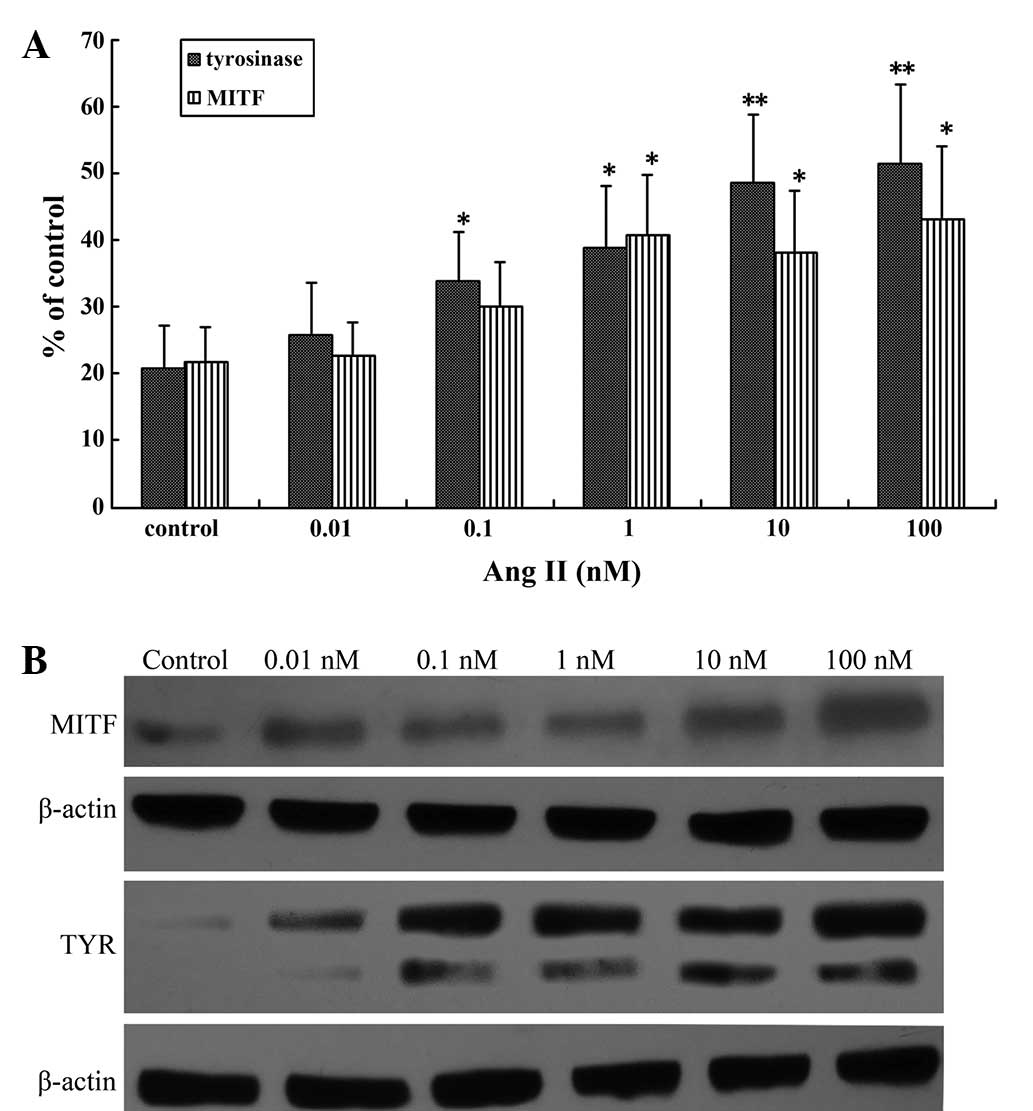
Ang II regulates MITF and TYR mRNA and
protein expression
MITF is the master regulator of melanogenesis,
regulating the expression of TYR (7,25), the
key regulatory enzyme in melanin biosynthesis (26). Investigation of the effects of Ang II
on MITF and TYR mRNA and protein expression levels in human
melanocytes by RT-qPCR and western blot analysis revealed that
there was a significant increase in transcription following a 24-h
incubation with higher concentrations of Ang II (0.1–100 nM;
Fig. 2A). A maximal increase was
achieved with 100 nM Ang II (P<0.01; Fig. 2B).
PKC inhibitor inhibits Ang II-induced
melanogenesis
Previous studies have reported that PKA and PKC
activation stimulates melanogenesis (27,28).
Therefore, in the present study, H-89 (a PKA inhibitor) and
Ro-32-0432 (a PKC inhibitor) were used to evaluate the molecular
mechanisms by which Ang II induces melanogenesis. Melanin content
and TYR activity were detected under the combined effect of 100 nM
Ang II and 1 µM H-89 or Ro-32-0432. Ro-32-0432 completely
attenuated the Ang II-induced increases in melanin content and TYR
activity while H-89 had no effect on melanin synthesis (Fig. 3A). TYR mRNA and protein expression
levels were detected following the combined treatment with 100 nM
Ang II and Ro-32-0432. RT-qPCR and western blot analysis revealed
that TYR expression was markedly decreased following the combined
Ang II and Ro-32-0432 treatment (Fig.
3B).
Discussion
The role of Ang II in wound healing has not been
fully elucidated; however, previous studies have indicated that Ang
II is a potent accelerator of cutaneous wound repair, which certain
studies have demonstrated by wounding healing skin (7,8,29). Notably, previous studies have
demonstrated significant AT1 receptor expression in primary human
melanocytes (12), and found that
inhibition of the AT1 receptor limited murine melanoma growth
(13). To the best of our knowledge,
the present study provides the first indication of the role of Ang
II in human melanocyte melanogenesis and elucidates the possible
mechanisms involved.
Melanogenesis is controlled by complex regulatory
mechanisms, and TYR is the rate-limiting enzyme in melanogenesis.
In order to investigate the effect of Ang II on human melanocyte
melanogenesis, melanocyte TYR activity and melanin content were
evaluated. The results indicate that Ang II significantly increased
TYR activity and melanin synthesis, suggesting that Ang II
stimulates melanogenesis.
Melanin synthesis is stimulated by numerous
effectors, including cAMP- elevating agents, ultraviolet (UV)
light, and TYR. The primary transcription factor in the regulation
of TYR gene expression is MITF, which serves a crucial function in
the regulation of various aspects of melanocyte survival and
differentiation (30). Studies
suggest that MITF mediates cAMP-induced increases in the expression
of a number of melanogenic proteins (27,31). In
order to evaluate the mechanism by which Ang II induces
pigmentation, the effects of Ang II on MITF and TYR mRNA and
protein expression levels were investigated in human melanocytes.
MITF expression was significantly increased following treatment
with 100 nM Ang II. RT-qPCR and western blot analysis revealed that
Ang II upregulated the expression levels of pigment cell-specific
genes, specifically TYR and MITF, in melanocytes, suggesting that
Ang II stimulates melanin synthesis by upregulating MITF in
melanocytes.
Previous studies of melanin synthesis signaling
pathways suggest that the cAMP/PKA and diacylglycerol (DAG)/PKC
pathways are involved in melanin synthesis; a number of
inextricably linked signaling pathways have been identified
(16–18). Previously, research on melanocyte
proliferation and melanogenesis signal transduction has focused on
the role of cAMP, where the cAMP pathway is a key modulator of
melanogenesis. Studies have shown that cAMP-induced increases in
the expression of numerous melanogenic proteins are mediated by
MITF (27,31). The cAMP pathway upregulates MITF,
which is crucial for key melanogenic proteins such as TYR,
TYR-related protein-1 (TRP-1) and TRP-2. Notably, Park et al
demonstrated that the cAMP pathway also upregulates PKC-β
expression via MITF (32). In the
present study, H-89 (PKA inhibitor) and Ro-32-0432 (PKC inhibitor)
were used to evaluate the molecular mechanism by which Ang II
induces melanogenesis. It was observed that the PKC inhibitor
completely attenuated the Ang II-stimulated increase in melanin
content and TYR activity, suggesting that Ang II stimulated
melanogenesis via the PKC pathway. The PKC pathway has been
identified to be an intracellular signaling pathway that regulates
melanogenesis. A previous study observed that adding
diacylglycerol, an endogenous PKC activator, to cultured human
melanocytes caused a rapid 3–4-fold increase in total melanin
content, and that a PKC inhibitor blocked this increase (17). Conversely, topical application of a
selective PKC inhibitor reduced pigmentation and blocked UV-induced
pigmentation in guinea pig skin (33). In cultured pigment cells, PKC
depletion reduced the melanin content markedly (34,35) and
blocked α-melanocyte-stimulating hormone-induced melanogenesis
completely (35). In addition, the
results of the present study indicate that H-89 had no effect on
melanin synthesis, suggesting that Ang II does not stimulate the
PKA pathway in human melanocytes. The present results are
consistent with the findings of Mehta and Griendling (21), which were that PKA activation should
not occur under Ang II-stimulated conditions. Molnar et al
(36) established that PKA is not
involved in Ang II-mediated cAMP response-binding element
activation in smooth muscle cells, which contradicts a study by
Funakoshi et al (37), which
reported that PKA was activated in response to Ang II stimulation.
Previous studies have also shown that Ang II mediates various
cellular functions involving PKC signaling in cardiovascular
function and disease (38). Müller
et al (39) reported that Ang
II inhibits renin gene transcription via the PKC pathway. The
results of the present study suggest that the PKC pathway mediates
the induction of TYR mRNA and protein expression by Ang II.
In summary, to the best of our knowledge, the
present study is the first to demonstrate the effects of Ang II on
melanogenesis and to identify the molecular mechanisms by which it
induces melanogenesis. Specifically, the present results
demonstrate that the Ang II-induced pigmentation effect in human
melanocytes requires PKC and that the mechanism involves the PKC
pathway and MITF upregulation. Collectively, these results may
provide a new strategy for the cosmetic study of
hyperpigmentation.
Acknowledgements
The authors thank the native English speaking
scientists of Elixigen Corporation for editing this manuscript.
References
|
1
|
Hirobe T: Proliferation of epidermal
melanocytes during the healing of skin wounds in newborn mice. J
Exp Zool. 227:423–431. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tobin DJ: The cell biology of human hair
follicle pigmentation. Pigment Cell Melanoma Res. 24:75–88. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishimura EK: Melanocyte stem cells: A
melanocyte reservoir in hair follicles for hair and skin
pigmentation. Pigment Cell Melanoma Res. 24:401–410. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ito M, Liu Y, Yang Z, Nguyen J, Liang F,
Morris RJ and Cotsarelis G: Stem cells in the hair follicle bulge
contribute to wound repair but not to homeostasis of the epidermis.
Nat Med. 11:1351–1354. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
McKinney CA, Fattah C, Loughrey CM,
Milligan G and Nicklin SA: Angiotensin-(1–7) and angiotensin-(1–9):
Function in cardiac and vascular remodelling. Clin Sci (Lond).
126:815–827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
RuizOrtega M, Lorenzo O, Rupérez M,
Esteban V, Suzuki Y, Mezzano S, Plaza JJ and Egido J: Role of the
renin-angiotensin system in vascular diseases: Expanding the field.
Hypertension. 38:1382–1387. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takeda H, Katagata Y, Hozumi Y and Kondo
S: Effects of angiotensin II receptor signaling during skin wound
healing. Am J Pathol. 165:1653–1662. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodgers K, Abiko M, Girgis W, St Amand K,
Campeau J and di Zerega G: Acceleration of dermal tissue repair by
angiotensin II. Wound Repair Regen. 5:175–183. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rodgers KE, DeCherney AH, St Amand KM,
Dougherty WR, Felix JC, Girgis WW and di Zerega GS: Histologic
alterations in dermal repair after thermal injury effects of
topical angiotensin II. J Burn Care Rehabil. 18:381–388. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nozawa Y, Matsuura N, Miyake H, Yamada S
and Kimura R: Effects of TH-142177 on angiotensin II-induced
proliferation, migration and intracellular signaling in vascular
smooth muscle cells and on neointimal thickening after balloon
injury. Life Sci. 64:2061–2070. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steckelings UM, Wollschläger T, Peters J,
Henz BM, Hermes B and Artuc M: Human skin: Source of and target
organ for angiotensin II. Exp Dermatol. 13:148–154. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Steckelings UM, Henz BM, Wiehstutz S,
Unger T and Artuc M: Differential expression of angiotensin
receptors in human cutaneous wound healing. Br J Dermatol.
153:887–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Otake AH, Mattar AL, Freitas HC, Machado
CM, Nonogaki S, Fujihara CK, Zatz R and Chammas R: Inhibition of
angiotensin II receptor 1 limits tumor-associated angiogenesis and
attenuates growth of murine melanoma. Cancer Chemother Pharmacol.
66:79–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Widlund HR and Fisher DE:
Microphthalamia-associated transcription factor: A critical
regulator of pigment cell development and survival. Oncogene.
22:3035–3041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee AY and Noh M: The regulation of
epidermal melanogenesis via cAMP and/or PKC signaling pathways:
Insights for the development of hypopigmenting agents. Arch Pharm
Res. 36:792–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buscà R and Ballotti R: Cyclic AMP a key
messenger in the regulation of skin pigmentation. Pigment Cell Res.
13:60–69. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gordon PR and Gilchrest BA: Human
melanogenesis is stimulated by diacylglycerol. J Invest Dermatol.
93:700–702. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park HY and Gilchrest BA: Signaling
pathways mediating melanogenesis. Cell Mol Biol (Noisy-le-grand).
45:919–930. 1999.PubMed/NCBI
|
|
19
|
LeiteMoreira AF, CastroChaves P,
PimentelNunes P, LimaCarneiro A, Guerra MS, Soares JB and
Ferreira-Martins J: Angiotensin II acutely decreases myocardial
stiffness: A novel AT1, PKC and Na+/H+
exchanger-mediated effect. Br J Pharmacol. 147:690–697. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berthold H, Just A, Kirchheim HR and Ehmke
H: Interaction between nitric oxide and endogenous vasoconstrictors
in control of renal blood flow. Hypertension. 34:1254–1258. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mehta PK and Griendling KK: Angiotensin II
cell signaling: Physiological and pathological effects in the
cardiovascular system. Am J Physiol Cell Physiol. 292:C82–C97.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei TC, Virador VM, Vieira WD and Hearing
VJ: A melanocyte-keratinocyte coculture model to assess regulators
of pigmentation in vitro. Anal Biochem. 305:260–268. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Virador VM, Kobayashi N, Matsunaga J and
Hearing VJ: A standardized protocol for assessing regulators of
pigmentation. Anal Biochem. 270:207–219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marin-Castaño ME, Elliot SJ, Potier M,
Karl M, Striker LJ, Striker GE, Csaky KG and Cousins SW: Regulation
of estrogen receptors and MMP-2 expression by estrogens in human
retinal pigment epithelium. Invest Ophthalmol Vis Sci. 44:50–59.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aksan I and Goding CR: Targeting the
microphthalmia basic helix-loop-helix-leucine zipper transcription
factor to a subset of E-box elements in vitro and in
vivo. Mol Cell Biol. 18:6930–6938. 1998.PubMed/NCBI
|
|
26
|
Halaban R, Pomerantz SH, Marshall S,
Lambert DT and Lerner AB: Regulation of tyrosinase in human
melanocytes grown in culture. J Cell Biol. 97:480–488. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bertolotto C, Abbe P, Hemesath TJ, Bille
K, Fisher DE, Ortonne JP and Ballotti R: Microphthalmia gene
product as a signal transducer in cAMP-induced differentiation of
melanocytes. J Cell Biol. 142:827–835. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Price ER, Horstmann MA, Wells AG,
Weilbaecher KN, Takemoto CM, Landis MW and Fisher DE:
alpha-Melanocyte-stimulating hormone signaling regulates expression
of microphthalmia, a gene deficient in Waardenburg syndrome. J Biol
Chem. 273:33042–33047. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Steckelings UM, Artuc M, Paul M, Stoll M
and Henz BM: Angiotensin II stimulates proliferation of primary
human keratinocytes via a non-AT1, non-AT2 angiotensin receptor.
Biochem Biophys Res Commun. 229:329–333. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gaggioli C, Buscà R, Abbe P, Ortonne JP
and Ballotti R: Microphthalmia-associated transcription factor
(MITF) is required but is not sufficient to induce the expression
of melanogenic genes. Pigment Cell Res. 16:374–382. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shibahara S, Takeda K, Yasumoto K, Udono
T, Watanabe K, Saito H and Takahashi K: Microphthalmia-associated
transcription factor (MITF): Multiplicity in structure, function,
and regulation. J Investig Dermatol Symp Proc. 6:99–104. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park HY, Wu C, Yonemoto L, MurphySmith M,
Wu H, Stachur CM and Gilchrest BA: MITF mediates cAMP-induced
protein kinase C-beta expression in human melanocytes. Biochem J.
395:571–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park HY, Lee J, González S, MiddelkampHup
MA, Kapasi S, Peterson S and Gilchrest BA: Topical application of a
protein kinase C inhibitor reduces skin and hair pigmentation. J
Invest Dermatol. 122:159–166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Niles RM and Loewy BP: Induction of
protein kinase C in mouse melanoma cells by retinoic acid. Cancer
Res. 49:4483–4487. 1989.PubMed/NCBI
|
|
35
|
Park HY, Russakovsky V, Ao Y, Fernandez E
and Gilchrest BA: Alpha-melanocyte stimulating hormone-induced
pigmentation is blocked by depletion of protein kinase C. Exp Cell
Res. 227:70–79. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Molnar P, Perrault R, Louis S and Zahradka
P: The cyclic AMP response element-binding protein (CREB) mediates
smooth muscle cell proliferation in response to angiotensin II. J
Cell Commun Signal. 8:29–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Funakoshi Y, Ichiki T, Takeda K, Tokuno T,
Iino N and Takeshita A: Critical role of cAMP-response
element-binding protein for angiotensin II-induced hypertrophy of
vascular smooth muscle cells. J Biol Chem. 277:18710–18717. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chintalgattu V and Katwa LC: Role of
protein kinase C-delta in angiotensin II induced cardiac fibrosis.
Biochem Biophys Res Commun. 386:612–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Müller MW, Todorov V, Krämer BK and Kurtz
A: Angiotensin II inhibits renin gene transcription via the protein
kinase C pathway. Pflugers Arch. 444:499–505. 2002. View Article : Google Scholar : PubMed/NCBI
|