Introduction
Plaque psoriasis is the most common form of
psoriatic disease, corresponding to a chronic inflammatory skin
disorder (1,2). Treatment of severe psoriatic plaques
may involve biological agents that block the action of tumor
necrosis factor (TNF)-α, such as etanercept and infliximab
(2–6). Etanercept is a recombinant human
TNF-receptor fusion protein that binds free TNF-α (7,8).
Infliximab is a monoclonal anti-TNF-α antibody that can bind both
soluble and membrane-bound TNF-α and effectively neutralize its
activity (9). The mechanism
underlying the action of these drugs as anti-TNF-α agents in
psoriasis is not yet clear; however, the application of etanercept
and infliximab has been shown to reduce multiple pro-inflammatory
pathways in psoriatic plaques (10,11). The
efficacy of anti-TNF-α biological treatment supports a role of
innate immunity in the pathogenesis of psoriatic disease.
Toll-like receptors (TLRs) have been demonstrated to
be essential elements of the innate immune system (12,13). To
date, ~10 different types of TLRs (TLR 1–10) have been found in
humans. Each TLR is activated by a different microbial component,
although they trigger a common myeloid differentiation factor 88
(MyD88)-dependent pathway, leading, via nuclear factor (NF)-κB, to
the production of pro-inflammatory cytokines and chemokines, such
as TNF-α (12). TLR-2 is a type of
TLR that is highly expressed in keratinocytes, and Langerhans and
mast cells of psoriatic plaques (14–16) and
is activated by various microorganism antigens (17–20) or
endogenous heat-shock proteins present at sites of tissue injury
and inflammation (21). TLR-9 is
another type of TLR that has been observed to be elevated in the
keratinocytes of psoriatic skin lesions (22) and is activated by unmethylated DNA
sequences (CpG dinucleotides) that are present in bacterial DNA and
viruses (17).
A member of the interleukin-1 receptor (IL-1R)/TLR
superfamily, IL-33, has been recognized as a pro-inflammatory
molecule that is predominantly expressed in the nucleus of cells
with a barrier function, such as endothelial and epithelial cells
(23,24). These cells share a common
intracellular domain (TIR domain) that may, through MyD88, initiate
a signaling cascade, leading to NF-κB translocation (23). IL-33 has been found to be expressed
at elevated levels in affected psoriatic skin, compared with
healthy skin, and it has also been proposed to represent a novel
marker of psoriasis, relative to other inflammatory skin disorders
(25–28). Balato et al (25) recently demonstrated that inflammatory
cytokines, such as TNF-α, induce the secretion of IL-33 from
immortalized keratinocytes (24) and
support the hypothesis that IL-33 has an important role in the
effect of anti-TNF therapy on psoriatic skin (25,29).
In the present study, the aim was to investigate the
effect of anti-TNF-α treatment with etanercept or infliximab on
pro-inflammatory IL-33, TLR-2 and -9 transcriptional levels in
psoriatic plaques. For the purpose of this study the mRNA levels of
IL-33, TLR-2 and -9 genes were identified using a precise reverse
transcription-quantitative polymerase chain reaction (qPCR)
analysis, with biopsies of psoriatic plaques obtained from patients
at the start and end of anti-TNF-α therapy.
Subjects and methods
Patients
Seventeen adult patients (mean age ± standard
deviation, 46.4±9.6 years; males, 13; and females, 4) with
moderate-to-severe psoriasis attending the Department of
Dermatology outpatient clinic of the University Hospital of Larissa
(Larissa, Greece) were included in an open-label study. Skin biopsy
confirmed the diagnosis of plaque psoriasis in these patients.
Thirteen of the patients (11 males and 2 females; median age, 48.5
years) were treated for 3 months with etanercept (Enbrel®; Immunex
Corp., Thousand Oaks, CA, USA), and 4 of them (2 male and 2 female)
were treated with infliximab (Remicade®; Janssen Biotech, Inc.,
Titusville, NJ, USA). The psoriasis area severity index (PASI)
(30) was calculated prior to
(PASI-1; range, 10–45.5; median, 20.3) and subsequent to (PASI-2;
range, 1.2–20.4; median, 5.2) the treatment.
Tissue collection
Punch skin biopsies (6-mm) were collected from
psoriatic plaques of patients prior to initiation of treatment
(baseline, B) and following 12 weeks of treatment (post-treatment,
P). Biopsies were taken under local anesthesia with 1% lidocaine
from one lesion of each patient. The skin biopsies were immediately
cryopreserved at −80°C, where they were kept until molecular
analysis. Part of the biopsy was used for histological examination.
This study was approved by the local Ethics Committee (University
Hospital of Larissa), and performed in accordance with the
Declaration of Helsinki; all patients gave their informed
consent.
Quantitative gene expression
analysis
An RNAeasy® Fibrous Tissue Mini kit (Qiagen, Inc.,
Valencia, CA, USA) was used for total RNA isolation from skin
biopsies, and a QuantiFast™ Reverse Transcription kit (Qiagen,
Inc.) was used for cDNA synthesis according to the manufacturer's
instructions. qPCR analysis of IL-33 (NM_033439), TLR-2 (NM_003264)
and -9 (NM_017442) mRNAs was performed using specific primers and
dual-labeled probes (QuantiFast® Probe assay; Qiagen, Inc.) by
applying Rotor Gene 6.1 (Qiagen, Inc.) according to the
manufacturer's instructions. The PCR cycling conditions were as
follows: Initial activation at 95°C for 5 min, followed by 40
cycles of denaturation at 95°C for 10 sec, annealing at 55°C for 15
sec and extension at 72°C for 15 sec. The human porphobilinogen
deaminase (hPBGD) gene was used as a reference-control, as
described previously (31). The
quantification of the mRNAs was achieved by creating a standard
curve of serial dilutions of hPBGD gene copies. The mRNA expression
levels of each IL-33, TLR-2 and -9 target gene were addressed as
ratios of target mRNA to control hPBGD mRNAs (target/control mRNA
ratios); therefore, target/control mRNA ratios <1.0 or ≥1.0 were
assigned as low or elevated mRNA levels, respectively.
Additionally, the mRNA expression of each target gene, IL-33, TLR-2
and -9, at P was compared with that at B and expressed as a P/B
mRNA ratio (relative expression).
Statistical analysis
The Wilcoxon signed-rank test was used for the
evaluation of changes in IL-33, TLR-2 and -9 mRNA values, as well
as in PASI scores, between B and P, which had skewed distributions
(P-values reported for one-tailed test). Pearson coefficients were
computed in order to investigate associations among
IL-33/TLR-2/TLR-9 expression or between the expression of
pro-inflammatory molecules and PASI scores (P-values reported for
two-tailed test). Analysis of variance was used to investigate
changes in PASI score according to the relative expression of
IL-33, TLR-2 and -9. All P-values were reported for the two-tailed
test. Statistical significance was set at 0.05 and analyses were
carried out using GraphPad Prism 6 software (GraphPad Software, San
Diego, CA, USA).
Results
Clinical response
In all patients, the PASI improved with treatment;
the mean ± standard deviation PASI score was reduced from 21.2±8.8
at B to 5.0±4.8 at P (ΔPASI=16.2, P<0.0001; Fig 1A). In patients who received
etanercept, the mean PASI score was decreased significantly from
20.3±6.7 at B to 5.2 ±5.3 at P (ΔPASI=15.1, P<0.0001; Wilcoxon
signed-rank test; Fig 1A).
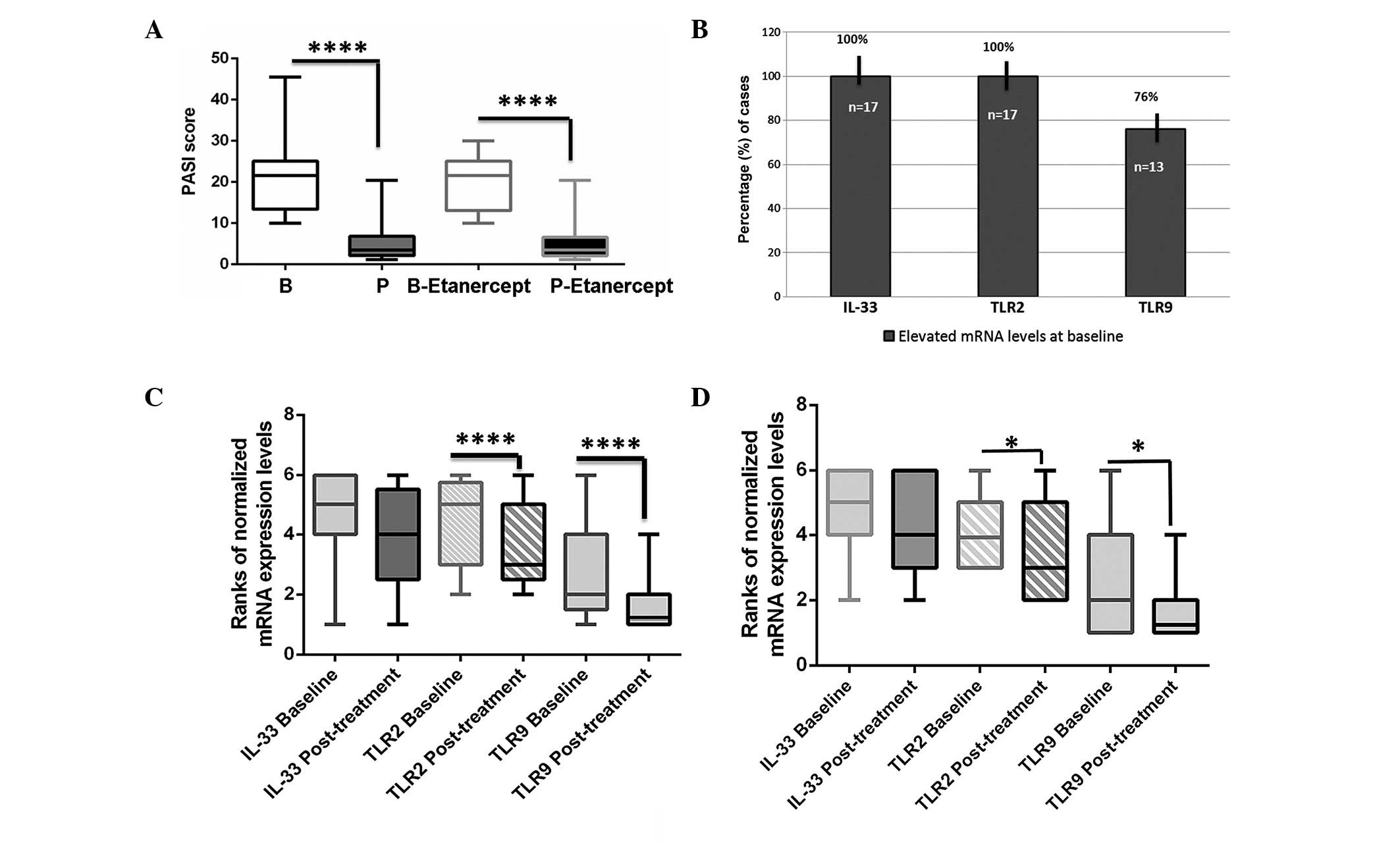 | Figure 1.Transcriptional changes of the
pro-inflammatory molecules IL-33, TLR-2 and TLR-9, and changes in
PASI scores, of psoriatic plaques prior to (baseline, B) and at the
end (post-treatment, P) of anti-TNF-α treatment. (A) Statistically
significant differences of PASI scores post-treatment vs. baseline
in psoriatic skin lesions treated with TNF-α inhibitors, and
particularly by etanercept (Wilcoxon matched-pairs signed rank
test; one-tailed). (B) Percentages of plaque psoriatic cases (%)
presenting elevated (target/hPBGD ≥1) mRNA levels of IL-33, TLR-2
and TLR-9 at baseline in psoriatic skin. Differences in the
expression levels of IL-33, TLR-2 and TLR-9 mRNA post-treatment vs.
baseline in (C) the whole treatment group and (D) in male patients
treated with etanercept (Wilcoxon matched-pairs signed rank test;
one-tailed. Graphs were created using Graph Pad Prism 6 software.
The boxplots represent the mean ranks of mRNAs evaluated by
quantitative polymerase chain reaction. The upper line indicates
the highest value, the lower line the lowest value and the middle
line the mean of normalized quantities of each variable.
*P<0.01; ****P<0.0001. IL, interleukin; TLR, Toll-like
receptor; PASI, psoriasis area severity index; TNF, tumor necrosis
factor; hPBGD, human porphobilinogen deaminase. |
TNF-α inhibitors reduce the expression
of pro-inflammatory IL-33, TLR-2 and TLR-9 at the transcriptional
levels in psoriatic plaques
To investigate the effect of the TNF-α inhibitors
etanercept and infliximab on the expression of pro-inflammatory
molecules in plaque psoriasis, the transcriptional levels of IL-33,
TLR-2 and TLR-9 were assessed in psoriatic lesions before and after
treatment. The quantification of IL-33, TLR-2 and TLR-9 expression
data revealed that at B, the mRNA levels of IL-33 and TLR-2 were
elevated in all psoriatic skin lesions, while those of TLR-9 were
elevated in the majority (76%) of cases (Fig 1B). At P, TLR-2 and -9 exhibited
significantly lower transcriptional levels compared with those at B
(P<0.0001, Wilcoxon signed-rank test), whereas the levels of
IL-33 mRNA were reduced, although not significantly (Fig 1C). The etanercept group was the most
affected. Paired t-test analysis showed significant reductions of
TLR-2 and TLR-9 mRNA levels at the end of etanercept therapy
compared with those at B (P=0.017 and P=0.0239, respectively;
Fig 1D), particularly in males
(P=0.0210 and P=0.0415, respectively).
Transcriptional levels of
pro-inflammatory IL-33, TLR-2 and TLR-9 show significant linear
Pearson's correlations
Significant linear Pearson's correlations were
observed between the transcriptional levels of IL-33 and TLR-2 at B
and P (r=0.953814 and r=0.782228, respectively; P<0.0001).
Moreover, significant positive correlations were observed at B
between IL-33 and TLR-9 and between TLR-2 and TLR-9 (r=0.914635 and
r=0.763433, respectively; P<0.0001), which were was less strong
at the end of anti-TNF-α treatment (r=0.534675 and r=0.668582,
respectively; P<0.05).
Additionally, strong linear Pearson's correlations
were identified between the relative (P/B) mRNA expression ratios
of IL-33 and TLR-2, IL-33 and TLR-9 (Fig
2A-a) or TLR-2 and TLR-9 (Fig.
2A-b; r=0.804390, r=0.876705 and r=0.916274, respectively;
P<0.0001), particularly in male patients who received etanercept
therapy (r=0.803771, r=0.886865 and r=0.92, respectively;
P<0.0003).
ΔPASI scores present significant
linear Pearson's correlations with changes in TLR-2 or TLR-9 mRNA
expression levels
Pearson's correlation analysis revealed a
significant linear correlation between the change in PASI score
(ΔPASI) and the relative (P/B) mRNA expression ratios of TLR-2 and
TLR-9 in the psoriatic plaques of males treated with TNF-α
inhibitors (r=0.556958 and r=0.555675, P<0.05; respectively),
particularly in the etanercept group (r=0.764774 or r=0.725006,
respectively; P<0.0001; Fig 2B).
No significant correlation was found between ΔPASI and changes in
IL-33 mRNA in the etanercept group by Pearson's analysis.
Discussion
The present findings have provided evidence of the
efficacy of anti-TNF-α therapy in reducing the innate immune
response, indicating that the pro-inflammatory factors TLR-2, -9
and IL-33 play a role in the pathogenic mechanism of plaque
psoriasis. The results support the involvement of innate immune
response elements in the pathophysiology of psoriasis, in line with
previous studies (12,17,22–24,26,27,32–36),
although some previous studies have reported conflicting results
regarding TLR-9 expression in psoriasis (22,34). The
activation of IL-33, TLR-2 and TLR-9 in psoriatic plaques may be
important since such activation has been previously associated with
the activation of NF-κB (12,23,37).
The present findings showed that TLR-2 and -9 were
most affected by anti-TNF-α therapy, indicating that they play a
critical role in the psoriatic innate immune response. IL-33 can
act as both a pro- and anti-inflammatory factor (38). It is currently considered that
biologically active IL-33 is released during necrosis as an
endogenous danger or ‘alarm’ signal; during apoptosis, IL-33 is
cleaved and inactivated (3,27). The present results indicate that the
pro-inflammatory function of IL-33 in psoriatic skin can be
inhibited by TNF-α blockers, in agreement with previous studies by
Balato et al (25) and Li
et al (39) which have
described the regulation of IL33 by TNF-α.
In the present study, the efficacy of the TNF-α
inhibitors etanercept and infliximab in plaque psoriasis therapy
has been clearly demonstrated, in agreement with previous studies
(2–6). Furthermore, concerning the small number
of infliximab-treated cases or female patients, this study revealed
notable observations regarding correlations among pro-inflammatory
genes, anti-TNF-α treatment type and gender. It was observed that
etanercept and infliximab exhibited similar effects on the
expression of the three innate immune response factors, IL-33,
TLR-2 and -9, in psoriatic skin lesions; however, the present data
indicate that TLR-2 and IL-33 may share a common stimulation
pattern in psoriatic plaques, in line with a previous report on
inflamed skin (39). Male patients
may exhibit a distinct biological response to etanercept compared
to females, implying a different pathophysiological mechanism of
psoriatic plaques. This finding is consistent with a previous
report, which suggested that males exhibit more severe psoriasis,
as compared with females (40).
Additionally, TLR-2 and -9 may play a role as indices of severe
psoriasis, since their mRNA alterations showed significant positive
correlations with ΔPASI-2 in male patients that received
etanercept.
In conclusion, the transcriptional levels of the
three pro-inflammatory factors, IL-33, TLR-2 and -9, were examined
prior to and subsequent to 3 months of anti-TNF-α treatment for
psoriatic plaques. Despite the small number of study cases, the
results support the efficacy of the TNF-α inhibitors etanercept or
infliximab in reducing the innate immune response and indicate that
the pro-inflammatory factors IL-33, TLR-2 and -9 play a role in
psoriatic biology. Etanercept tended to be more effective in innate
immune response inhibition in males. The present findings support
the instigation of further investigations into innate immune
response elements, particularly TLR-2 and -9, under anti-TNF-α
treatment, including a more extensive group of patients, in order
to clarify the possible pathophysiological mechanisms of psoriatic
plaques and TNF-α therapy mechanisms according to their action and
efficacy, as well as the associations with gender.
References
|
1
|
Langley RG, Krueger GG and Griffiths CE:
Psoriasis: Epidemiology, clinical features, and quality of life.
Ann Rheum Dis. 64:(Suppl 2). 18–25. 2005. View Article : Google Scholar
|
|
2
|
Weger W: Current status and new
developments in the treatment of psoriasis and psoriatic arthritis
with biological agents. Br J Pharmacol. 160:810–820. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prieto-Pérez R, Cabaleiro T, Daudén E,
Ochoa D, Roman M and Abad-Santos F: Genetics of psoriasis and
pharmacogenetics of biological drugs. Autoimmune Dis.
2013:6130862013.PubMed/NCBI
|
|
4
|
Rodgers M, Epstein D, Bojke L, Yang H,
Craig D, Fonseca T, Myers L, Bruce I, Chalmers R, Bujkiewicz S, et
al: Etanercept, infliximab and adalimumab for the treatment of
psoriatic arthritis: A systematic review and economic evaluation.
Health Technol Assess. 15:1–329. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ayroldi E, Bastianelli A, Cannarile L,
Petrillo MG, Delfino DV and Fierabracci A: A pathogenetic approach
to autoimmune skin disease therapy: Psoriasis and biological drugs,
unresolved issues and future directions. Curr Pharm Des.
17:3176–3190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Winterfield LS, Menter A, Gordon K and
Gottlieb A: Psoriasis treatment: Current and emerging directed
therapies (Review). Ann Rheum Dis. 64:(Suppl 2). 87–90. 2005.
View Article : Google Scholar
|
|
7
|
Papp KA: Etanercept in psoriasis. Expert
Opin Pharmacother. 5:2139–2146. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nestorov I, Zitnik R, DeVries T, Nakanishi
AM, Wang A and Banfield C: Pharmacokinetics of subcutaneously
administered etanercept in subjects with psoriasis. Br J Clin
Pharmacol. 62:435–445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Knight DM, Trinh H, Le J, Siegel S, Shealy
D, McDonough M, Scallon B, Moore MA, Vilcek J, Daddona P, et al:
Construction and initial characterization of a mouse-human chimeric
anti-TNF antibody. Mol Immunol. 30:1443–1453. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gottlieb AB: Tumor necrosis factor
blockade: Mechanism of action. J Investig Dermatol Symp Proc.
12:1–4. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gottlieb AB, Chamian F, Masud S, Cardinale
I, Abello MV, Lowes MA, Chen F, Magliocco M and Krueger JG: TNF
inhibition rapidly down-regulates multiple proinflammatory pathways
in psoriasis plaques. J Immunol. 175:2721–2729. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akira S and Takeda K: Toll-like receptor
signalling. Nat Rev Immunol. 4:499–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Terhorst D, Kalali BN, Ollert M, Ring J
and Mempel M: The role of toll-like receptors in host defenses and
their relevance to dermatologic diseases. Am J Clin Dermatol.
11:1–10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Begon E, Michel L, Flageul B, Beaudoin I,
Jean-Louis F, Bachelez H, Dubertret L and Musette P: Expression,
subcellular localization and cytokinic modulation of Toll-like
receptors (TLRs) in normal human keratinocytes: TLR-2 up-regulation
in psoriatic skin. Eur J Dermatol. 17:497–506. 2007.PubMed/NCBI
|
|
15
|
Miller LS and Modlin RL: Toll-like
receptors in the skin. Semin Immunopathol. 29:15–26. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sandig H and Bulfone-Paus S: TLR signaling
in mast cells: Common and unique features. Front Immunol.
3:1852012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miller LS: Toll-like receptors in skin.
Adv Dermatol. 24:71–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krishna S, Ray A, Dubey SK, Larrouy-Maumus
G, Chalut C, Castanier R, Noguera A, Gilleron M, Puzo G, Vercellone
A, et al: Lipoglycans contribute to innate immune detection of
mycobacteria. PLoS One. 6:e284762011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zughaier SM: Neisseria meningitidis
capsular polysaccharides induce inflammatory responses via TLR-2
and TLR4-MD-2. J Leukoc Biol. 89:469–480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bieback K, Lien E, Klagge IM, Avota E,
Schneider-Schaulies J, Duprex WP, Wagner H, Kirschning CJ, Ter
Meulen V and Schneider-Schaulies S: Hemagglutinin protein of
wild-type measles virus activates toll-like receptor 2 signaling. J
Virol. 76:8729–8736. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sloane JA, Blitz D, Margolin Z and
Vartanian T: A clear and present danger: Endogenous ligands of
toll-like receptors. Neuromolecular Med. 12:149–163. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morizane S, Yamasaki K, Mühleisen B, Kotol
PF, Murakami M, Aoyama Y, Iwatsuki K, Hata T and Gallo RL:
Cathelicidin antimicrobial peptide LL-37 in psoriasis enables
keratinocyte reactivity against TLR-9 ligands. J Invest Dermatol.
132:135–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miller AM: Role of IL-33 in inflammation
and disease. J Inflamm (Lond). 8:222011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balato A, Lembo S, Mattii M, Schiattarella
M, Marino R, De Paulis A, Balato N and Ayala F: IL-33 is secreted
by psoriatic keratinocytes and induces pro-inflammatory cytokines
via keratinocyte and mast cell activation. Exp Dermatol.
21:892–894. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Balato A, Schiattarella M, Lembo S, Mattii
M, Prevete N, Balato N and Ayala F: Interleukin-1 family members
are enhanced in psoriasis and suppressed by vitamin D and retinoic
acid. Arch Dermatol Res. 305(3): 255–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Psoriasis and skin pain: instrumental and
biological evaluations. Patruno C, Napolitano M, Balato N, Ayala F,
Megna M, Patrì A, Cirillo T and Balato A: Acta Derm Venereol.
95(4): 432–438. 2015.PubMed/NCBI
|
|
27
|
Theoharides TC, Zhang B, Kempuraj D, Tagen
M, Vasiadi M, Angelidou A, Alysandratos KD, Kalogeromitros D, Asadi
S, Stavrianeas N, et al: IL-33 augments substance P-induced VEGF
secretion from human mast cells and is increased in psoriatic skin.
Proc Natl Acad Sci USA. 107:4448–4453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hueber AJ, Alves-Filho JC, Asquith DL,
Michels C, Millar NL, Reilly JH, Graham GJ, Liew FY, Miller AM and
McInnes IB: IL-33 induces skin inflammation with mast cell and
neutrophil activation. Eur J Immunol. 41:2229–2237. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Balato A, Di Caprio R, Canta L, Mattii M,
Lembo S, Raimondo A, Schiattarella M, Balato N and Ayala F: IL-33
is regulated by TNF-α in normal and psoriatic skin. Arch Dermatol
Res. 306:299–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Puzenat E, Bronsard V, Prey S, Gourraud
PA, Aractingi S, Bagot M, Cribier B, Joly P, Julien D, Le Maitre M,
et al: What are the best outcome measures for assessing plaque
psoriasis severity? A systematic review of the literature. J Eur
Acad Dermatol Venereol. 24:(Suppl 2). 10–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vageli D, Daniil Z, Dahabreh J, Karagianni
E, Vamvakopoulou DN, Ioannou MG, Scarpinato K, Vamvakopoulos NC,
Gourgoulianis KI and Koukoulis GK: Phenotypic mismatch repair hMSH2
and hMLH1b gene expression profiles in primary non-small cell lung
carcinomas. Lung Cancer. 64:282–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saraceno R, Saggini A, Pietroleonardo L
and Chimenti S: Infliximab in the treatment of plaque type
psoriasis. Clin Cosmet Investig Dermatol. 2:27–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Panzer R, Blobel C, Fölster-Holst R and
Proksch E: TLR-2 and TLR4 expression in atopic dermatitis, contact
dermatitis and psoriasis. Exp Dermatol. 23:364–366. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hata TR, Afshar M, Miller J, Two AM, Kotol
P, Jackson M, Alexandrescu DT, Kabigting F, Gerber M, Lai Y and
Gallo RL: Etanercept decreases the innate immune wounding response
in psoriasis. Exp Dermatol. 22:599–601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garcia Rodriguez, Arias-Santiago S,
Perandrés-López R, Castellote L, Zumaquero E, Navarro P,
Buendía-Eisman A, Ruiz JC, Orgaz-Molina J and Sancho J: Increased
gene expression of Toll-like receptor 4 on peripheral blood
mononuclear cells in patients with psoriasis. J Eur Acad Dermatol
Venereol. 27:242–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meephansan J, Komine M, Tsuda H, Karakawa
M, Tominaga S and Ohtsuki M: Expression of IL-33 in the epidermis:
The mechanism of induction by IL-17. J Dermatol Sci. 71:107–114.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Choi YS, Park JA, Kim J, Rho SS, Park H,
Kim YM and Kwon YG: Nuclear IL-33 is a transcriptional regulator of
NF-κB p65 and induces endothelial cell activation. Biochem Biophys
Res Commun. 421:305–311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ali S, Mohs A, Thomas M, Klare J, Ross R,
Schmitz ML and Martin MU: The dual function cytokine IL-33
interacts with the transcription factor NF-κB to dampen
NF-κB-stimulated gene transcription. J Immunol. 187:1609–1616.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li C, Li H, Jiang Z, Zhang T, Wang Y, Li
Z, Wu Y, Ji S, Xiao S, Ryffel B, et al: Interleukin-33 increases
antibacterial defense by activation of inducible nitric oxide
synthase in skin. PLoS Pathog. 10:e10039182014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hägg D, Eriksson M, Sundström A and
Schmitt-Egenolf M: The higher proportion of men with psoriasis
treated with biologics may be explained by more severe disease in
men. PLoS One. 8:e636192013. View Article : Google Scholar : PubMed/NCBI
|
















