Introduction
Polycystic ovary syndrome (PCOS) is a common cause
of reproductive endocrinopathy in women and is characterized by
hyperandrogenism, chronic oligo-anovulation and insulin-resistance
(1). Previous studies have suggested
that PCOS not only leads to disorders of the reproductive axis and
reproductive function, but also contributes to the abnormal
metabolism of glucose and aliphatic acid, increasing the risk of
endometrial and breast cancers (2,3). For
infertile woman with PCOS, clomiphene citrate (CC) remains the
first-line treatment; however, 15–40% of women do not resume
ovulation following CC treatment, which is defined as CC-resistance
(4).
Currently, the most common treatments for
CC-resistant PCOS are laparoscopic ovarian drilling (LOD) and
gonadotropin treatment. Successful pregnancy outcomes for both
treatments have been reported (5).
There are, however, disadvantages to LOD, as it requires
hospitalization and general anesthesia and may lead to pelvic
adhesion and ovarian function decrease, which would hinder any
subsequent pregnancies. Due to the high sensitivity of the ovaries
to gonadotropin stimulation, treatment with human menopausal
gonadotropin or pure follicle-stimulating hormone (FSH) is
challenging to control and is individually administered to induce
several ovulatory follicles, which incurs a substantial increased
risk of multiple pregnancies and ovarian hyperstimulation syndrome
(OHSS) (6). In addition, the cost of
gonadotropin treatment could add a financial burden to the
infertile patient; therefore, a convenient, economic and safe
treatment method for CC-resistant PCOS is required (7).
Letrozole (LE) is a potent and selective
third-generation aromatase inhibitor (AI), which can effectively
and highly selectively block the production of estrogen without
disturbing other steroidogenic pathways. LE was first used to treat
breast cancer and was found to be superior to the previous gold
standard, tamoxifen, and more effective than other AIs. Mitwally
and Casper (8,9) introduced LE to the ovulation induction
field; since then, numerous investigations into LE-induced
ovulation have been performed (10–12).
According to the reports, the ovulation rate in women with
CC-resistant PCOS is between 54.6 and 84.4%. The aim of the present
study was to compare LE with LOD, in order to determine a safer,
more efficacious and economical method of treating CC-resistant
PCOS.
Patients and methods
Patient selection
The present study followed 141 women attending the
Center for Reproductive Medicine of Tongji University (Shanghai,
China). The women were diagnosed with PCOS based on the Revised
2003 Consensus Diagnostic Criteria for PCOS (13). This study was approved by Tongji
Hospital Research Ethics Committee (Shanghai, China), and all
participants provided informed consent prior to inclusion in the
trial.
Inclusion criteria
The criteria for inclusion in the trial were as
follows: Clomiphene resistance, i.e. failure to ovulate following
100 mg CC for 5 days for at least three cycles; patent fallopian
tubes, confirmed by hysterosalpingography or hysteroscopic
diagnosis; normal semen analysis parameters of the patients'
spouses according to the modified criteria of the World Health
Organization (14); normal serum
prolactin, thyroid stimulating hormone and 17-OH progesterone; no
systemic disease; no gonadotropin or other hormonal drug treatment
during the preceding 3 months; normal blood count and blood
chemistry, including glutamic-pyruvic transaminase,
glutamic-oxaloacetic transaminase, urea nitrogen, creatinine,
glucose and urine analysis. The semen of the patients' spouses was
tested to strengthen the comparibility between the two groups.
During the period of treatment, all patients were requested to
follow a normal diet and rest regime and to avoid intense physical
activities in any form and mental stress and fatigue.
Exclusion criteria
The exclusion criteria were as follows: Infertility
induced by reasons other than PCOS; uterine cavity lesions or
ovarian cyst; >40 years old; body mass index (BMI) >26
kg/m2; contraindications to general anesthesia; history
of pelvic surgery; other endocrine diseases; or a history of liver
or kidney disease.
Hormone assays and transvaginal
ultrasound
The patients underwent baseline hormone assays for
FSH, luteinizing hormone (LH), estradiol (E2) and free
testosterone on the third day of menses, and the LH/FSH ratio was
calculated. At the same time, the ovary volume, antral follicle
counts and endometrial thickness were measured by transvaginal
ultrasound. For patients with amenorrhea or with irregular cycles,
the baseline hormone assays were taken improvisationally.
Intervention and follow-up
The women were randomly allocated into the either
the LE or LOD group (groups A and B, respectively). No medical
leading was made during the decision making process. Once the
patients had been allocated to one of the two groups, the treatment
was revealed to the investigator; however, the doctor responsible
for performing the transvaginal ultrasound follow-up assessment was
blinded to the treatment groups.
In group A, 2.5 mg LE oral tablets (Adooq
Bioscience, Nanjing, China) were administered on the fifth day of
menses and then every day for 5 days. Treatment was repeated for up
to six cycles if the patient failed to conceive. In group B,
laparoscopy was performed under intravenous general anesthesia
(Diprivan; AstraZeneca S.p.A., Rome, Italy) with the patient in a
supine position. A 5-mm incision was made in the navel, through
which a long sheath punctured into the abdominal cavity, and the
inflatable pneumoperitoneum (Guangxi University, Yuannan, China)
was placed. Another two 5-mm incisions were made on the right and
left lower abdomen and the surgical instruments were inserted into
the abdominal cavity. The patient was adjusted into a position with
the head high up, the pelvic organs were exposed and a
comprehensive exploration of the pelvic organs was made, focusing
on the structure and position of the adjacent organs of the
bilateral ovaries. Once immobilized, each ovary was cauterized at
4–6 points, each for 4 sec at 40 W, at a depth of 7–8 mm and a
diameter of 3–5 mm, using a monopolar electrosurgical needle
(Kirgen Co., Shanghai, China), according to the size of each ovary.
Following cauterization, a bilateral tubal hydrotubation with
methylene blue was performed. During the procedure, small pieces of
the ovaries were obtained for pathological analysis. The pelvis was
irrigated using physiological saline. Ringer's solution (ZiQi
Bioscience, Shanghai, China) plus dexamethasone was added into the
abdominal cavity to avoid adhesion. The total duration of the
procedure, as well as any intra-operative or post-operative
complications, was noted. The patients were followed-up for 6
months after the procedure.
In both groups, hormone levels were monitored every
third day of menstruation in each cycle following treatment, and
comparisons were made with the baseline data in the second
menstruation after LE treatment or LOD surgery. The endometrial
thickness and follicle size were monitored on days 10, 12 and 14 of
menses and the subsequent surveillance time-point was adjusted
according to the individual situation until ovulation. Ovulation
frequency and mean follicular diameters were recorded in both
groups during the six cycles. When at least one dominant follicle
reached a diameter of 18–22 mm, 8,000 IU human chorionic
gonadotropin (hCG; ZiQi Bioscience) was injected and natural
intercourse was advised for 36–48 h. The serum hCG concentration
was measured 14 days after hCG injection. Biochemical pregnancy was
considered when hCG was >2.5 mIU/ml in the absence of
menstruation, and clinical pregnancy was defined by a fetal heart
beat monitored by ultrasound at 6 weeks of gestation. Comparisons
of biochemical and clinical pregnancy rates between the two groups
were made.
Statistical analysis
Data were collected and analyzed using the
Statistical Package for Social Sciences software version 21.0 (IBM
SPSS, Armonk, NY, USA). The measurement data are presented as the
mean ± standard deviation. Proportions were compared using the
χ2 test. A P-value of <0.01 was considered to
indicate a statistically significant difference.
Results
Patient data
Of the 147 patients assessed for eligibility, four
did not meet the inclusion criteria and two did not consent to
participate. The remaining 141 patients were randomly assigned to
group A (LE treatment, n=71) or group B (LOD, n=70). No
statistically significant differences were found between the two
groups in terms of age, BMI, duration of infertility, ovarian
volume, amenorrhea rates or baseline hormone levels, including LH,
FSH, LH/FSH, E2 and free testosterone. The baseline
hormone levels were taken at the third day of menstruation
(Table I).
 | Table I.Baseline clinical and hormonal
profiles of the study participants. |
Table I.
Baseline clinical and hormonal
profiles of the study participants.
| Parameter | Group A, n=71 | Group B, n=70 | P-value |
|---|
| Agea, years | 29.50±3.26 | 28.08±3.61 | 0.707 |
| BMIa, kg/m2 | 22.50±1.46 | 22.41±2.07 | 0.253 |
|
Amenorrheab, n/total n (%) | 9/71
(11.2) | 12/70 (17.1) | 0.456 |
| Years of
infertilitya |
3.35±0.43 |
3.16±0.66 | 0.120 |
| Volume of
ovarya, ml | 11.47±1.45 | 12.20±1.11 | 0.262 |
| D3 LHa, mIU/ml | 12.25±1.34 | 12.55±1.17 | 0.359 |
| D3 FSHa, mIU/ml |
5.28±0.31 |
5.21±0.29 | 0.117 |
| D3 LH/FSH
ratioa |
2.57±0.24 |
2.48±0.27 | 0.176 |
| D3
E2a,
pg/ml | 50.82±9.49 | 50.21±9.86 | 0.323 |
|
Hyperandrogenismb,c, n/total n (%) | 22/71 (20.4) | 19/70 (27.1) | 0.615 |
Hormonal characteristics
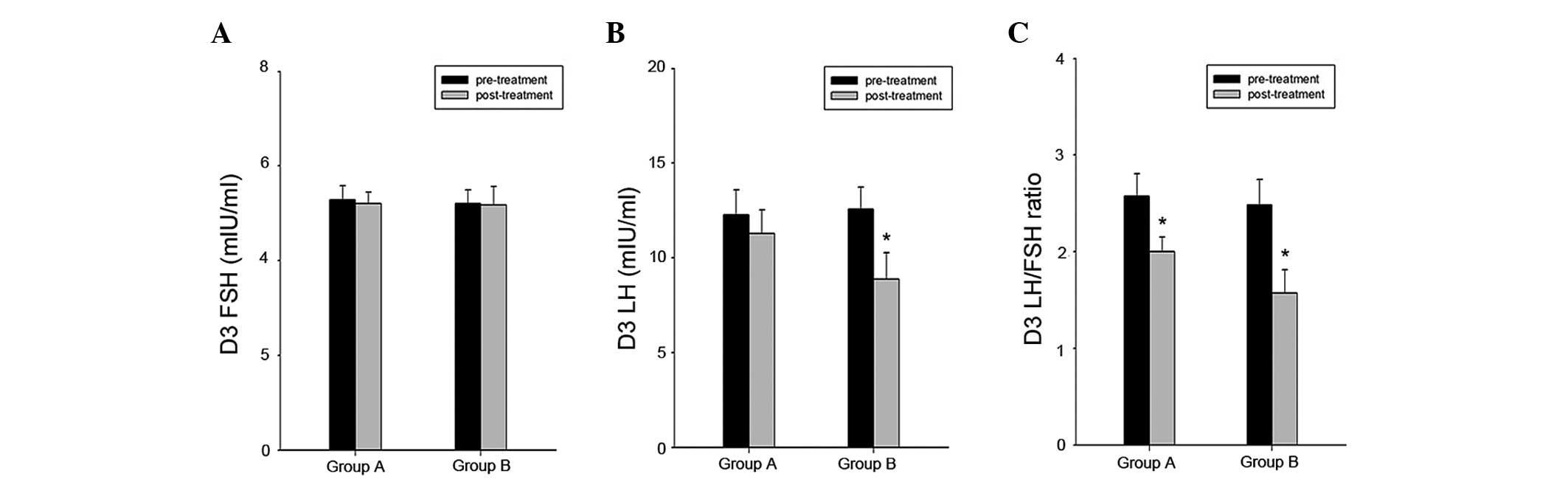
In the second cycle after treatment, the hormone
levels were again measured in the two groups (Table II). Compared with the group B
patients, the group A patients had a significantly higher LH level
(11.30±1.22 vs. 8.89±1.40, P<0.01) and LH/FSH ratio (2.00±0.15
vs. 1.57±0.24, P<0.01). The two groups had a similar level of
FSH, E2 and free testosterone. When comparing the pre-
and post-treatment hormone levels in the two groups (Fig. 1), no clear change in the FSH level
was found in either of the groups. There was, however, a marked
decrease in the level of LH in the group B. A reduction was also
observed in the LH/FSH ratio in the two groups, which was
statistically significant in both groups.
 | Table II.Hormonal characteristics of patients
in the second cycle. |
Table II.
Hormonal characteristics of patients
in the second cycle.
| Hormone | Group A, n=71 | Group B, n=70 | P-value |
|---|
| D3 LHa, mIU/ml | 11.30±1.22 |
8.89±1.40 | <0.001 |
| D3 FSHa, mIU/ml |
5.20±0.24 |
5.17±0.40 |
0.332 |
| D3 LH/FSH
ratioa |
2.00±0.15 |
1.57±0.24 | <0.001 |
| D3
E2a,
pg/ml | 50.23±9.86 | 52.46±9.42 |
0.271 |
|
Hyperandrogenismb,c, n/total n (%) | 20/71 (28.2) | 4/70 (5.7) | <0.001 |
Reproductive outcomes
The clinical and reproductive outcomes are presented
in Table III. The women were
studied for 382 (group A) and 358 (group B) cycles. Ovulation
occurred in 305 out of 382 cycles (79.8%) in group A (the LE group)
and 237 out of 358 cycles (66.2%) in group B (the LOD group)
(P<0.01). In the 305 cycles with ovulation in group A, there
were 249 synchronous cycles, in which the endometrium matched with
follicle development, which favored implantation; by comparison,
132 cycles out of the 237 in group B were synchronous. The
difference between the two groups was statistically significant
(81.6 vs. 55.7%, P<0.01). Typical synchronous and
non-synchronous images are shown in Fig.
2. The ultrasound images were captured on the day of hCG
injection. The endometrial thickness measured on the day of the hCG
injection was observed to be significantly increased in group A
compared with that in group B (7.82±1.7 vs. 6.21±1.46 mm,
P<0.01).
 | Table III.Reproductive outcomes following
treatment in the two groups. |
Table III.
Reproductive outcomes following
treatment in the two groups.
| Reproductive
outcome | Group A, n=71 | Group B, n=70 | P-value |
|---|
| Ovulation rate,
n/total n (%) | 305/382 (79.8) | 237/358 (66.2) | <0.001 |
| Endometrial
thicknessa, mm | 7.82±1.70 | 6.21±1.46 |
0.324 |
| Synchronous cycles,
n/total n (%) | 249/305 (81.6) | 132/237 (55.7) | <0.001 |
| Clinical pregnancy
rate, n/total n (%) | 29/71 (40.8) | 19/70 (27.1) |
0.086 |
| Spontaneous
abortion rate, n/total n (%) | 2/29 (6.9) | 3/19
(15.8) |
0.372 |
| Live birth rate,
n/total n (%) | 27/71 (38.0) | 16/70 (22.9) |
0.050 |
Clinical pregnancy occurred in 29 out of 71 (40.8%)
women in group A and 19 out of 70 (27.1%) in group B (P>0.01).
Two out of the 29 (6.9%) women in group A had a spontaneous
abortion, whereas in group B, 3 out of the 19 (15.8%) women
underwent spontaneous abortion; despite the fact that the
spontaneous abortion rate was lower in group A, the difference was
not statistically significant (P>0.01). The two groups had a
comparable live birth rate (38.0 vs. 22.9% in groups A and B,
respectively, P=0.05). One case of twin pregnancy was observed in
group A and no case of OHSS occurred in either group.
Discussion
Previous studies (15,16) have
suggested that the mechanism by which LE stimulates ovulation may
have two parts: The central and the peripheral mechanisms. In the
central mechanism, LE acts on the hypothalamus and pituitary in the
early follicular phase, and aromatase is then is inhibited. The
conversion of testosterone to estrogen is hindered and levels of
estrogen in the body are reduced to terminate the negative feedback
effect of the hypothalamus or pituitary. FSH is secreted and
promotes follicular maturation and ovulation. In the peripheral
mechanism, aromatase is a rate-limiting enzyme for testosterone
production. LE mainly acts as an AI and prevents the conversion of
testosterone to estrogen; testosterone rapidly accumulates in the
ovary and FSH receptor gene expression is amplified directly or
indirectly; therefore, the follicle is more sensitive to FSH. In
addition, testosterone can stimulate insulin-like growth factor, as
well as other endocrine and paracrine factors, which promotes the
follicular development and ovulation together with FSH (17).
Surgical treatment for PCOS first took place in 1935
(18), when laparotomy ovarian wedge
resection was the only way to treat anovulatory PCOS; however, with
the development of the retroperitoneal laparoscopic technique, LOD
gradually replaced laparotomy ovarian wedge resection. We
hypothesize that burning and puncturing the follicle is the main
mechanism underlying the efficacy of LOD, as it encourages
follicular fluid flow and reduces or eliminates the influence of
abnormal hormone and factor levels in the follicle on ovarian
function. Furthermore, surgery destroys some of the abnormal
structure of the ovary and partially mitigates the abnormal
function; therefore, the synthesis of hormones and regulating and
growth factors in the ovary is subsequently normalized (19). While the stimulatory and inhibitory
interaction between various hormones and factors results in the
functionality of the hypothalamus-pituitary-ovarian axis,
improvements in the internal ovarian environment can induce normal
local control. Patients with PCOS can thus eventually acquire
normal ovulatory function (20).
Several studies have been conducted regarding the
treatment of CC-resistant PCOS with LE or LOD, and positive results
have been achieved; however, there have been few studies comparing
the effects of the two treatments (21–23). The
present study demonstrated that LE had a superior effect in
treating CC-resistant PCOS compared with LOD, and the endometrium
was significantly thicker in the LE group than that in the LOD
group on the day of hCG injection, which may have resulted from
improved angiogenesis of the uterus. In addition, LE has a
relatively short half-life of 45 h (CC is 28 days) and, therefore,
the lack of estrogen caused by LE would not effect the endometrium
and cervix for long (24,25). Furthermore, on the day of hCG
injection, ovulation was better synchronized with endometrial
development in the LE group, compared with that in the LOD group,
which was likely due to LE stimulating more follicles than LOD.
The present study indicated that the LE group had a
lower miscarriage rate, although no statistically significant
difference was found between the two groups, which have may been
due to the small sample size. According to the statistical results,
the data showed that the LE group had a better follicle quality,
thicker endometrium and more synchronization. In conclusion,
compared with LOD treatment, LE treatment is more easily
administered and more affordable. In addition, it was shown to
improve the ovulation and pregnancy rate of patients with
refractory PCOS, especially when combined with LOD. Therefore, LE
may be considered a first-line treatment for PCOS.
Acknowledgements
The present study was supported by the Shanghai
Natural Science Foundation (grant no. 12ZR1434200).
References
|
1
|
Tehrani FR, Simbar M, Tohidi M,
Hosseinpanah F and Azizi F: The prevalence of polycystic ovary
syndrome in a community sample of Iranian population: Iranian PCOS
prevalence study. Reprod Biol Endocrinol. 9:392011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brown J, Farquhar C, Beck J, Boothroyd C
and Hughes E: Clomiphene and anti-oestrogens for ovulation
induction in PCOS. Cochrane Database Syst Rev CD002249. 2009.
View Article : Google Scholar
|
|
3
|
Legro RS, Barnhart HX, Schlaff WD, Carr
BR, Diamond MP, Carson SA, Steinkampf MP, Coutifaris C, McGovern
PG, Cataldo NA, et al: Cooperative Multicenter Reproductive
Medicine Network: Clomiphene, metformin, or both for infertility in
the polycystic ovary syndrome. N Engl J Med. 356:551–566. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National Institute for Health and Care
Excellence: Fertility: Assessment and Treatment for People with
Fertility Problems. Guidance. National Collaborating Centre for
Women's and Children's Health; London, UK: 2004
|
|
5
|
Palomba S, Falbo A and Zullo F: Management
strategies for ovulation induction in women with polycystic ovary
syndrome and known clomifene citrate resistance. Curr Opin Obstet
Gynecol. 21:465–473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bayram N, van Wely M, Kaaijk EM, Bossuyt
PM and van der Veen F: Using an electrocautery strategy or
recombinant follicle stimulating hormone to induce ovulation in
polycystic ovary syndrome: Randomised controlled trial. BMJ.
328:1922004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farquhar CM, Williamson K, Gudex G,
Johnson NP, Garland J and Sadler L: A randomized controlled trial
of laparoscopic ovarian diathermy vs. gonadotropin therapy for
women with clomiphene citrate-resistant polycystic ovary syndrome.
Fertil Steril. 78:404–411. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitwally MF and Casper RF: Use of an
aromatase inhibitor for induction of ovulation in patients with an
inadequate response to clomiphene citrate. Fertil Steril.
75:305–309. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitwally MF and Casper RF: Aromatase
inhibition reduces gonadotrophin dose required for controlled
ovarian stimulation in women with unexplained infertility. Hum
Reprod. 18:1588–1597. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Casper RF and Mitwally MF: Use of
aromatase inhibitor letrozole for ovulation induction in women with
polycystic ovarian syndrome. Clin Obstet Gynecol. 4:685–695. 2011.
View Article : Google Scholar
|
|
11
|
Ramezanzadeh F, Nasiri R, Sarafraz Yazdi M
and Baghrei M: A randomized trial of ovulation induction with two
different doses of Letrozole in women with PCOS. Arch Gynecol
Obstet. 284:1029–1034. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang HY and Zheng PS: A comparison of the
efficacy of two doses of letrozole alone or with continuous
recombinant follicle-stimulating hormone for ovulation induction in
anovulatory women. Gynecol Obstet Invest. 4:250–255. 2015.
|
|
13
|
Rotterdam ESHRE/ASRM-Sponsored PCOS
consensus workshop group: Revised 2003 consensus on diagnostic
criteria and long-term health risks related to polycystic ovary
syndrome (PCOS). Hum Reprod. 19:41–47. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sills ES, Fryman JT, Perloe M, Michels KB
and Tucker MJ: Chromatin fluorescence characteristics and standard
semen analysis parameters: Correlations observed in andrology
testing among 136 males referred for infertility evaluation. J
Obstet Gynaecol. 24:74–77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elnashar A, Fouad H, Eldosoky M and Saeid
N: Letrozole induction of ovulation in women with clomiphene
citrate-resistant polycystic ovary syndrome may not depend on the
period of infertility, the body mass index, or the luteinizing
hormone/follicle-stimulating hormone ratio. Fertil Steril.
85:511–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al Omari, Sulaiman WR and Al-Hadithi N:
Comparison of two aromatase inhibitors in women with
clomiphene-resistant polycystic ovary syndrome. Int J Gynaecol
Obstet. 85:289–291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baruah J, Roy KK, Rahman SM, Kumar S,
Sharma JB and Karmakar D: Endometrial effects of letrozole and
clomiphene citrate in women with polycystic ovary syndrome using
spiral artery Doppler. Arch Gynecol Obstet. 279:311–314. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Unlu C and Atabekoglu CS: Surgical
treatment in polycystic ovary syndrome. Curr Opin Obstet Gynecol.
18:286–292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salah IM: Office microlaparoscopic ovarian
drilling (OMLOD) versus conventional laparoscopic ovarian drilling
(LOD) for women with polycystic ovary syndrome. Arch Gynecol
Obstet. 287:361–367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vendola K, Zhou J, Wang J, Famuyiwa OA,
Bievre M and Bondy CA: Androgens promote oocyte insulin-like growth
factor I expression and initiation of follicle development in the
primate ovary. Biol Reprod. 61:353–357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siebert TI, Kruger TF, Steyn DW and
Nosarka S: Is the addition of metformin efficacious in the
treatment of clomiphene citrate-resistant patients with polycystic
ovary syndrome? A structured literature review. Fertil Steril.
86:1432–1437. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fisher SA, Reid RL, Van Vugt DA and Casper
RF: A randomized double-blind comparison of the effects of
clomiphene citrate and the aromatase inhibitor letrozole on
ovulatory function in normal women. Fertil Steril. 78:280–285.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abdellah MS: Reproductive outcome after
letrozole vs. laparoscopic ovarian drilling for
clomiphene-resistant polycystic ovary syndrome. Int J Gynaecol
Obstet. 113:218–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hendawy SF, Samaha HE and Elkholy MF:
Letrozole vs. clomiphene citrate for induction of ovulation in
patients with polycystic ovarian syndrome undergoing intrauterine
insemination. Clin Med Insights Reprod Health. 5:11–16.
2011.PubMed/NCBI
|
|
25
|
Abu Hashim H, Mashaly AM and Badawy A:
Letrozole vs. laparoscopic ovarian diathermy for ovulation
induction in clomiphene-resistant women with polycystic ovary
syndrome: A randomized controlled trial. Arch Gynecol Obstet.
282:567–571. 2010. View Article : Google Scholar : PubMed/NCBI
|