Introduction
Choroidal neovascularization (CNV) is a pathological
neovascularization that affects choroids, as these new vessels have
high permeability, structural defects, and a high susceptibility to
bleeding and seepage. Such defective vasculature can lead to the
formation of scars, which can greatly affect visual function
(1); thus, CNV is one of the leading
causes of blindness. The pathogenesis of CNV is not yet completely
understood and studies have indicated that retinal pigment
epithelial cells (RPE cells) exposed to hypoxia play an important
role in the development of CNV (2).
RPE cells are located between the neural retina and choroids. In
normal eyes, the retinal pigment epithelium (RPE), together with
Bruch's membrane and choriocapillaris, form a complex. During CNV,
choroidal capillary endothelial cells of the complex keep dividing
and proliferating continuously, forming new blood vessels and
entering into the site between Bruch's membrane and the RPE layer,
or between the RPE layer and the neurosensory retina (3). It has been suggested that in the RPE,
hypoxia is caused by the reduction of choroid local blood flow and
the oxygen diffusion barrier, which ranges from choroids to the RPE
and neuroepithelium. Hypoxic RPE cells secrete large amounts of
cytokines and factors that induce angiogenesis, such as vascular
endothelial growth factor (VEGF), basic fibroblast growth factor
(bFGF), as well as transforming growth factor (TGF), angiopoietin
(Ang), and receptor tyrosine kinases Tie1 and Tie2 (4,5). VEGF
plays an important role in angiogenesis and leakage and is involved
in all stages of diabetic retinopathy (6). It has been demonstrated that the
expression of VEGF in RPE cells, retinal endothelial cells,
pericytes and Müller cells increases under disease conditions
(5). Hypoxia induces the production
of hypoxia-inducible factor (HIF), which elevates the transcription
and stability of VEGF and VEGF receptor (VEGFR), and enhances the
biological effects of VEGF. When normoxia is restored, the
VEGF mRNA levels decrease to baseline levels (7). Thus, by controlling the activity of RPE
cells, it may be possible to inhibit the induction of VEGF and
attenuate the formation of new blood vessels.
It is known that the VEGFA/VEGFR-2 axis is a key
regulated signaling pathway of angiogenesis (8). In vascular endothelial cells, the
presence of VEGFR-2 along with its ligand, VEGFA, promote
endothelial cell mitosis and chemotactic response, thus leading to
the formation of new blood vessels (9). Drugs such as ranibizumab, bevacizumab,
pegaptanib, as well as others block the VEGFA/VEGFR-2 axis and
inhibit the development of CNV. These drugs have achieved some
therapeutic effects, which has provided hope for the treatment of
retinopathy and CNV. However, these drugs only have a single
application point (single point of action) and their therapeutic
effects are limited (10). It has
been demonstrated that VEGF inhibitors, although effective during
the early stages of treatment, gradually lose effectiveness due to
the development of drug resistance, and do not effectively inhibit
angiogenesis in long-term therapy (11). However, the inhibition of VEGF alone
may lead to the activation of other types of pro-angiogenic factors
released from histiocytes, which can also promote angiogenesis
(12). Therefore, it is necessary to
further explore other signaling pathways of angiogenesis as
therapeutic targets.
It has previously been suggested that VEGFR-3 is
only associated with the formation of lymphatic vessels (13). However, it has also been found that
embryonic VEGFR-3 is also involved in angiogenesis (14). VEGFR3 expression is confined to the
lymphatic vasculature in benign lesions; however, its expression
increases during wound healing and tumor angiogenesis (15,16). As
shown in the study by Yuasa et al, the expression of VEGFR-3
may be a suitable biomarker to predicet response to renal disease
(17), which suggests that the
activation of VEGFR-3 plays an important role in angiogenesis
(3). Tammela et al reported
that the inhibition of VEGFR-3 with a monoclonal antibody reduced
vascular sprouting, vascular branches and endothelial cell
proliferation during embryonic development and tumor growth
(14), which suggests that VEGFR-3
is a novel target in the treatment of CNV.
Fenofibrate is a common lipid-lowering drug, which
reduces plasma triglyceride and low-density lipoprotein cholesterol
levels, and increases high-density lipoprotein cholesterol levels
in patients with hyperlipidemia. Apart from its lipid-lowering
effects, fenofibrate has several other effects, such as the
improvement of vascular endothelial function, anti-inflammatory and
antioxidant effects and the inhibition of angiogenesis (18–20). In
recent years, fibrate lipid-lowering drugs have been studied
extensively regarding diabetic retinal neovascularization (21–23);
however, to the best of our knowledge, there is no information
available to date on their effects on CNV. Researchers have focused
on the association between VEGFA-VEGFR-2 and neovascular disease
(24), but not on VEGFC-VEGFR-3.
Thus, in the present study, we examined the mechanisms of action of
fenofibrate using an RPE cell model of hypoxia, in an aim to
determine whether fenofibrate exerts an effect on RPE cells to
influence the secretion of VEGFC, thus altering the function of
endothelial cells.
Materials and methods
Reagents and kits
Dulbecco's modified Eagle's medium (DMEM) containing
L-glutamine, fetal bovine serum (FBS), penicillin-streptomycin and
0.25% pancreatin-ethylenediaminetetraacetic acid (EDTA) were all
purchased from Gibco/Invitrogen, Grand Island, NY, USA. ECA medium
was obtained from ScienCell (San Diego, CA, USA); the cell culture
plate was from Corning Inc., Corning, NY, USA; the 0.22 µm
disposable filter was obtained from Millipore (Billerica, MA, USA);
MTT reagent and cobalt(II) chloride (CoCl2) were both
from Aladdin Reagent (Shanghai) Co., Ltd., Shanghai, China; the
superoxide anion probe was from Beyotime, Shanghai, China;
fenofibrate was obtained from Sigma Chemical Co., St. Louis, MO,
USA; Matrigel was purchased from BD Biosciences, Bedford, MA, USA;
anti-VEGFR-3 antibody (20712-1-AP) was from Proteintech, Chicago,
IL, USA; anti-VEGFC antibody (sc-9047) was from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; anti-glyceraldehyde
3-phosphate dehydrogenase (GAPDH) antibody (P30008) and IgG-HRP
(M21002) were from Abmart (Shanghai, China); PVDF membranes were
purchased from Millipore; skim milk was purchased from BD
Biosciences; HRP-substrate coloring solution was obtained from
Millipore; the VEGFC enzyme-linked immunosorbent assay (ELISA) kit
and the VEGFR-3 ELISA kit were from USCN Life Science, Inc., Wuhan,
China.
Cell culture
RPE cells (CRL-2302) were obtained from ATCC
(Manassas, VA, USA) and human umbilical vein endothelial cells
(HUVECs) were from ScienCell. The RPE cell model of hypoxia was
established as follows: the RPE cells were cultured in a 37°C, 5%
CO2 saturated humidity incubator with DMEM (containing
1% penicillin-streptomycin and 10% FBS). The cells were grown to
60–70% cell density in DMEM containing 1% FBS for 24 h, and the
medium was then changed to 1% FBS DMEM containing 200 µmol/l
CoCl2, for the induction of hypoxia. Following culture
for 24 h, the old medium was replaced with DMEM (with 1% FBS)
containing 100 µmol/l fenofibrate + 200 µmol/l CoCl2 and
culture was continued for 24 h. Cells in the control group were
treated as follows: the cells were cultured in DMEM (with 1% FBS)
for 48 h, followed by culture in DMEM (with 1% FBS) containing 200
µmol/l CoCl2 for 48 h, and then with DMEM (with 1% FBS)
containing 100 µmol/l fenofibrate for 24 h. Cell culture was
conducted in in a 37°C and 5% CO2 saturated humidity
incubator. Following treatment with CoCl2 for 48 h, a
superoxide anion probe was used to measure the production of
superoxide anion, which is indicative of hypoxic conditions. The
HUVECs were cultured in a 37°C and 5% CO2 saturated
humidity incubator with extracellular matrix (ECM) medium (with 1%
penicillin-streptomycin, 10% FBS). The HUVECs were cultured with
RPE culture supernatant that was collected following the exposure
of the RPE cells to hypoxia, and the control cells were cultured in
DMEM (with 1% FBS). There were 8 samples in each group.
MTT assay
The cells were treated as described above. The cells
were seeded in a 96-well culture plate with 1×104
cells/well, and the following day, the cells were treated according
to their grouping. Following culture for 48 h, the culture medium
was removed and 100 µl MTT solution were added, followed by
incubation for 4 h in an incubator at 37°C; the MTT solution was
then removed and 100 µl DMSO were added to each well, followed by
mixing for 10 min, and the OD value was then measured at 570 nm
using a spectral scanning multimode reader (Varioskan Flash, Thermo
Fisher Scientific Inc., Waltham, MA, USA).
ELISA
Following subculture for 48 h, the RPE cells were
divided into 4 groups: group 1, CoCl2-induced hypoxia
for 48 h; group 2, exposure to CoCl2 for 48 h and 100
µmol/l fenofibrate treatment for 24 h; group 3, treatment with 100
µmol/l fenofibrate for 24 h; group 4, the control group was treated
with DMEM (with 1% FBS). The supernatant was then collected; 8
samples were taken from each group. Following filtration with a
0.22 µm filter, ELISA was used to detect VEGFC and VEGFR-3 protein
expression in the supernatant.
RT-qPCR
The RPE cells were divided into the following
groups: group 1, CoCl2-induced hypoxia for 48 h; group
2, exposure to CoCl2 for 48 h and fenofibrate treatment
for 24 h; group 3, treatment with fenofibrate for 24 h; group 4,
the control cells were treated with DMEM containing 1% FBS.
Following treatment, TRIzol reagent was used to extract the total
RNA. The RevertAid First Strand cDNA synthesis kit (Thermo Fisher
Scientific Inc.) was used for the synthesis of first strand of cDNA
by reverse transcription. The PCR conditions were as follows: cDNA
template 1 µl, forward primer 0.5 µl, reverse primer 0.5 µl
(Table I), FastStart Universal
SYBR-Green Master (Rox; Roche Life Science, Branford, CT, USA) 10
µl, nuclease-free water 8 µl, mixing, total volume of 20 µl, in
96-well PCR plates; triplicate wells for each gene in each sample
were taken, qPCR was carried out using an ABI 7300 qPCR instrument
(Applied Biosystems, Foster City, CA, USA). PCR reactions were
carried out as follows: stage 1: 95°C, 3 min; stage 2 (for 40
cycles): step 1, 95°C, 15 sec; step 2, 60°C, 30 sec. Melting curve
analysis was carried out as follows: 95°C: 15 sec; 60°C: 30 sec;
95°C: 15 sec. All experiments were repeated 3 times. The
2−ΔΔCt method was used to analyze the results.
 | Table I.Primers used for RT-qPCR. |
Table I.
Primers used for RT-qPCR.
| Human gene | Forward primer | Reverse primer |
|---|
| VEGFC (103
bp) |
5′-GTGTCCAGTGTAGATGAA-3′ |
5′-CCTGTTCTCTGTTATGTTG-3′ |
| VEGFR-3 (123
bp) |
5′-GAGGGAAAGAATAAGACT-3′ |
5′-GGTCACATAGAAGTAGAT-3′ |
| GAPDH (80
bp) |
5′-AAAGGGTCATCATCTCTG-3′ |
5′-GCTGTTGTCATACTTCTC-3′ |
Western blot analysis
The RPE cells were grouped in the same manner as in
RT-qPCR. Following digestion with 0.25% trypsin, total protein was
extracted from the cells in each group using RIPA buffer and
subjected to SDS-PAGE electrophoresis and transferred onto PVDF
membranes. The PVDF membranes were blocked with 5% skim milk at
room temperature for 1 h, followed by incubation with anti-VEGFC
and anti-VEGFR-3 antibodies overnight at 4°C (VEGFR-3, 1:200;
VEGFC, 1:500), and then with HRP-labeled universal secondary
antibody (1:2,000, Abmart) at room temperature for 2 h. GAPDH
antibody (1:1,000) was used as a loading control. The protein bands
were visualized with HRP-substrate coloring solution for 1 min and
ImageJ software was used to analyze the density of the bands and
protein quantity.
Wound healing assay
In vitro, the scratch-wound assay was used to
detect the migration of HUVECs, as previously described (25). The HUVECs were seeded in a 6-well
culture plate, and when the cells had attached completely, a
vertical straight line was drawn in each well using a 10 µl aseptic
suction head, by scraping the cells. The medium was then removed
and the cells were washed 2–3 times with phosphate-buffered saline
(PBS), followed by incubation with the culture supernatant from the
RPE cells exposed to CoCl2-induced hypoxia. The control
HUVECs were incubated in DMEM (with 1% FBS). They were cultured in
a 37°C, 5% CO2 incubator. A total of 8 samples was taken
from each group and images were captured under a microscope
(DMI3000 B, Leica Microsystems, Mannheim, Germany) at x200
magnification, at 24 and 0 h after scratching. ImageJ software was
used to measure the wound width.
Cell lumen formation
The cells were plated with Matrigel, and the HUVECs
were stimulated to form capillary-like lumen, as previously
described (26). Matrigel (60 µl)
was added to a well of a 96-well plate, and the culture plate was
shaken gently. Following Matrigel solidification, a 100 µl
suspension of HUVECs was added to each well at a cell density of
1×104. The cells were then treated with the supernatant
from RPE cells exposed to hypoxia, and cultured in a 37°C, 5%
CO2 incubator for 48 h. Vwssel lumen formation was
observed at x50 magnification and counted (at least 5 horizons were
randomly selected, counted and averaged; 8 samples were taken from
each group). The number of lumen formed represented the angiogenic
ability of the HUVECs.
Statistical analysis
Data are presented as the means ± standard deviation
(SD). SPSS 18.0 software (SPSS. Inc., Chicago, IL, USA) was used
for statistical analysis. One-way analysis of variance was used to
make comparisons between groups, and the t-test was used for
comparisons between 2 groups. A value of P<0.05 was considered
to indicate a statistically significant difference.
Results
CoCl2-induced hypoxia in
RPE cells
The RPE cells were treated with CoCl2 for
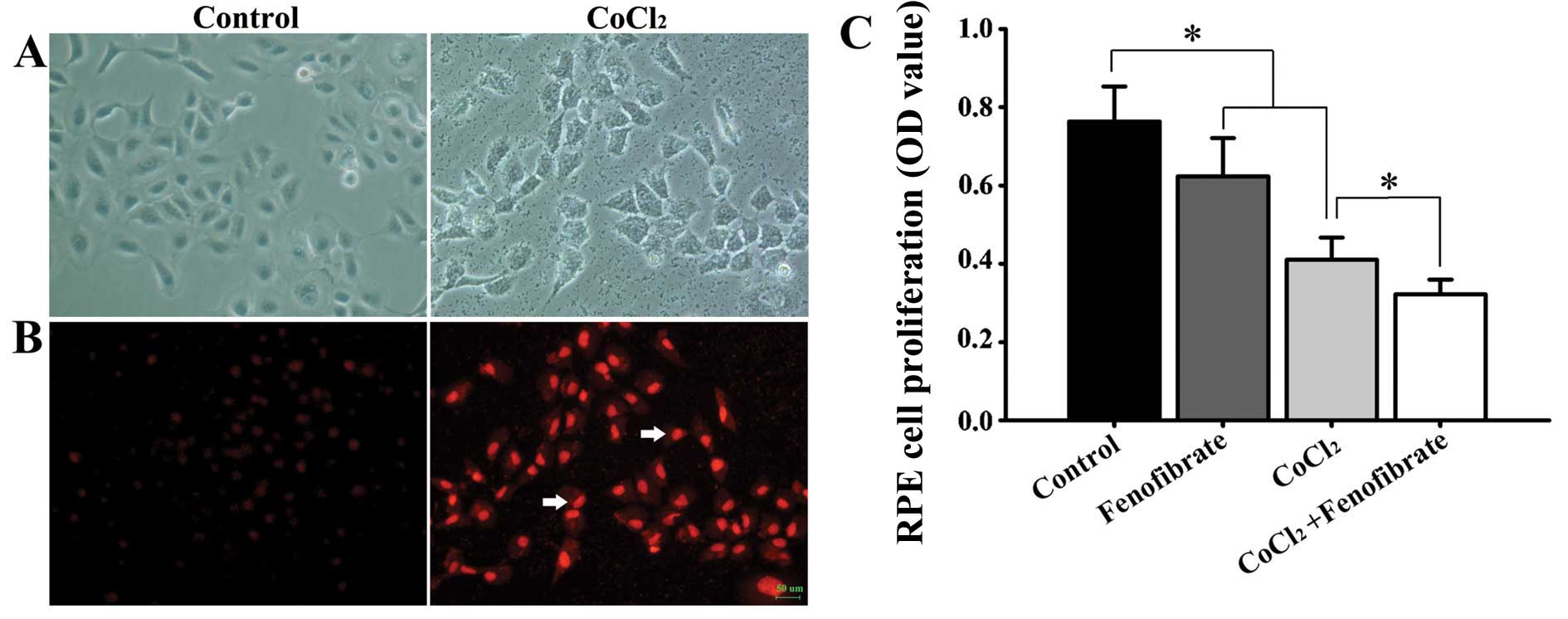
48 h to induce chemical hypoxia. Compared with the control group,
the cell body became round in the treated cells, the volume became
enlarged (Fig. 1A), the cell growth
rate was reduced, and the production of superoxide anion was
increased (Fig. 1B). Cell viability
decreased after the RPE cells were epxosed to hypoxia. The addition
of fenofibrate to the normal RPE cells led to a decrease in cell
viability. Treatment of the hypoxic RPE cells with fenofibrate
decreased the cell viability even further (Fig. 1C).
VEGFC and VEGFR-3 protein levels in
the RPE cell supernatant
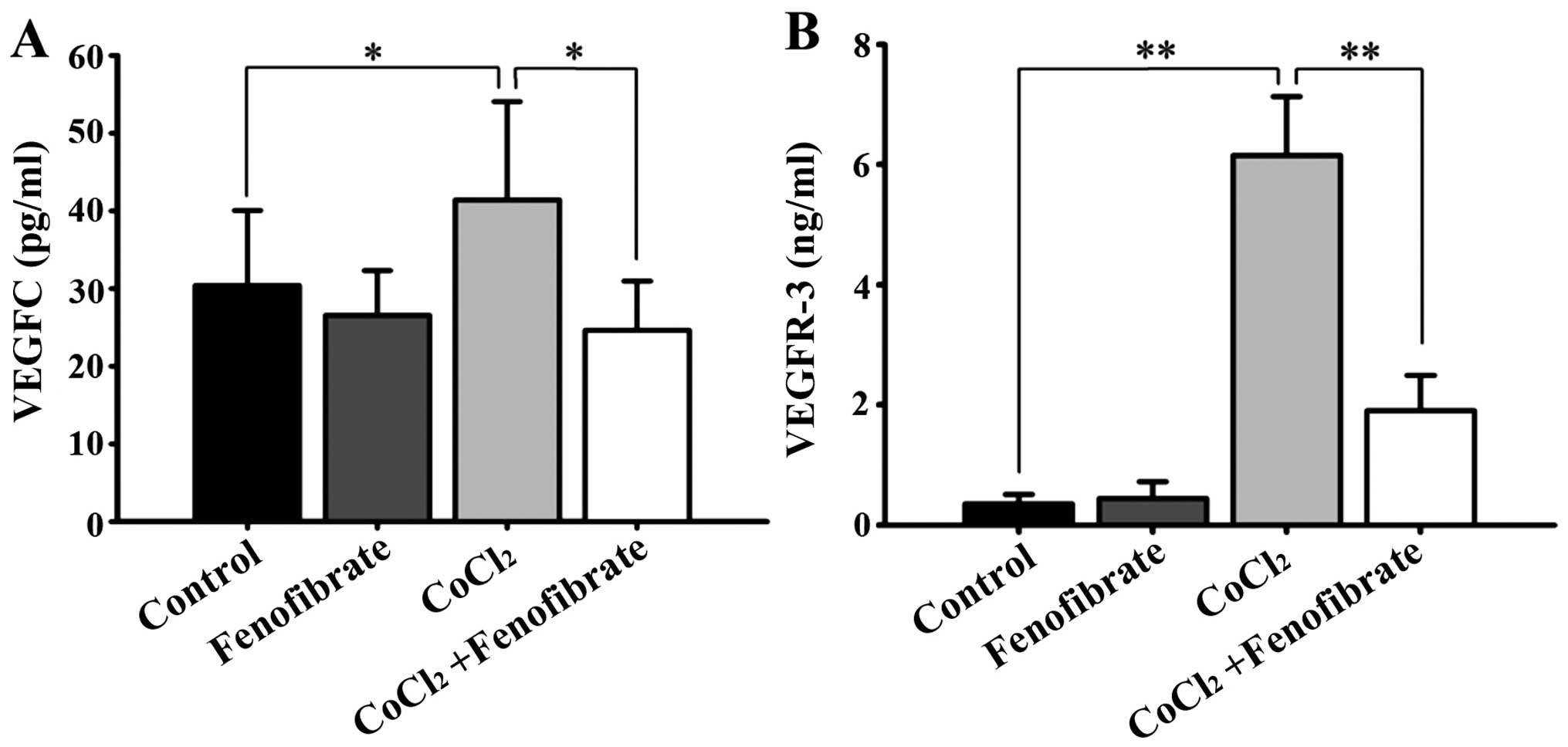
The normal RPE cells expressed VEGFR-3 at low
levels, but expressed a detectable amount of VEGFC. Following
CoCl2-induced hypoxia, the amount of VEGFC and VEGFR-3,
which was synthesized and secreted by the RPE cells into the cell
culture medium, increased significantly (Fig. 2). Fenofibrate had little effect on
the expression of VEGFC and VEGFR-3 in the normal RPE cells;
however, it significantly decreased the expression of VEGFC and
VEGFR-3 in the hypoxic RPE cells (Fig.
2).
Effects of fenofibrate on VEGFC and
VEGFR-3 expression in hypoxic RPE cells
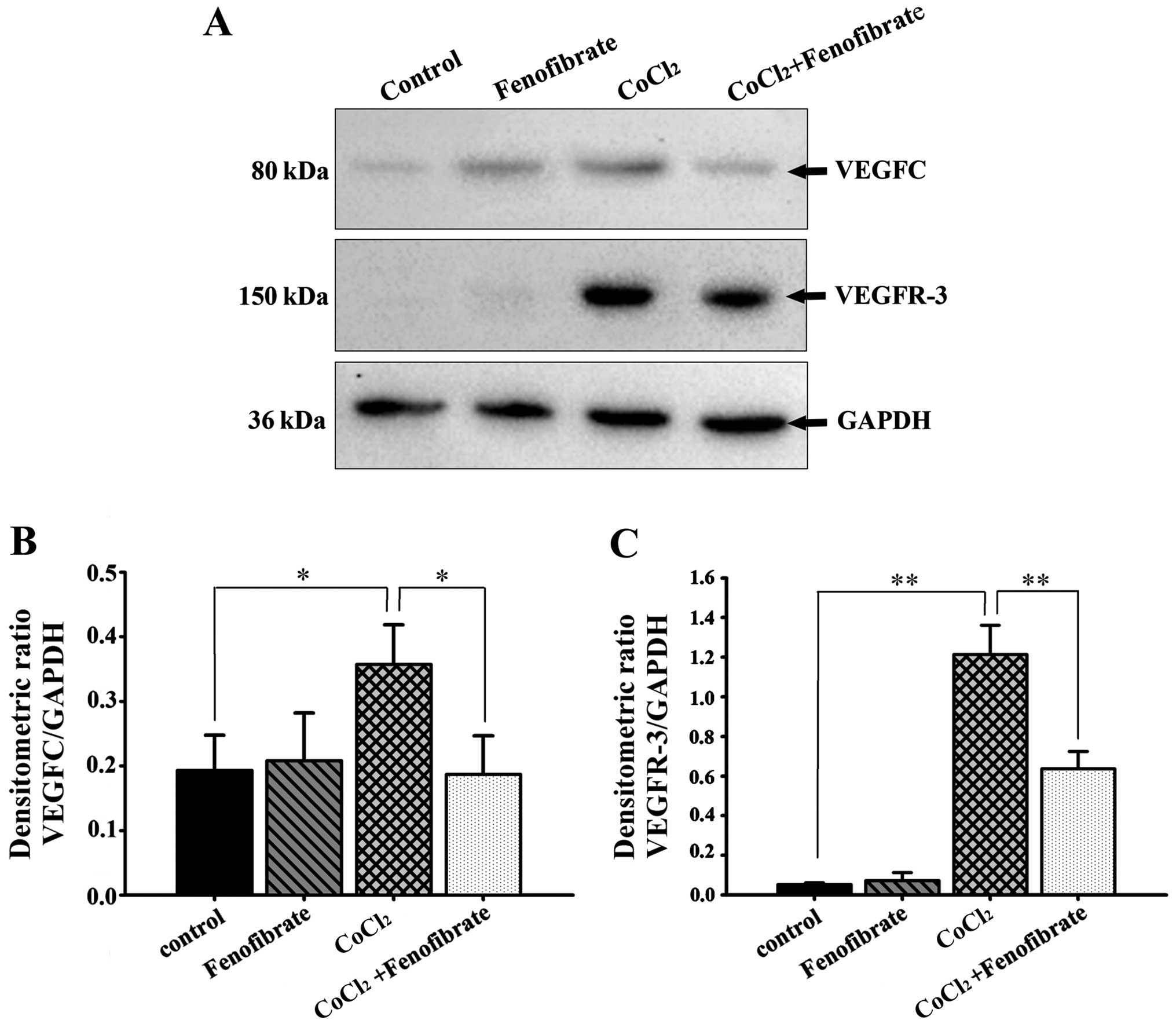
The expression levels of VEGFC and VEGFR-3 in the
cells that were cultured under hypoxic conditions with or without
fenofibrate, were measured by western blot analysis and RT-qPCR. In
comparison to the housekeeping gene, GAPDH, we calculated the
relative expression and found that there were detectable levels of
VEGFC expression in the normoxic RPE cells, but very low levels of
VEGFR-3 protein expression (Fig.
3A). The expression of both VEGFC and VEGFR-3 in the RPE cells
increased following the induction of hypoxia for 48 h; the increase
in VEGFR-3 expression was more significant. Following exposure to
hypoxia, the addition of fenofibrate and further incubation for 24
h significantly decreased the expression of VEGFC and VEGFR-3 in
the RPE cells (Fig. 3B and C). The
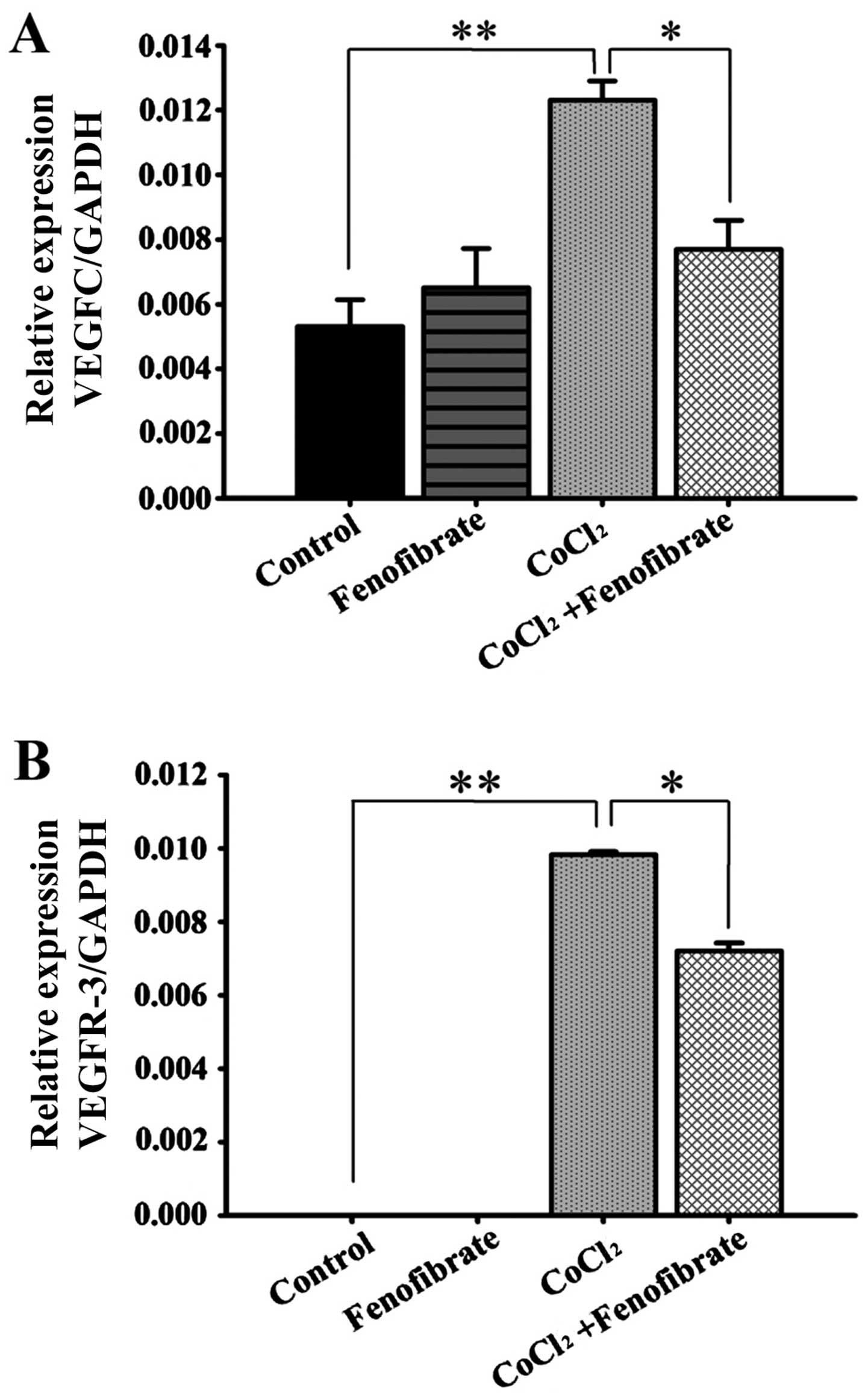
changes in the VEGFC and VEGFR-3 mRNA levels
(Fig. 4) were consistent with the
trends observed with the protein levels, determined by western blot
analysis (Fig. 3).
Effects of culture with the culture
supernatant of hypoxic RPE cells on HUVEC proliferation, migration
and tube formation
Cell culture medium obtained from the cultures of
hypoxic RPE cells at 48 h, was used to culture the HUVECs directly.
By MTT assay, scratch-wound assay and tube formation assay, the
effects of hypoxia-conditioned cell supernatant on the angiogenic
activity of the HUVECs were determined. The results revealed that
compared with the control group, 48 h after the addition of the
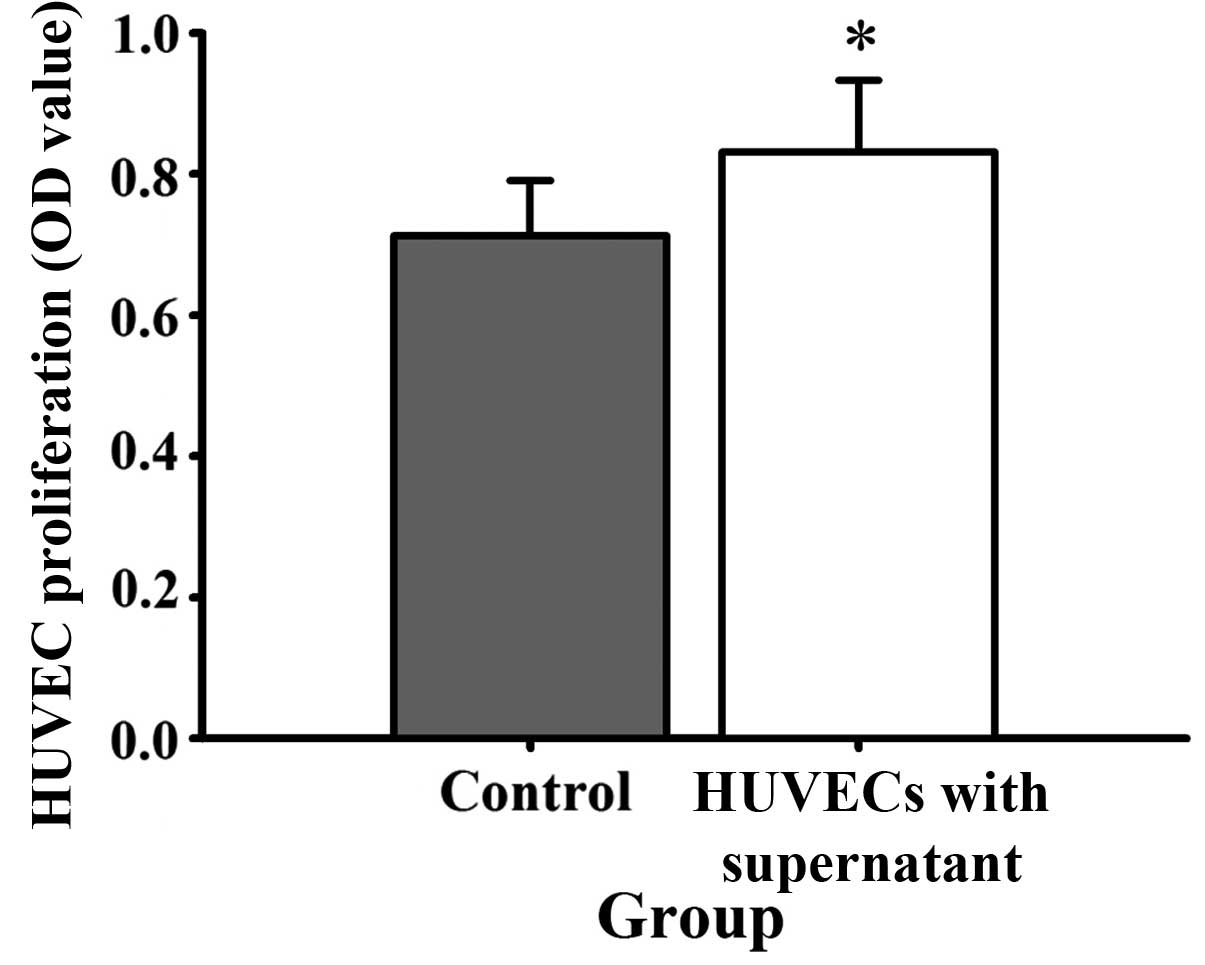
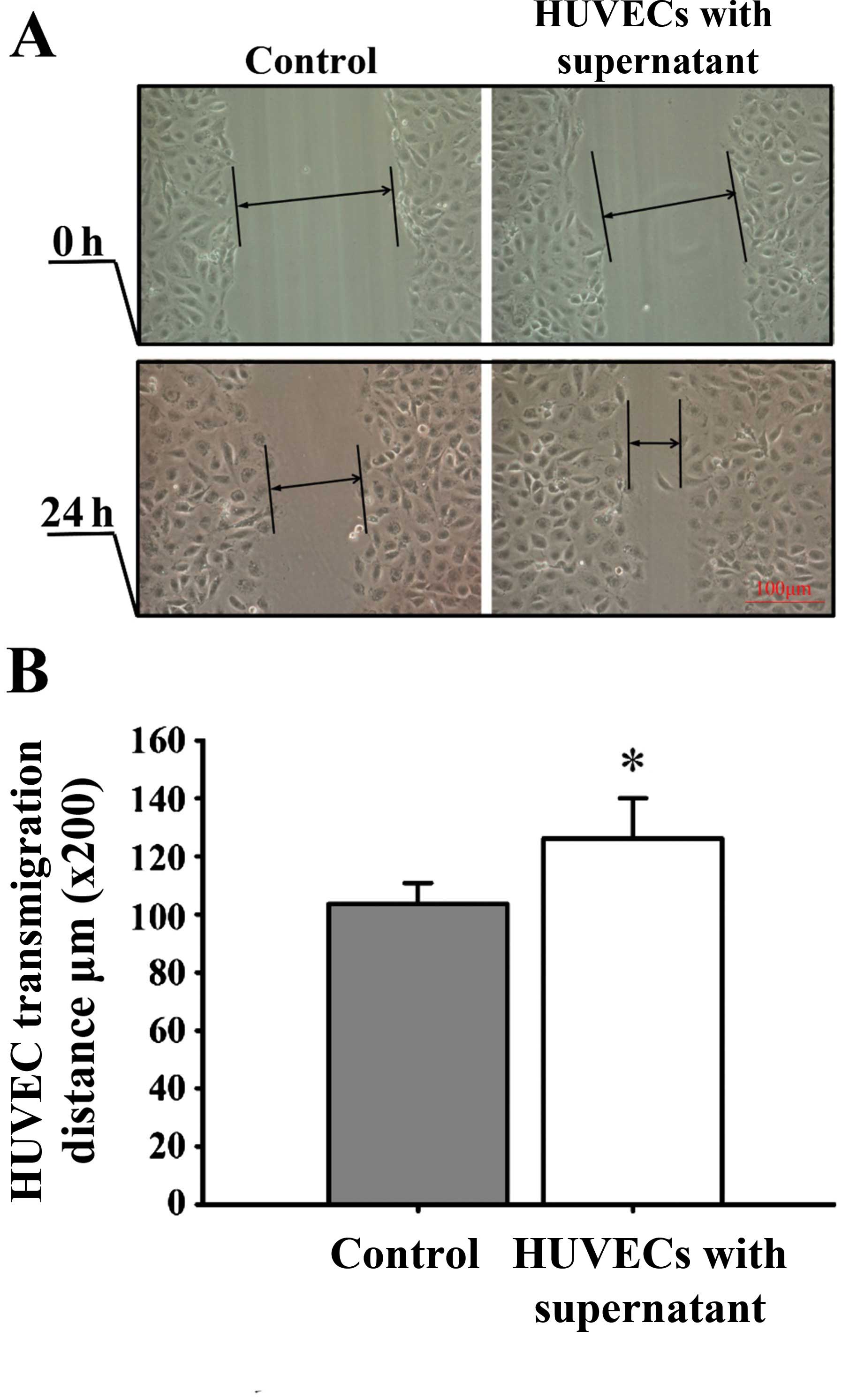
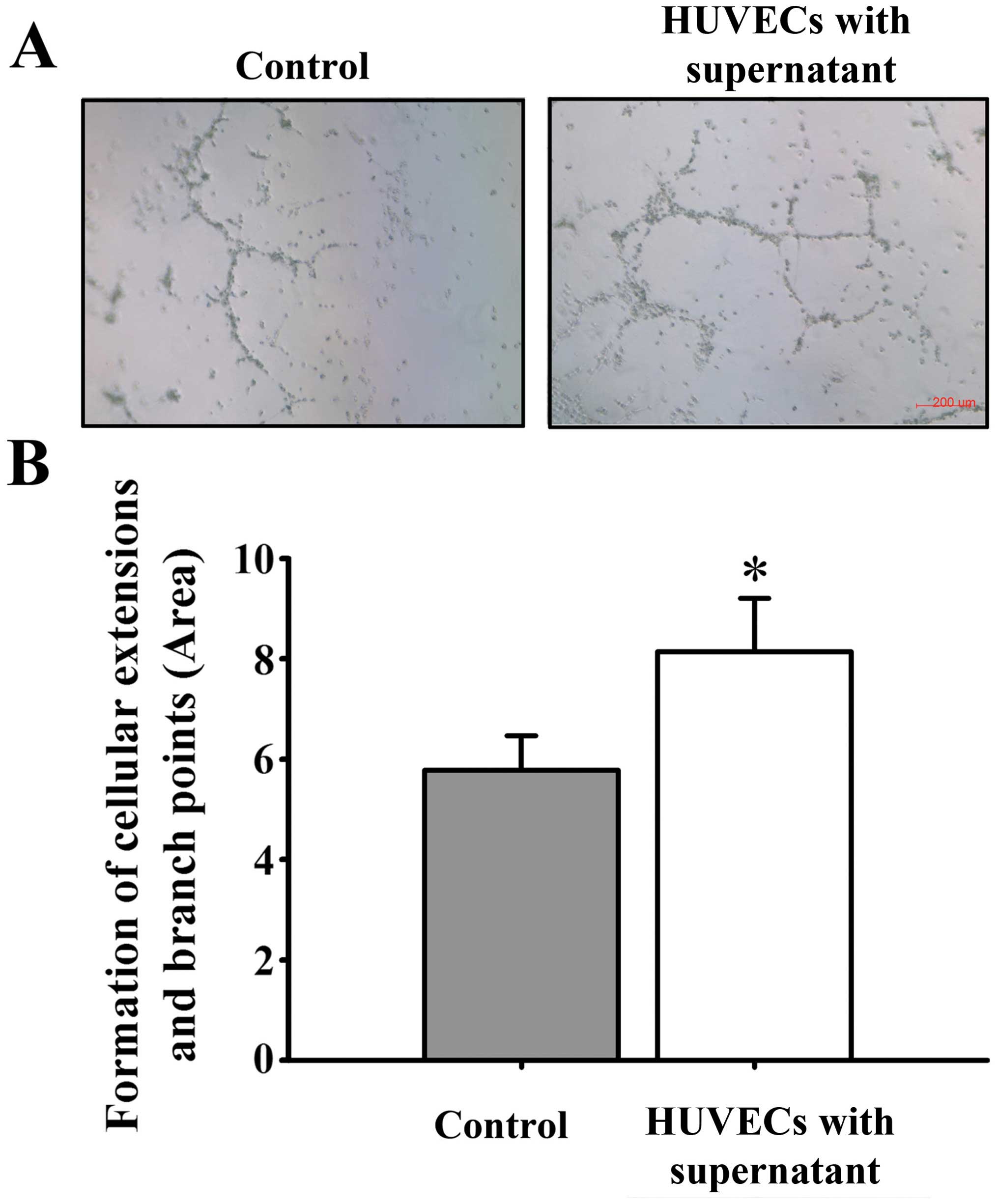
culture medium, HUVEC proliferation (Fig. 5), mobility (Fig. 6) and tube formation ability (Fig. 7) were significantly enhanced.
Discussion
The damage to and destruction of the RPE-Bruch's
membrane-choriocapillaris complex forms the anatomical basis for
the formation of CNV (27). The
changes in the extracellular microenvironment in vivo,
promote the formation of neovascularization. Studies have
demonstrated that there are three main reasons for the formation of
intraocular neovascularization: hypoxia, inflammation and oncogene
products (28,29). Neovascularization often occurs to
meet the needs of physiology and metabolism of local tissues. VEGF
is the most powerful cytokine known thus far, that can promote the
formation of new vessels and it is considered that hypoxia induces
the release of adenosine in tissue, which binds with its receptor
and stimulates endothelial cells to synthesize VEGF. Recent studies
have demonstrated that hypoxia induces the upregulation of
intracellular HIF1 (30–32), which regulates the expression of a
number of hypoxia stress proteins and upregulates VEGF, as well as
many angiogenic factors in tumor tissues, and thus promotes the
formation of new vessels. The process of choroid neovascularization
has a close association with the functional changes of the RPE.
VEGF expression has been detected in the RPE in animals, and VEGF
expression is closely related to the development of CNV (33).
VEGFC belongs to the vascular endothelial growth
factor/platelet derived growth factor (VEGF/PDGF) family, and
VEGFR-3 is the receptor of VEGFC, which can only bind with VEGFC
(or VEGFD). In the physiological state, VEGFR-3 only exists in the
human body in lymphatic vessels and in some reticular endothelial
cells. However, under pathological conditions, it plays an
important role in promoting angiogenesis, such as tumor growth and
wounding (34). During the course of
embryonic development, the deletion of VEGFR-3 has been shown to
lead to the developmental failure of the cardiovascular system,
which suggests that VEGFR-3 plays a key role in the formation of
blood vessels (35). Previous
research on angiogenesis has focused on VEGF and its receptor,
VEGFR-2, and research on the inhibition of angiogenesis has mainly
focused on blocking the VEGF/VEGFR-2 signaling pathway (36). Tammela et al (14) found that silencing VEGFR-3 expression
reduces vascular sprouting during tumor and embryonic development.
The expression of VEGF and its receptors has also been found in
mammalian RPE cells and in the ECM (37). To the best of our knowledge, the
expression of VEGFC and its receptor, VEGFR-3, in RPE cells has not
been reported to date. The present study demonstrated that normal
RPE cells cultured in vitro expressed VEGFC, but not
VEGFR-3. After the RPE cells were exposed to hypoxia in
vitro, the expression of VEGFC and VEGFR-3 increased, which
suggests that under pathological conditions, particularly when
tissues are subjected to ischemia and hypoxia, the expression of
VEGFC and VEGFR-3 is upregulated in RPE cells, and thus VEGFC and
VEGFR-3 can be secreted into the extracellular medium. We found
that HUVEC proliferation and tube formation increased when the
cells were cultured in the culture supernatant from RPE cells
exposed to hypoxia, which contained high levels of VEGFC and
VEGFR-3 expression. However, whether VEGFC and VEGFR-3 have a
direct effect on HUVECs, or whether RPE cells can also express
other VEGF members remains unknown (33,38). It
can be inferred that RPE cells play an important role in the
development of CNV. Moreover, the upregulation of VEGFC and VEGFR-3
is closely related to the development of CNV.
Fenofibrate is a lipid-regulating drug, which is an
agonist of the peroxisome proliferator-activated receptor (PPAR)
(39). In addition to its
hypolipidemic effects, fenofibrate also inhibits angiogenesiss. It
has been demonstrated that fenofibrate inhibits the proliferation
of capillary endothelial cells induced by bFGF and the
proliferation and migration activity of HUVECs induced by VEGF
(40). At the same time, fenofibrate
downregulates VEGFR-2 expression in HUVECs, thus inhibiting the
formation and development of neovascularization (41). It has been found that fenofibrate
inhibits the formation of micro-blood vessels through various
mechanisms, such as downregulating SP1 activity, blocking the
signaling pathway of VEGF and Wnt, and inhibiting the proliferation
of vascular endothelial cells and capillary tube formation
(42). Although it has been found
that fenofibrate has some potential effects on vascular formation
and protection, our knowledge on its effects on RPE cells, which
play an important role in the development of CNV, is still limited.
In this study, we found that fenofibrate downregulated the
expression of VEGFC and VEGFR-3 in the RPE cells in a hypoxic
environment. Thus, we hypothesized that fenofibrate inhibits the
development of CNV through the downregulation of VEGFC and VEGFR-3
in RPE cells. It has been previously demonstrated that fenofibrate
inhibits the activation of HIF-1, and this results in the
downregulation of VEGF (43). Thus,
it possible that fenofibrate downregulates VEGFC expression through
the same pathway. However, in our experiments, the level of free
VEGFR-3 was elevated in the culture supernatant, indicating that
for RPE cells, VEGFR-3 can also be released in to the extracellular
environment, and that fenofibrate can downregulate the expression
of VEGFR-3 in the supernatant. In this study, after the HUVECs were
cultured with the culture supernatant from hypoxic RPE cells, the
expression of VEGFR-3 was significantly increased, and cell
proliferation and tube formation were significantly enhanced. We
hypothesized that although the expression of other VEGFs is
increased in RPE cells following epxosure to hypoxia, the
upregulation of the VEGFC receptor, VEGFR-3, confirmed that the
expression of VEGFR-3 in HUVECs increases when the extracellular
expression of VEGFC increases. It is possible that VEGFC plays a
significant role in the development of CNV. Tammela et al
(14) found that blocking the
VEGFR-3 signaling pathway significantly reduced the number of
capillary branches and sprouting in vascular endothelial cells
(14). Currently, to the best of our
knowledge, no reports are available indicating that fenofibrate
inhibits the VEGFR-3 pathway, and thus, we hypothesized that
fenofibrate inhibits the development of CNV mainly through the
downregulation of VEGFC.
In conclusion, in the present study, we found that
under hypoxic conditions, the expression of VEGFC and that of its
receptor, VEGFR-3, was upregulated in RPE cells and that VEGFC and
VEGFR-3 were secreted into the ECM. VEGFC increased the expression
of VEGFR-3 in HUVECs. Treatment with fenofibrate decreased the
expression of VEGFC and VEGFR-3 in the RPE cells, thereby
suppressing HUVEC proliferation and capillary tube formation, which
were induced by culture with the superntant of hypoxic RPE cells.
Thus, it appears that treatment with fenofibrate may provide a new
treatment strategy with which to prevent the development of
CNV.
Acknowledgements
This study was supported by the National Natural
Science Foundation (grant no. 81060077); the International
Cooperation Fund for the Social Development of Science and
Technology Program in Yunnan (grant no. 2010CA006). The authors are
grateful to Professor Xianqun Fan from the Ninth People's Hospital
affiliated to Shanghai Jiao Tong University for providing the
necessary facilities, constant encouragement and valuable comments
throughout this study.
References
|
1
|
Nowak JZ: Age-related macular degeneration
(AMD): Pathogenesis and therapy. Pharmacol Rep. 58:353–363.
2006.PubMed/NCBI
|
|
2
|
Das A and McGuire PG: Retinal and
choroidal angiogenesis: Pathophysiology and strategies for
inhibition. Prog Retin Eye Res. 22:721–748. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campochiaro PA, Soloway P, Ryan SJ and
Miller JW: The pathogenesis of choroidal neovascularization in
patients with age-related macular degeneration. Mol Vis.
5:341999.PubMed/NCBI
|
|
4
|
Ambati J, Ambati BK, Yoo SH, Ianchulev S
and Adamis AP: Age-related macular degeneration: Etiology,
pathogenesis, and therapeutic strategies. Surv Ophthalmol.
48:257–293. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blaauwgeers HG, Holtkamp GM, Rutten H,
Witmer AN, Koolwijk P, Partanen TA, Alitalo K, Kroon ME, Kijlstra
A, van Hinsbergh VW, et al: Polarized vascular endothelial growth
factor secretion by human retinal pigment epithelium and
localization of vascular endothelial growth factor receptors on the
inner choriocapillaris. Evidence for a trophic paracrine relation.
Am J Pathol. 155:421–428. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ozturk BT, Bozkurt B, Kerimoglu H, Okka M,
Kamis U and Gunduz K: Effect of serum cytokines and VEGF levels on
diabetic retinopathy and macular thickness. Mol Vis. 15:1906–1914.
2009.PubMed/NCBI
|
|
7
|
Zhang P, Wang Y, Hui Y, Hu D, Wang H, Zhou
J and Du H: Inhibition of VEGF expression by targeting HIF-1 alpha
with small interference RNA in human RPE cells. Ophthalmologica.
221:411–417. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gerhardt H, Golding M, Fruttiger M,
Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C,
Alitalo K, Shima D, et al: VEGF guides angiogenic sprouting
utilizing endothelial tip cell filopodia. J Cell Biol.
161:1163–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gaengel K and Betsholtz C: Endocytosis
regulates VEGF signalling during angiogenesis. Nat Cell Biol.
15:233–235. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carvalho B, Freitas-Costa P,
Pinheiro-Costa J, Falcêo M, Carneiro  and Falcão-Reis F:
Evaluation of antiangiogenic treatment results in choroidal
neovascularization related to pathological myopia. Acta Med Port.
27:49–58. 2014.(In Portuguese). PubMed/NCBI
|
|
11
|
Casanovas O, Hicklin DJ, Bergers G,
Hanahan D, Carneiro  and Falcão-Reis F: Drug resistance by evasion
of antiangiogenic targeting of VEGF signaling in late-stage
pancreatic islet tumors. Cancer Cell. 8:299–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones D, Xu Z, Zhang H, He Y, Kluger MS,
Chen H and Min W: Functional analyses of the bone marrow kinase in
the X chromosome in vascular endothelial growth factor-induced
lymphangiogenesis. Arterioscler Thromb Vasc Biol. 30:2553–2561.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tammela T, Zarkada G, Wallgard E,
Murtomäki A, Suchting S, Wirzenius M, Waltari M, Hellström M,
Schomber T, Peltonen R, et al: Blocking VEGFR-3 suppresses
angiogenic sprouting and vascular network formation. Nature.
454:656–660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clarijs R, Schalkwijk L, Hofmann UB,
Ruiter DJ and de Waal RM: Induction of vascular endothelial growth
factor receptor-3 expression on tumor microvasculature as a new
progression marker in human cutaneous melanoma. Cancer Res.
62:7059–7065. 2002.PubMed/NCBI
|
|
16
|
Paavonen K, Puolakkainen P, Jussila L,
Jahkola T and Alitalo K: Vascular endothelial growth factor
receptor-3 in lymphangiogenesis in wound healing. Am J Pathol.
156:1499–1504. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuasa T, Takahashi S, Hatake K, Yonese J
and Fukui I: Biomarkers to predict response to sunitinib therapy
and prognosis in metastatic renal cell cancer. Cancer Sci.
102:1949–1957. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Hu Y, Lin M, Jenkins AJ, Keech AC,
Mott R, Lyons TJ and Ma JX: Therapeutic effects of PPARα agonists
on diabetic retinopathy in type 1 diabetes models. Diabetes.
62:261–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trudeau K and Roy S, Guo W, Hernández C,
Villarroel M, Simó R and Roy S: Fenofibric acid reduces fibronectin
and collagen type IV overexpression in human retinal pigment
epithelial cells grown in conditions mimicking the diabetic milieu:
Functional implications in retinal permeability. Invest Ophthalmol
Vis Sci. 52:6348–6354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Walker AE, Kaplon RE, Lucking SM,
Russell-Nowlan MJ, Eckel RH and Seals DR: Fenofibrate improves
vascular endothelial function by reducing oxidative stress while
increasing endothelial nitric oxide synthase in healthy
normolipidemic older adults. Hypertension. 60:1517–1523. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharma N, Ooi JL, Ong J and Newman D: The
use of fenofibrate in the management of patients with diabetic
retinopathy: an evidence-based review. Aust Fam Physician.
44:367–370. 2015.PubMed/NCBI
|
|
22
|
Fagan XJ and Chong EW: Fenofibrate and
diabetic retinopathy. Clin Experiment Ophthalmol. 43:297–299. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Simó R, Simó-Servat O and Hernández C: Is
fenofibrate a reasonable treatment for diabetic microvascular
disease? Curr Diab Rep. 15:242015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lazzeri S, Orlandi P, Figus M, Fioravanti
A, Cascio E, Di Desidero T, Agosta E, Canu B, Sartini MS, Danesi R,
et al: The rs2071559 AA VEGFR-2 genotype frequency is significantly
lower in neovascular age-related macular degeneration patients.
ScientificWorldJournal. 2012:4201902015.
|
|
25
|
Izuta H, Chikaraishi Y, Shimazawa M,
Mishima S and Hara H: 10-Hydroxy-2-decenoic acid, a major fatty
acid from royal jelly, inhibits VEGF-induced angiogenesis in human
umbilical vein endothelial cells. Evid Based Complement Alternat
Med. 6:489–494. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pourgholami MH, Khachigian LM, Fahmy RG,
Badar S, Wang L, Chu SW and Morris DL: Albendazole inhibits
endothelial cell migration, tube formation, vasopermeability, VEGF
receptor-2 expression and suppresses retinal neovascularization in
ROP model of angiogenesis. Biochem Biophys Res Commun. 397:729–734.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu HA, Liu YL, Ma ZZ, Wang JC and Zhang
Q: A lipid nanoparticle system improves siRNA efficacy in RPE cells
and a laser-induced murine CNV model. Invest Ophthalmol Vis Sci.
52:4789–4794. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao J, Zhao L, Li Y, Liu Y, Xiao W, Song
Y, Luo L, Huang D, Yancopoulos GD, Wiegand SJ, et al: A subretinal
matrigel rat choroidal neovascularization (CNV) model and
inhibition of CNV and associated inflammation and fibrosis by VEGF
trap. Invest Ophthalmol Vis Sci. 51:6009–6017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Querques G, Thirkill CE, Hagege H,
Soubrane G and Souied EH: Choroidal neovascularization associated
with cancer-associated retinopathy. Acta Ophthalmol. 88:571–575.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Du J and Xi Q: HIF-1α ODD
polypeptides increased the expression of HIF1 and VEGF in hypoxic
rat cortical neuron. Neurol Sci. 32:1029–1033. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miyasaka A, Oda K, Ikeda Y, Sone K, Fukuda
T, Inaba K, Makii C, Enomoto A, Hosoya N, Tanikawa M, et al:
PI3K/mTOR pathway inhibition overcomes radioresistance via
suppression of the HIF1-α/VEGF pathway in endometrial cancer.
Gynecol Oncol. 138:174–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakajima T, Anayama T, Koike T, Shingyoji
M, Castle L, Kimura H, Yoshino I and Yasufuku K: Endobronchial
ultrasound doppler image features correlate with mRNA expression of
HIF1-α and VEGF-C in patients with non-small-cell lung cancer. J
Thorac Oncol. 7:1661–1667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang H, Geisen P, Wittchen ES, King B,
Burridge K, D'Amore PA and Hartnett ME: The role of RPE
cell-associated VEGF189 in choroidal endothelial cell
transmigration across the RPE. Invest Ophthalmol Vis Sci.
52:570–578. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Padera TP and Jain RK: VEGFR3: A new
target for antiangiogenesis therapy? Dev Cell. 15:178–179. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dumont DJ, Jussila L, Taipale J,
Lymboussaki A, Mustonen T, Pajusola K, Breitman M and Alitalo K:
Cardiovascular failure in mouse embryos deficient in VEGF
receptor-3. Science. 282:946–949. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wittig C, Scheuer C, Parakenings J, Menger
MD and Laschke MW: Geraniol suppresses angiogenesis by
downregulating vascular endothelial growth factor (VEGF)/VEGFR-2
signaling. PLoS One. 10:e01319462015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yi X, Mai LC, Uyama M and Yew DT:
Time-course expression of vascular endothelial growth factor as
related to the development of the retinochoroidal vasculature in
rats. Exp Brain Res. 118:155–160. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Le YZ, Bai Y, Zhu M and Zheng L: Temporal
requirement of RPE-derived VEGF in the development of choroidal
vasculature. J Neurochem. 112:1584–1592. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Milosavljevic D, Griglio S, Le Naour G and
Chapman MJ: Preferential reduction of very low density
lipoprotein-1 particle number by fenofibrate in type IIB
hyperlipidemia: Consequences for lipid accumulation in human
monocyte-derived macrophages. Atherosclerosis. 155:251–260. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Panigrahy D, Kaipainen A, Huang S,
Butterfield CE, Barnés CM, Fannon M, Laforme AM, Chaponis DM,
Folkman J and Kieran MW: PPARalpha agonist fenofibrate suppresses
tumor growth through direct and indirect angiogenesis inhibition.
Proc Natl Acad Sci USA. 105:985–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Meissner M, Stein M, Urbich C, Reisinger
K, Suske G, Staels B, Kaufmann R and Gille J: PPARalpha activators
inhibit vascular endothelial growth factor receptor-2 expression by
repressing Sp1-dependent DNA binding and transactivation. Circ Res.
94:324–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Noonan JE, Jenkins AJ, Ma JX, Keech AC,
Wang JJ and Lamoureux EL: An update on the molecular actions of
fenofibrate and its clinical effects on diabetic retinopathy and
other microvascular end points in patients with diabetes. Diabetes.
62:3968–3975. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ge Y, Liu J, Yang X, Zhu H, Yang B, Zhao
K, Wu Z, Cheng G, Wang F, Ni F, et al: Fenofibrate enhances
radiosensitivity of esophageal squamous cell carcinoma by
suppressing hypoxia-inducible factor-1α expression. Tumour Biol.
35:10765–10771. 2014. View Article : Google Scholar : PubMed/NCBI
|