Introduction
Jean Marjolin first described malignant change
arising in a skin ulcer in 1828. This condition was subsequently
described by Smith in 1850 and Da Costa in 1903 (1,2). As
these ulcers have not been extensively studied, the mechanisms
underlying carcinomatous change remain unclear. Marjolin's ulcers
are frequently induced by scarring following deep burns caused by
hot ceramic, metal or soil (3–5).
Marjolin's ulcers are usually considered to be highly aggressive
tumors, with a rapid rate of regional metastases. Radical excision
is the primary treatment option; however, there is currently no
consensus regarding the efficacy of lymph node dissection.
Marjolin's ulcers are typically associated with a poor prognosis,
and may be life threatening. As living standards improve, the
incidence of Marjolin's ulcers should gradually decrease. Although
there are few reports of patients with Marjolin's ulcer in China
(6), this is not a rare disease,
even in the relatively well-developed Pearl River Delta region. The
Departments of Plastic Surgery of the Affiliated Foshan Hospital
(Foshan, China) and the Second Affiliated Hospital (Guangzhou,
China) of Sun Yat-sen University, located in the Pearl River Delta
region, treated 51 patients with Marjolin's ulcers between January
2001 and September 2013.
Materials and methods
Patients and data collection
Fifty-one patients who were treated for Marjolin's
ulcers between January 2001 and September 2013 were retrospectively
reviewed. Follow-up was continued for more than one year. The
diagnoses were verified by incisional biopsies in all cases. The
specimens received by our laboratory were fixed by formalin and
processed using routine hematoxylin and eosin staining. Data
collected included age, gender, time from initial ulceration to
carcinomatous change, cause of initial ulceration, history of ulcer
treatment, surgical treatment and follow-up results. The
associations between pathological type and metastasis and between
the location of squamous cell carcinoma and metastasis were
analyzed. Eleven patients with deep, aggressive squamous cell
carcinoma or melanoma and suspected sentinel lymph node metastasis
underwent 18F-fluorodeoxyglucose positron emission
tomography-computed tomography (PET-CT) and B-mode
ultrasound-guided biopsy, with a 100% accuracy rate, for the
detection of sentinel node metastasis. This retrospective study was
approved by the ethical review boards of the participating
instututions and written informed consent was obtained from all
patients or their next of kin.
Results
Patients
The 51 patients with Marjolin's ulcers included 22
males (43.14%) and 29 females (56.86%) with a mean age of 64.15
years (range, 32–89 years). The mean time from initial ulceration
to diagnosis of squamous cell carcinoma was 13.42 years (range, 6
months-54 years) and to diagnosis of melanoma was 2.47 years
(range, 3 months-10 years). One patient developed epithelioid
sarcoma after two years and one developed basal cell carcinoma
after three years. Squamous cell carcinomas were located on the
lower limb in 31 cases, the upper limb in seven cases, the head in
four cases and the chest in one case. The six cases of melanoma
were all located on the foot. One case of basal cell carcinoma was
located over the occipital area and one case of epithelioid sarcoma
was located on the foot.
The underlying injury causing ulceration was a burn
scar in 35 cases and a traumatic wound scar in 16 cases. Ulceration
was usually present for a long time prior to carcinomatous change.
The non-healing of ulcers was associated with ineffective initial
treatment. Of the 51 patients, seven (13.73%) received treatment in
a large general hospital, eight (15.69%) received conservative
treatment in the outpatient clinic of a community hospital, 23
(45.10%) received external application of Chinese herbs at home and
13 (25.49%) did not receive any treatment.
The pathological type was squamous cell carcinoma in
43 cases (84.31%), including 42 cases of well-differentiated
squamous cell carcinoma (Broder's Grade I) and one case of
moderately differentiated squamous cell carcinoma (Broder's Grade
II), melanoma in six cases (11.76%), basal cell carcinoma in one
case and epithelioid sarcoma in one case. The rate of metastasis
varied among the pathological types. In patients with squamous cell
carcinoma, the rate of sentinel lymph node metastasis was 30.23%
and the rate of distant metastasis was 11.63%. In patients with
melanoma, the rate of sentinel lymph node metastasis was 66.67% and
the rate of distant metastasis was 33.33%. Lymph node and distant
metastasis were not detected in the patients with basal cell
carcinoma and epithelioid sarcoma (Table
I).
 | Table I.Metastasis according to the
pathological type of Marjolin's ulcer. |
Table I.
Metastasis according to the
pathological type of Marjolin's ulcer.
| Pathological
type | n | Patients with
sentinel lymph node metastasis, n (%) | Patients with distant
metastasis, n (%) |
|---|
| Squamous cell
carcinoma | 43 | 13 (30.23) | 5 (11.63) |
| Melanoma | 6 | 4
(66.67) | 2 (33.33) |
| Basal cell
carcinoma | 1 | 0
(0.00) | 0
(0.00) |
| Epithelioid
sarcoma | 1 | 0
(0.00) | 0
(0.00) |
The rates of lymph node metastasis and distant
metastasis in patients with squamous cell carcinoma varied
according to the location of the lesion. In patients with squamous
cell carcinoma of the lower limb, the rate of sentinel lymph node
metastasis was 35.48% and the rate of distant metastasis was
16.13%. In patients with squamous cell carcinoma of the upper limb,
the rate of sentinel lymph node metastasis was 28.57% and the rate
of distant metastasis was 0% (Table
II). Eleven patients with squamous cell carcinoma and two
patients with melanoma with deep, aggressive tumors and suspected
sentinel lymph node metastasis underwent
18F-fluorodeoxyglucose PET-CT and B-mode ultrasound
guided biopsy. These investigations had a 100% accuracy rate for
the detection of metastasis (Table
III).
 | Table II.Sentinel lymph node and distant
metastases according to the location of squamous cell
carcinoma. |
Table II.
Sentinel lymph node and distant
metastases according to the location of squamous cell
carcinoma.
| Location | n | Sentinel lymph node
metastasis, n (%) | Distant metastasis, n
(%) |
|---|
| Lower limb | 31 | 11 (35.48) | 5 (16.13) |
| Upper limb | 7 | 2
(28.57) | 0
(0.00) |
| Head | 4 | 0
(0.00) | 0
(0.00) |
| Chest | 1 | 0
(0.00) | 0
(0.00) |
 | Table III.PET-CT and B-mode ultrasound-guided
biopsy findings in 11 patients with aggressive tumors and suspected
lymph node metastasis. |
Table III.
PET-CT and B-mode ultrasound-guided
biopsy findings in 11 patients with aggressive tumors and suspected
lymph node metastasis.
| Case no. | Gender | Age (years) | Site of tumor | Type of tumor | Region of lymph node
metastasis indicated by PET-CT | Results of biopsy
under ultrasound B-mode |
|---|
| 1 | Female | 47 | Left toes | Well-differentiated
SCC | Left groin | Positive |
| 2 | Female | 37 | Left foot | Moderately
differentiated SCC | Left/right
groin |
Positive/positive |
| 3 | Male | 47 | Head | Well-differentiated
SCC | Head | Negative |
| 4 | Female | 83 | Left foot | Well-differentiated
SCC | Left groin | Positive |
| 5 | Male | 55 | Left ankle | Well-differentiated
SCC | Left groin | Negative |
|
|
|
|
|
| Left popliteal | Positive |
| 6 | Male | 57 | Right foot | Melanoma | Right groin | Positive |
| 7 | Male | 54 | Left forearm | Well-differentiated
SCC | Left axillary | Positive |
| 8 | Male | 64 | Right popliteal
fossa | Well-differentiated
SCC | Right groin | Positive |
| 9 | Female | 47 | Right hand | Well-differentiated
SCC | Right axillary | Positive |
| 10 | Male | 48 | Left toe | Well-differentiated
SCC | Left groin | Positive |
| 11 | Male | 53 | Right foot | Melanoma | Left groin | Positive |
Surgical methods and follow-up
results
One patient with basal cell carcinoma on the head
underwent extended resection and skin grafting, with no evidence of
relapse or metastasis after eight years of follow-up. One patient
with an epithelioid sarcoma over the occipital region underwent
extended resection and skin grafting, with no evidence of relapse
or metastasis after seven years of follow-up. Of the 43 patients
with squamous cell carcinoma, 27 did not develop aggressive tumors
or sentinel lymph node metastasis. These 27 patients underwent
extended resection and skin grafting or skin flap repair. One of
these patients succumbed to extensive metastasis after three years.
Five patients developed deep, aggressive tumors with no metastasis.
Four of these five patients underwent amputation and survived. One
patient refused amputation and underwent only resection of the
ulcer and surrounding tissues with skin grafting, and subsequently
developed metastasis and succumbed one year later. Eleven patients
developed deep, aggressive squamous cell carcinoma with inguinal,
popliteal or axillary sentinel lymph node metastasis. Nine of these
11 patients underwent amputation and sentinel lymph node
dissection, and eight patients survived. One patient who underwent
amputation and inguinal lymph node dissection succumbed two years
later due to pelvic lymph nodes and lung metastasis. One patient
refused surgery and developed metastasis, and succumbed two years
later. One patient developed deep, aggressive squamous cell
carcinoma with extensive sentinel lymph node metastasis and distant
pelvic lymph nodes metastasis. Radiotherapy was administered
instead of surgery and the patient succumbed one year later due to
lung metastasis (Table IV).
 | Table IV.Treatment and follow-up results in 43
patients with squamous cell carcinoma. |
Table IV.
Treatment and follow-up results in 43
patients with squamous cell carcinoma.
| Case no. | Age (years) | Tumor location | Tumor aggression,
lymphatic metastasis and distant metastasis | Surgical
method | Follow-up
(years) | Follow-up
results |
|---|
| 1 | 75 | Left calf | No | Extended resection,
skin grafting | 8 | Presence |
| 2 | 53 | Right foot | No | Extended resection,
skin grafting | 7 | Presence |
| 3 | 48 | Scalp | No | Extended resection,
local skin flap | 5 | Presence |
| 4 | 32 | Right popliteal
fossa | No | Extended resection,
skin grafting | 3 | Presence |
| 5 | 41 | Left thigh | No | Extended resection,
skin grafting | 2 | Presence |
| 6 | 56 | Left calf | No | Extended resection,
skin grafting | 1 | Presence |
| 7 | 73 | Right popliteal
fossa | No | Extended resection,
skin grafting | 2 | Presence |
| 8 | 62 | Left foot | No | Extended resection,
skin grafting | 4 | Presence |
| 9 | 37 | Left elbow | No | Extended resection,
skin grafting | 2 | Presence |
| 10 | 78 | Left forearm | No | Extended resection,
skin grafting | 3 | Presence |
| 11 | 56 | Right popliteal
fossa | No | Extended resection,
axial skin flap | 2 | Presence |
| 12 | 47 | Left calf | No | Extended resection,
Free skin flap | 3 | Presence |
| 13 | 89 | Right foot | No | Extended resection,
skin grafting | 4 | Presence |
| 14 | 75 | Left foot | No | Extended resection,
skin grafting | 2 | Presence |
| 15 | 51 | Left popliteal
fossa | No | Extended resection,
skin grafting | 8 | Presence |
| 16 | 61 | Left thigh | No | Extended resection,
skin grafting | 2 | Presence |
| 17 | 63 | Right foot | No | Extended resection,
skin grafting | 2 | Presence |
| 18 | 47 | Right foot | No | Extended resection,
skin grafting | 5 | Presence |
| 19 | 61 | Left thigh | No | Extended resection,
skin grafting | 2 | Presence |
| 20 | 75 | Left foot | No | Extended resection,
skin grafting | 1 | Presence |
| 21 | 68 | Left middle
finger | No | Extended resection,
skin grafting | 7 | Presence |
| 22 | 47 | Scalp | No | Extended resection,
local skin flap | 1 | Presence |
| 23 | 57 | Chest | No | Extended resection,
skin grafting | 3 | Presence |
| 24 | 75 | Scalp | No | Extended resection,
skin grafting | 1 | Presence |
| 25 | 46 | Scalp | No | Extended resection,
free skin flap | 4 | Presence |
| 26 | 67 | Right eyelid | No | Extended resection,
local skin flap | 7 | Presence |
| 27 | 37 | Left foot | No | Extended resection,
axial skin flap | 3 | Metastasized to
bilateral inguinal groove, left thigh and lung; mortality |
| 28 | 58 | Right
footplate | Deep aggression, no
metastasis | Amputation | 8 | Presence |
| 29 | 74 | Right
forefinger | Deep aggression, no
metastasis | Finger
amputation | 1 | Presence |
| 30 | 79 | Right
forefinger | Deep aggression, no
metastasis | Finger
amputation | 3 | Presence |
| 31 | 38 | Left footplate | Deep aggression, no
metastasis | Amputation | 2 | Presence |
| 32 | 89 | Left popliteal
fossa | Deep aggression, no
metastasis | Refused to
amputation; extended resection, skin grafting only | 1 | Metastasized to
left inguinal lymph node and lung; mortality |
| 33 | 64 | Right popliteal
fossa | Deep aggression;
metastasized to right inguinal lymph node | Amputation, right
inguinal lymph node dissection | 5 | Presence |
| 34 | 48 | Left toe | Deep aggression;
metastasized to left inguinal lymph node | Toe amputation,
left inguinal lymph node dissection | 2 | Presence |
| 35 | 55 | Left ankle | Deep aggression;
metastasized to left popliteal lymph node | Amputation, left
popliteal and inguinal lymph node dissection | 1 | Presence |
| 36 | 66 | Right foot | Deep aggression;
metastasized to right inguinal lymph node | Amputation, right
inguinal lymph node dissection | 5 | Presence |
| 37 | 47 | Right hand | Deep aggression;
metastasized to left axillary lymph node | Amputation, left
axillary lymph node dissection | 2 | Presence |
| 38 | 54 | Left forearm | Deep aggression;
metastasized to left axillary lymph node | Amputation, left
axillary lymph node dissection | 4 | Presence |
| 39 | 51 | Right popliteal
fossa | Deep aggression;
metastasized to left inguinal lymph node | Amputation, left
inguinal lymph node dissection | 1 | Presence |
| 40 | 47 | Left toe | Deep aggression;
metastasized to left inguinal lymph node | Amputation, left
inguinal lymph node dissection | 2 | Presence |
| 41 | 58 | Left popliteal
fossa | Deep aggression;
metastasized to left inguinal lymph node | Amputation, left
inguinal lymph node dissection | 2 | Metastasized to
pelvic lymph nodes and lung; mortality |
| 42 | 83 | Left foot | Deep aggression;
metastasized to left inguinal lymph node | Refused to undergo
surgery | 2 | Metastasized to
lung; mortality |
| 43 | 78 | Left thigh | Deep aggression;
metastasized to left inguinal lymph nodes and pelvic lymph
nodes | No surgery,
radiotherapy | 1 | Metastasized to
lung; mortality |
Two of the six patients with melanoma succumbed. One
patient with melanoma on the right foot, right inguinal lymph node
metastasis and lung metastasis was considered to have unresectable
disease and received interferon therapy, and one patient with
melanoma on the left foot and left inguinal lymph node metastasis
refused surgery. These two patients succumbed from lung metastasis
six months later. The other four patients survived. Two of these
four patients did not develop metastasis, and underwent extended
resection and skin grafting or skin flap reconstruction. The
remaining two patients with melanoma on the foot and inguinal lymph
node metastasis underwent extended resection and skin grafting or
amputation combined with inguinal lymph node dissection, and
survived with no evidence of relapse or metastasis (Table V).
 | Table V.Characteristics of six patients with
melanoma. |
Table V.
Characteristics of six patients with
melanoma.
| Case no. | Tumor location | Lymphatic and
distant metastases | Therapy | Follow-up
(months) | Follow-up
results |
|---|
| 1 | Right foot | Lung, right
inguinal lymph node metastasis | Interferon | 6 | Lung metastasis;
mortality |
| 2 | Left footplate | Left inguinal lymph
node metastasis | Refused the
treatment | 6 | Lung metastasis;
mortality |
| 3 | Left footplate | No metastasis | Extended resection,
skin grafting | 21 | Presence |
| 4 | Left footplate | No metastasis | Extended resection,
medial pedal flap of footplate | 41 | Presence |
| 5 | Right
footplate | Right inguinal
lymph node metastasis | Amputation, right
inguinal lymph node dissection | 31 | Presence |
| 6 | Right heel | Right inguinal
lymph node metastasis | Extended resection,
skin grafting, right inguinal lymph node dissection | 26 | Presence |
A number of patients had unusual presentations of
disease. In one patient, squamous cell carcinoma developed
simultaneously in traumatic skin ulcers on the lateral and medial
sides of the left ankle. One patient developed squamous cell
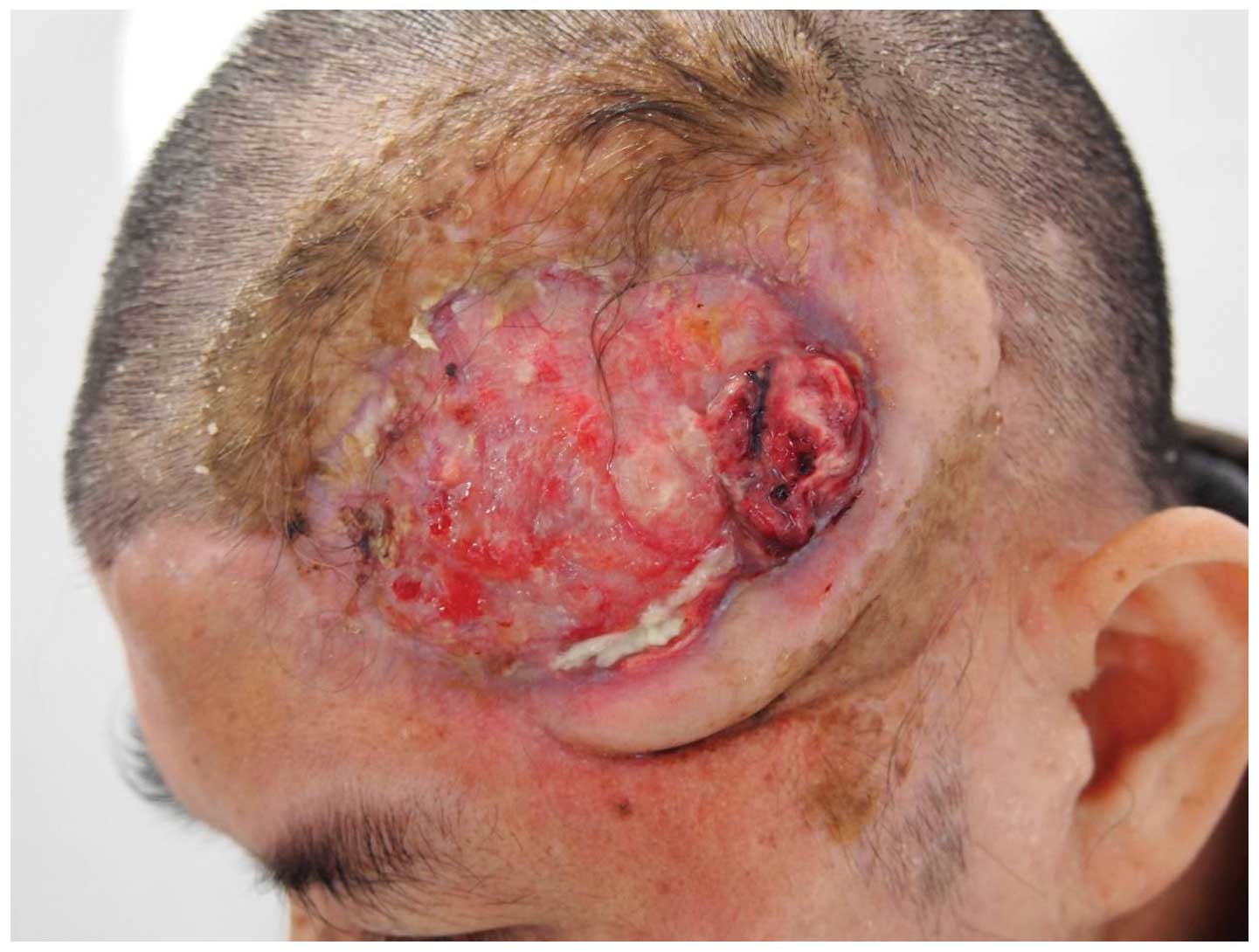
carcinoma in a burn scar ulcer over the temple, which extended
through the bone and dura mater into the brain (Fig. 1). Three patients developed squamous
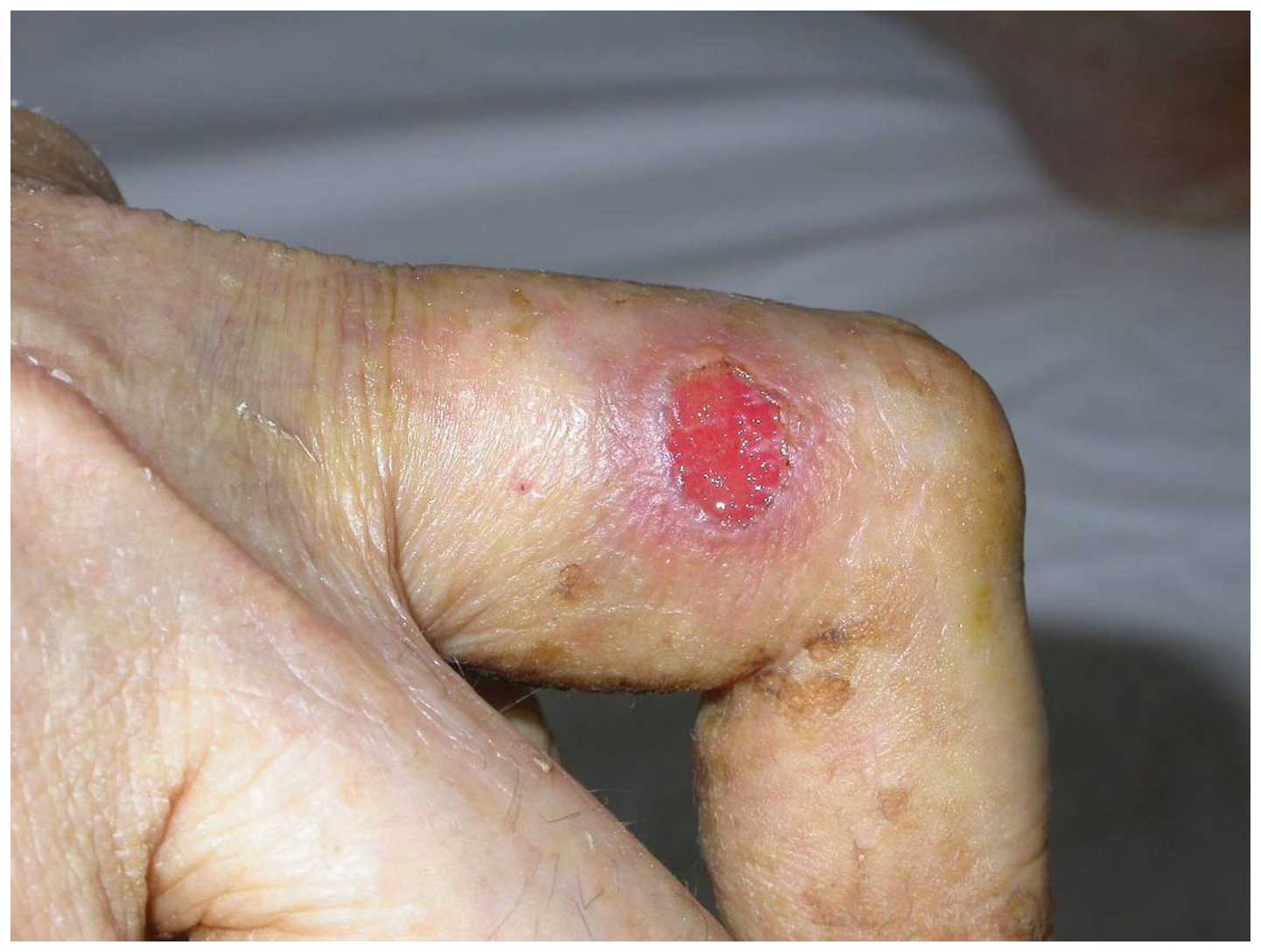
cell carcinoma in an ulcer on the finger (Fig. 2).
Case report
A 55-year-old male with a 20-year history of
ulceration over the lateral and medial aspects of his left ankle
presented with a two-month history of pain. In 1993, he had
developed chronic ulceration on either side of the ankle from
friction caused by his shoe. The wounds were originally treated
with saline irrigation by a rural doctor. The patient worked in a
paddy field and had poor economic circumstances.
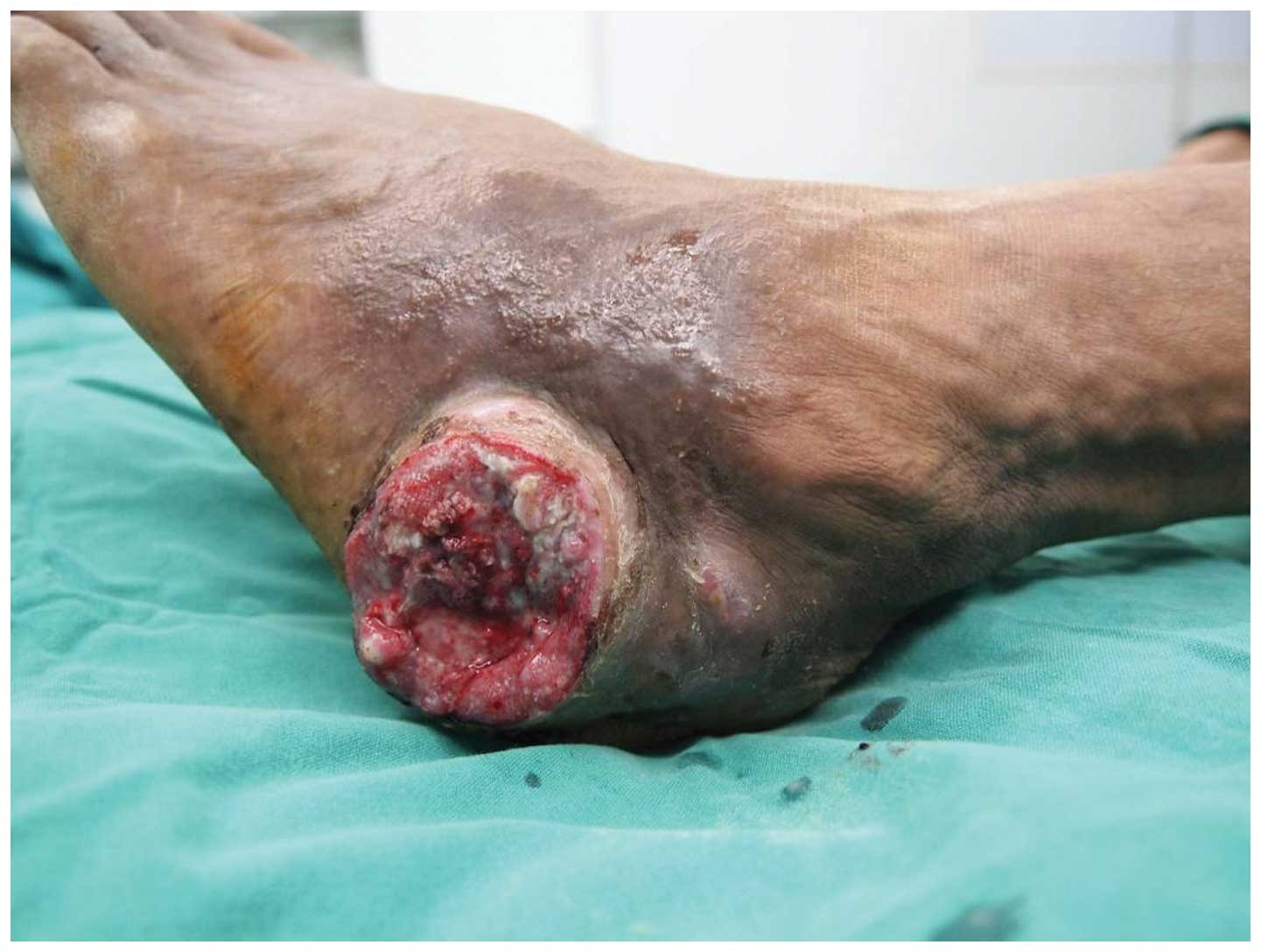
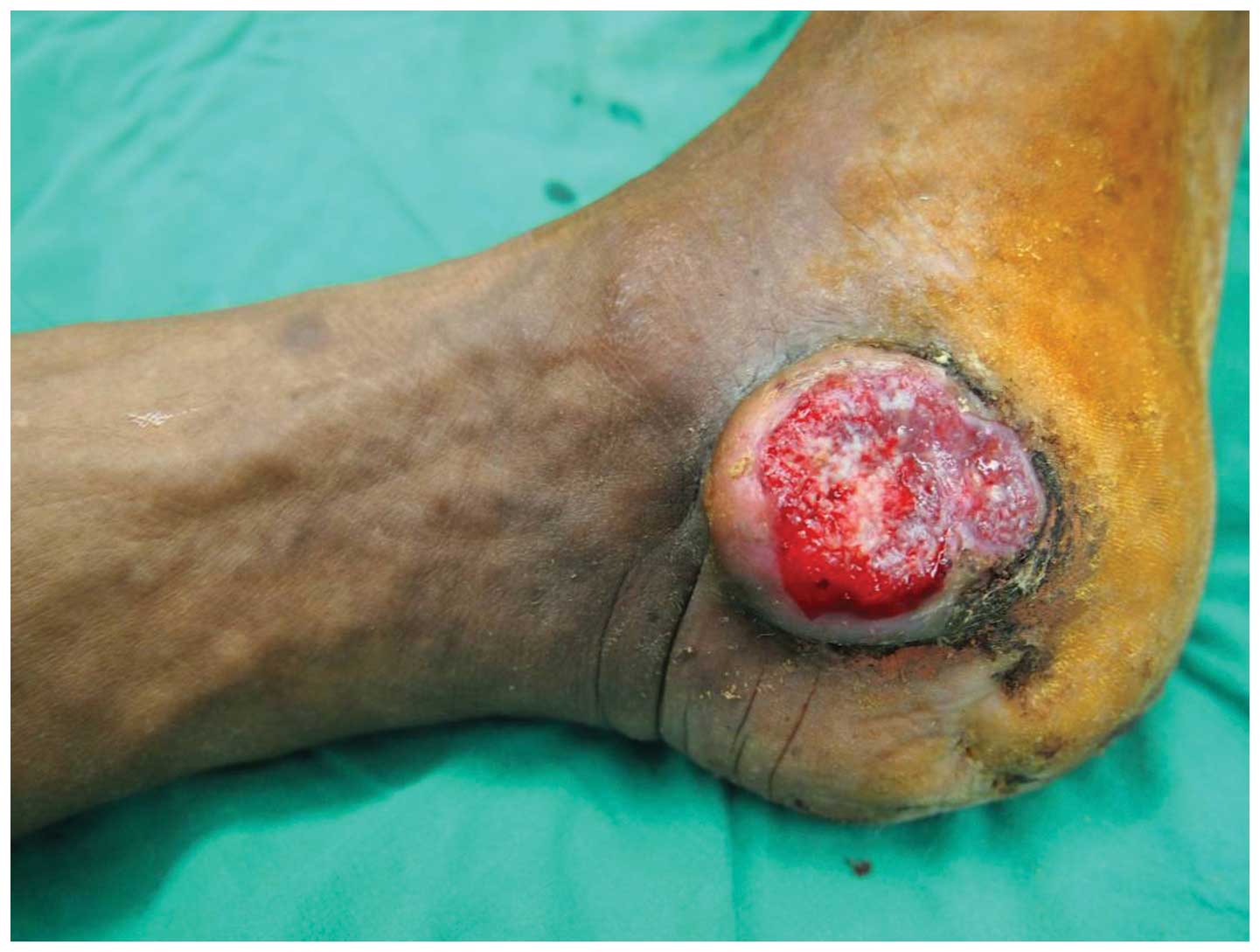
Physical examination revealed a 4.5-cm-diameter
ulcer over the medial aspect and a 5-cm-diameter ulcer over the
lateral aspect of the left ankle. A Marjolin's ulcer with similar
histological characteristics occurring in different parts of the
body simultaneously is a rarely reported occurrence. The
crater-shaped ulcers were dirty, necrotic and malodorous, with
surrounding tissue proliferation (Figs.
3 and 4).
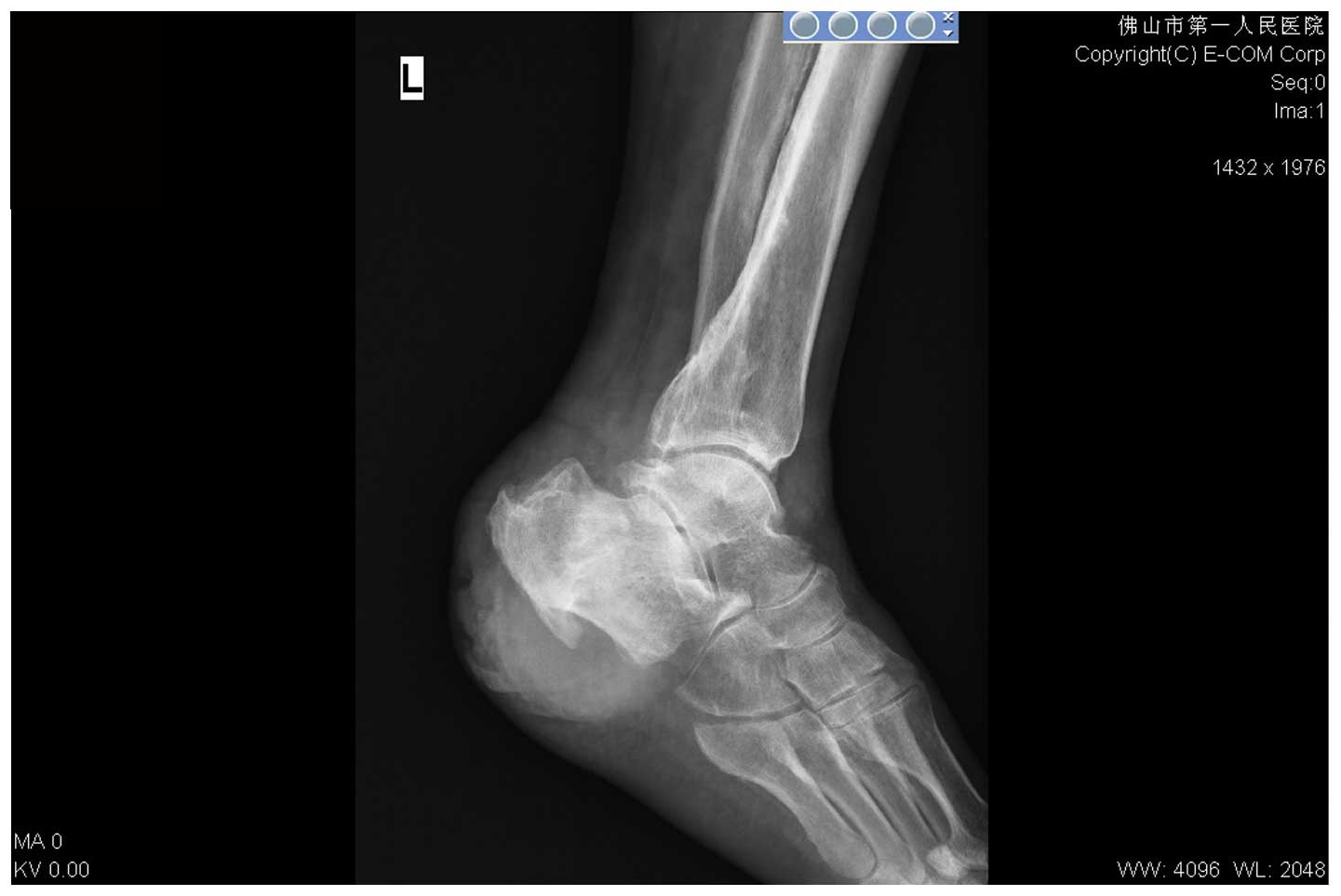
Radiography showed areas of dense cortical bone and
new periosteal bone formation in the middle and distal parts of the
left tibia and fibula, and in the calcaneus and talus. There was a
small area of bone destruction in the distal part of the tibia,
with signs of chronic osteomyelitis and surrounding soft tissue
swelling (Fig. 5). Bacterial
cultures of the wound surface revealed Proteus penneri.
In September 2012, the patient underwent partial
resection of the lesions. Pathological examination showed
well-differentiated squamous cell carcinoma in the two lesions
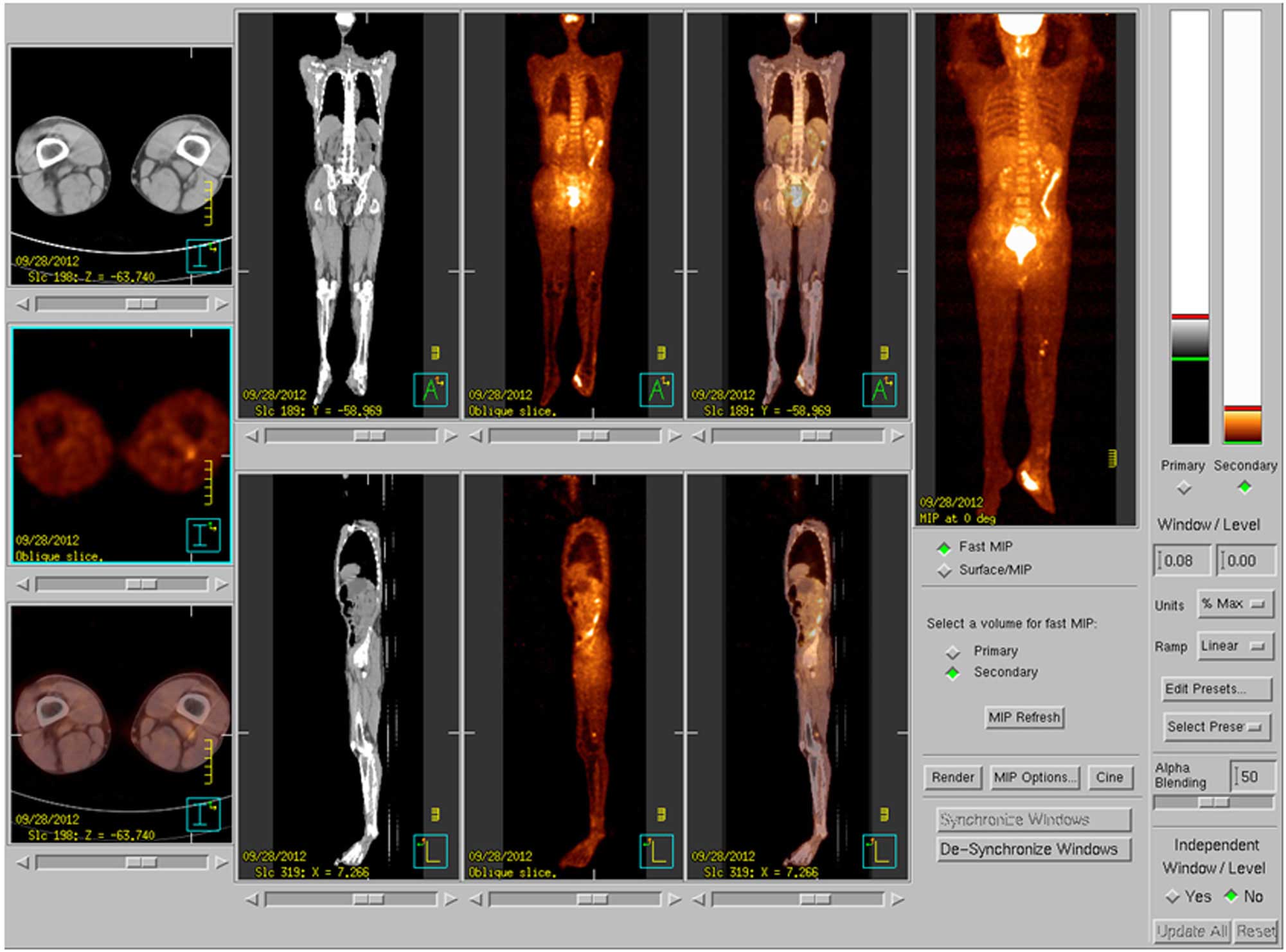
(Fig. 6). PET-CT showed abnormal
uptake in the lymph nodes of the left popliteal fossa and left
inguinal region, but it was unclear whether this represented wound
infection or tumor metastasis (Fig.
7).
In September 2012, the patient underwent below-knee
amputation of the left leg for these aggressive lesions. Two weeks
after surgery, PET-CT still showed increased uptake in the lymph
nodes of the left popliteal fossa and inguinal region, indicating
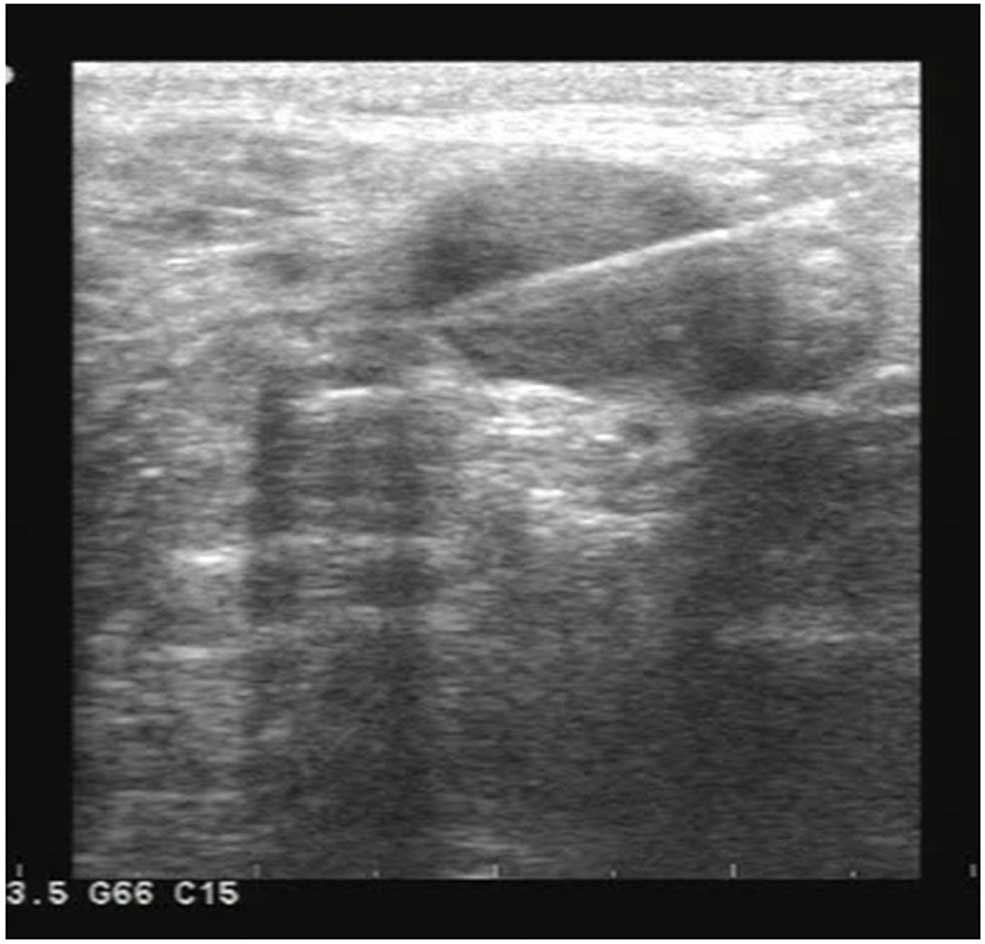
possible metastasis. The patient then underwent B-mode
ultrasound-guided biopsy of the left popliteal and inguinal lymph
nodes. Examination of the biopsy specimens showed metastasis in the
popliteal nodes, but not in the inguinal nodes (Fig. 8).
The patient underwent left popliteal and inguinal
lymph node dissection. Postoperative pathological examination
showed metastatic squamous cell carcinoma in the popliteal nodes
but not in the inguinal nodes, which was consistent with the
previous biopsy findings. There was no evidence of relapse or
metastasis after one year.
Discussion
Marjolin's ulcers are tumors that form in chronic
skin ulcers, predominantly on burn scar wounds. These tumors also
develop on other wounds, including pressure sores (7), venous stasis ulcers (8), traumatic wounds (9), osteomyelitis (10), fistulas (11), leprosy ulcers (12) and lacerations (13). Burn scars are reported to have a rate
of carcinomatous change of 2% (14).
The most common type of Marjolin's ulcer is squamous cell
carcinoma, followed by basal cell carcinoma, sarcoma and melanoma
(15,16). Kowal-Vern and Criswell (17) retrospectively reviewed 412 cases of
Marjolin's ulcers reported in 146 studies between 1923 and 2004,
and found that 71% had squamous cell carcinoma, 12% had basal cell
carcinoma, 6% had melanoma, 5% had sarcoma and 6% had other tumors.
The present study included 51 patients with Marjolin's ulcers,
including 43 (84.31%) with squamous cell carcinoma and six (11.76%)
with melanoma.
Kowal-Vern and Criswell (17) reported that the average period of
ulceration prior to carcinomatous change was 31 years. The present
study included more female (56.86%) than male (43.14%) patients.
The mean period of ulceration prior to carcinomatous change was
relatively short (13.42 years for squamous cell carcinoma and 2.47
years for melanoma). The rates of lymph node and distant metastasis
are higher in squamous cell carcinoma-type Marjolin's ulcer than in
primary cutaneous squamous cell carcinoma (4,18).
Kowal-Vern and Criswell (17)
reported regional or sentinel lymph node metastasis in 22% of cases
of squamous cell carcinoma-type Marjolin's ulcer, distant
metastasis in 14% and a resulting mortality rate of 21%. Novick
et al (19) reported a
metastasis rate of 54% from lower limb squamous cell carcinoma-type
Marjolin's ulcer, including metastases to the brain, liver, lung,
kidney and distant lymph nodes. In the present study, patients with
squamous cell carcinoma had a regional or sentinel lymph node
metastasis rate of 30.23% and a distant metastasis rate of 11.63%.
In patients with squamous cell carcinoma of the lower limb, the
rate of sentinel lymph node metastasis was 35.48% and the rate of
distant metastasis was 16.13%. In patients with squamous cell
carcinoma of the upper limb, the rate of sentinel lymph node
metastasis was 28.57% and the rate of distant metastasis was 0%.
The location of the tumor was strongly associated with the rate of
metastasis. Squamous cell carcinoma in the lower limb has
previously been reported to have a higher rate of metastasis
(20). Among patients with melanoma,
66.67% had sentinel lymph node metastasis and 33.33% had distant
metastasis.
Squamous cell carcinoma and melanoma are aggressive
types of tumor with high rates of metastasis. It is therefore
important to detect sentinel lymph node and distant metastases
prior to deciding the therapeutic regimen. Patients with sentinel
lymph node metastasis should undergo lymph node dissection
(21). PET-CT has a high sensitivity
for the detection of metastasis and has been reported to be useful
for the detection of lymph node metastasis in patients with
malignant melanoma (22). Sentinel
lymph node biopsy is a relatively non-traumatic method of screening
for lymph node metastasis in patients with squamous cell
carcinoma-type Marjolin's ulcers (23). In the present study, we were able to
identify sentinel lymph node metastasis by detecting areas of
increased uptake on PET-CT. However, B-mode ultrasound-guided
biopsy and surgical specimen examination findings showed that
certain nodes with increased uptake on PET-CT exhibited
inflammatory hyperplasia but not metastasis. The reasons for this
are unclear. PET-CT findings alone are therefore insufficient for
the definitive diagnosis of lymph node metastasis, and they should
be used in combination with ultrasound-guided biopsy findings. The
Affiliated Foshan Hospital started using a Philips Gemini PET-CT
scanner (Philips Healthcare, Best, the Netherlands) in February
2004. In the present study, only 11 patients underwent both PET-CT
and ultrasound guided biopsy, and the accuracy rate for diagnosis
of sentinel lymph node metastasis was 100% in these patients. Prior
to the introduction of PET-CT, patients with suspected sentinel
lymph node metastasis underwent B-mode ultrasound and CT
examinations, but the findings were less precise than those with
PET-CT. Distant metastasis can be detected early using PET-CT
alone, and patients with distant metastasis are considered to have
unresectable disease.
The pathogenesis of Marjolin's ulcers remains poorly
understood. Development of squamous cell carcinoma in burn scar
ulcers was reported to be associated with local Fas gene
mutation and deletion (24,25). Diagnosis of Marjolin's ulcers depends
on the pathological examination of biopsy specimens. Sampling from
different sites increases the diagnostic rate (16). Patients with chronic or recurrent
skin ulcers that do not heal after several months of conservative
treatment should undergo biopsy for early diagnosis. Marjolin's
ulcers should be treated by extended resection and skin grafting or
skin flap repair (26). The
resection margin should extend ≥2 cm beyond the edges of the lesion
(20). Amputation is necessary when
the tumor has invaded the bones, for aggressive tumors and for
tumors that cannot otherwise be resected with adequate margins.
Sentinel lymph node dissection is required in patients with
sentinel lymph node metastasis (9,20,26).
Patients with squamous cell carcinoma and sentinel lymph node
metastasis can undergo amputation and sentinel lymph node
dissection. The present data confirm that squamous cell
carcinoma-type Marjolin's ulcers can occur in different regions of
the body, but that sentinel lymph node metastasis most commonly
occurs in limb lesions, particularly of the lower limb. Patients
with limb lesions can therefore be treated by amputation and
sentinel lymph node dissection with satisfactory results.
Similar to patients with squamous cell carcinoma,
patients with melanoma who do not have metastasis should undergo
more extended resection and skin grafting or skin flap repair.
Patients with sentinel lymph node metastasis but no distant
metastasis should undergo amputation with lymph node dissection. In
the present study, all malignant melanoma-type ulcers occurred in
the lower limb. However, unlike with squamous cell carcinoma,
patients with melanoma and with distant or extensive lymph node
metastasis cannot be cured by surgical treatment, and interferon
therapy should be considered in these patients, despite its poor
curative effects.
There is no evidence that radiotherapy is a
successful first-line treatment choice for squamous cell carcinoma.
Squamous cell carcinoma in Marjolin's ulcers is usually well- or
moderately differentiated, and radiotherapy is therefore not
effective (16,21). Radiotherapy may also induce further
carcinomatous change. Radiotherapy was therefore not selected as
the first treatment choice in any of the patients in this
study.
Marjolin's ulcers are preventable. Chronic skin
ulcers should be actively treated to avoid carcinomatous change
(26,27). In this study, the mean patient age
was 64.15 years. The majority of the patients had been treated
ineffectively with Chinese herbs or other local remedies due to
their poor financial circumstances, and some did not receive any
treatment. This resulted in chronic ulceration that eventually
underwent carcinomatous change. Recently, a new cooperative medical
care system has been developed in rural areas of China, and the
Urban Employee Medical Insurance system has been established
(28,29). Patients with financial restrictions
can therefore be treated in hospital, which may help to reduce the
incidence of Marjolin's ulcers.
In conclusion, the results of the present study
strongly indicate that chronic skin ulcers should be treated as
early as possible and carefully followed-up. PET-CT combined with
B-mode ultrasound-guided biopsy can precisely detect sentinel lymph
node metastasis and guide clinical therapy. Patients with squamous
cell carcinoma- or melanoma-type Marjolin's ulcers and sentinel
lymph node metastasis should undergo amputation and sentinel lymph
node dissection, since such tumors predominantly occur in the limb,
particularly in the lower limb.
Acknowledgements
The study was supported by Medical Scientific
Research Foundation of Guangdong Province, China (no.
A2012637).
References
|
1
|
Steffen C: Marjolin's ulcer. Report of two
cases and evidence that Marjolin did not describe cancer arising in
scars of burns. Am J Dermatopathol. 6:187–193. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Da Costa JC III: Carcinomatous changes in
an area of chronic ulceration, or Marjolin's ulcer. Ann Surg.
37:496–502. 1903.PubMed/NCBI
|
|
3
|
Nayil K, Hafiz A, Dar H, et al: Kangri
cancer invading the brain in a Kashmiri lady (Marjolin ulcer): A
case report. Neurosurg Q. 22:69–71. 2012. View Article : Google Scholar
|
|
4
|
Fleming MD, Hunt JL, Purdue GF and
Sandstad J: Marjolin's ulcer: A review and reevaluation of a
difficult problem. J Burn Care Rehabil. 11:460–469. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wani I: Kangri cancer. Surgery.
147:586–588. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie EF, Li AO, Wang SL, et al: Burn scar
carcinoma: Case reports and review of the literature. Ann MBC.
5:1021992.
|
|
7
|
Eltorai IM, Montroy RE, Kobayashi M, et
al: Clinical notes: Marjolin's ulcer in patients with spinal cord
injury. J Spinal Cord Med. 25:191–195. 2002.PubMed/NCBI
|
|
8
|
Smith J, Mello LF, Nogueira Neto NC, et
al: Malignancy in chronic ulcers and scars of the leg (Marjolin's
ulcer): a study of 21 patients. Skeletal Radiol. 30:331–337. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ozek C, Celik N, Bilkay U, et al:
Marjolin's ulcer of scalp: report of fourteen cases and review of
the literature. J Burn Care Rehabil. 22:65–72. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bauer T, David T, Rimareix F, et al:
Marjolin's ulcer in chronic osteomyelitic: seven cases and a review
of the literature. Rev Chir Orthop Reparatrice Appar Mot. 93:63–71.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bauk VOZ, Assunção AM, Domingues RF, et
al: Marjolin's ulcer: a twelve-case report. An Bras Dermatol.
81:355–358. 2006.
|
|
12
|
Schoeman BJ: Squamous cell carcinoma in
neuropathic plantar ulcers in leprosy: another example of
Marjolin's ulcer. S Afr Med J. 86:966–969. 1996.(In Dutch).
PubMed/NCBI
|
|
13
|
Barr LH and Menard JW: Marjolin's ulcer:
The LSU experience. Cancer. 52:173–175. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gül U and Kiliç A: Squamous cell carcinoma
developing on burn scar. Ann Plast Surg. 56:406–408. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Copcu E and Culhaci N: Marjolin's ulcer on
the nose. Burns. 28:701–704. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozek C, Cankayali R, Bilkay U, et al:
Marjolin's ulcers arising in burn scars. J Burn Care Rehabil.
22:384–389. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kowal-Vern A and Criswell BK: Burn scar
neoplasms: a literature review and statistical analysis. Burns.
31:403–413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Treves N and Pack GT: The development of
cancer in burn scars. Surg Gynecol Obstel. 51:7491930.
|
|
19
|
Novick M, Gard DA, Hardy SB and Spira M:
Burn scar carcinoma: a review and analysis of 46 cases. J Trauma.
17:809–817. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sabin SR, Goldstein G, Rosenthal HG and
Haynes KK: Aggressive squamous cell carcinoma originating as a
Marjolin's ulcer. Dermatol Surg. 30:229–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ames FC and Hickey RC: Squamous cell
carcinoma of the skin of the extremities. Int Adv Surg Oncol.
3:179–199. 1980.PubMed/NCBI
|
|
22
|
Mijnhout GS, Hoekstra OS, van Tulder MW,
et al: Systematic review of the diagnostic accuracy of
(18)F-fluorodeoxyglucose positron emission tomography in melanoma
patients. Cancer. 91:1530–1542. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eastman AL, Erdman WA, Lindberg GM, et al:
Sentinel lymph node biopsy identifies occult nodal metastases in
patients with Marjolin's ulcer. J Burn Care Rehabil. 25:241–245.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee SH, Shin MS, Kim HS, et al: Somatic
mutations of Fas (Apo-1/CD95) gene in cutaneous squamous cell
carcinoma arising from a burn scar. J Invest Dermatol. 114:122–126.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baliarsing AS: Will Fas gene help to
diagnose burn scar squamous cell carcinoma? Plast Reconstr Surg.
108:5752001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Phillips TJ, Salman SM, Bhawan J and
Rogers GS: Burn scar carcinoma. Diagnosis and management. Dermatol
Surg. 24:561–565. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Spring PM, Myers JN, EI-Naggar AK and
Langstein HN: Malignant melanoma arising within a burn scar case
report and review of the literature. Ann Otol Rhinol Laryngeol.
110:369–376. 2001. View Article : Google Scholar
|
|
28
|
Shi L and Zhang D: China's new rural
cooperative medical scheme and underutilization of medical care
among adults over 45: Evidence from CHARLS pilot data. J Rural
Health. 29:(Suppl 1). s51–s61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou Q, Hong D, Lu J, Zheng D, Ashwani N
and Hu S: Pediatric medical care system in China has significantly
reduced abandonment of acute lymphoblastic leukemia treatment. J
Pediatr Hematol Oncol. 37:181–184. 2015. View Article : Google Scholar : PubMed/NCBI
|